Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extraction
2.3. Quality Assessment
3. Results
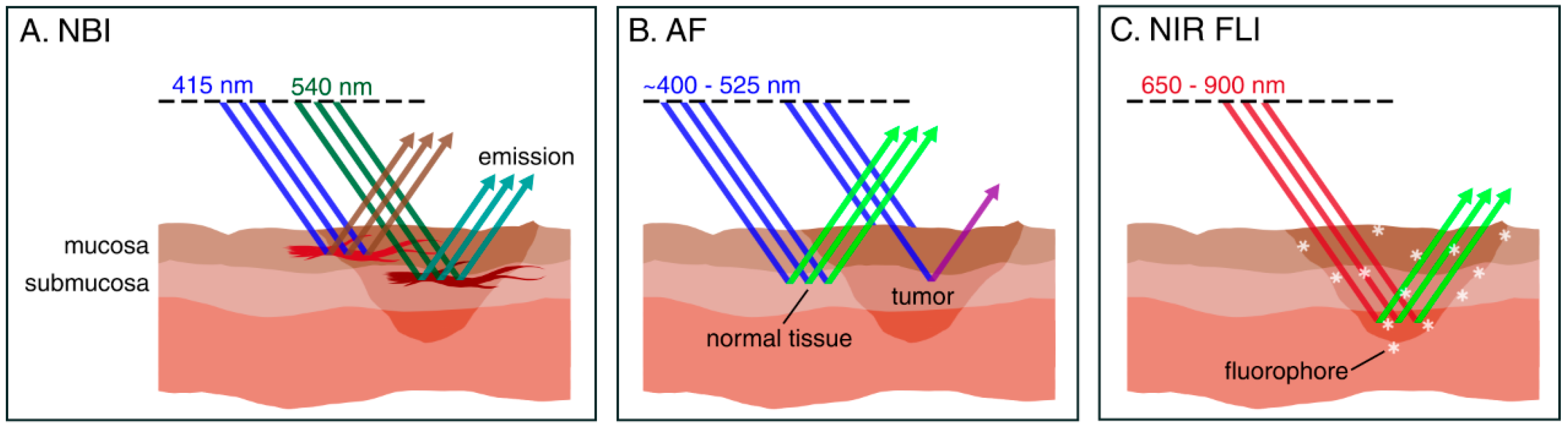
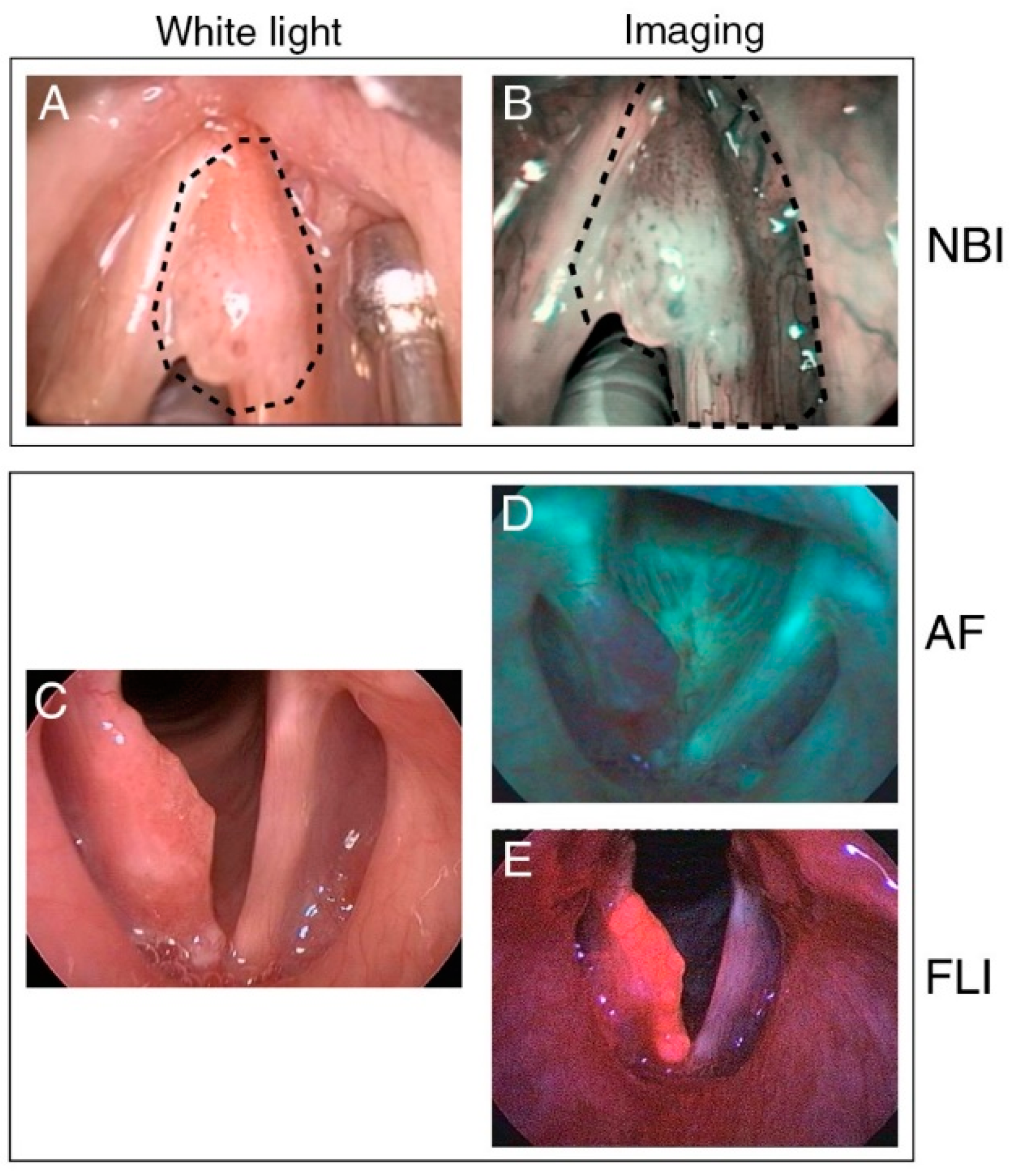
3.1. Intraoperative Narrow-Band Imaging
3.2. Intraoperative Autofluorescence Imaging
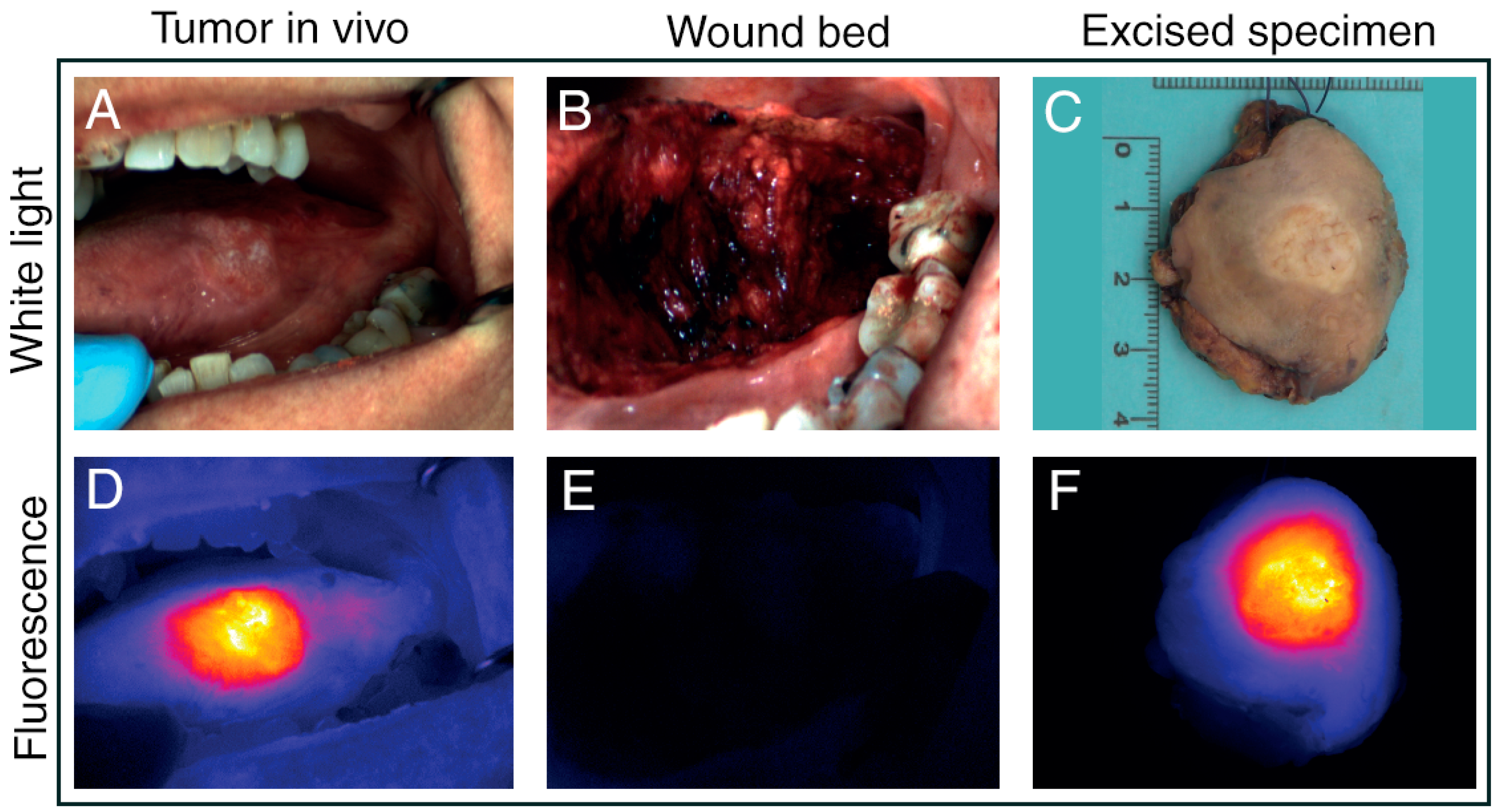
3.3. Intraoperative Fluorescence Imaging
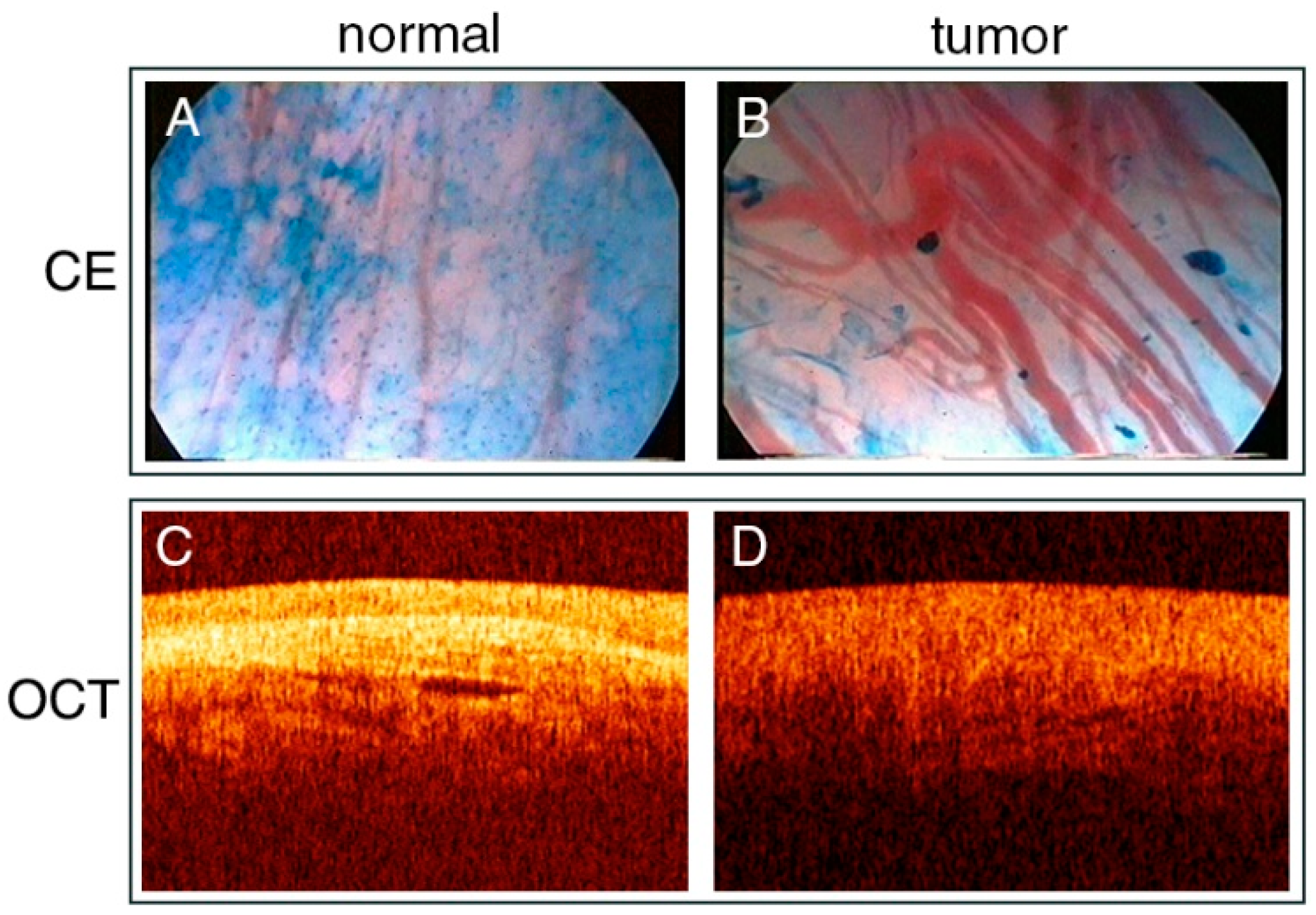
3.4. Contact Endoscopy
3.5. Optical Coherence Tomography
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Obid, R.; Redlich, M.; Tomeh, C. The Treatment of Laryngeal Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fiz, I.; Mazzola, F.; Fiz, F.; Marchi, F.; Filauro, M.; Paderno, A.; Parrinello, G.; Piazza, C.; Peretti, G. Impact of Close and Positive Margins in Transoral Laser Microsurgery for Tis–T2 Glottic Cancer. Front. Oncol. 2017, 7, 245. [Google Scholar] [CrossRef]
- Brandstorp-Boesen, J.; Falk, R.S.; Evensen, J.F.; Boysen, M.; Brøndbo, K. Risk of Recurrence in Laryngeal Cancer. PLoS ONE 2016, 11, e0164068. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Savard, C.; Topf, M.C.; Keane, W.; Luginbuhl, A.; Curry, J.; Cognetti, D. Association of Positive Initial Margins With Survival Among Patients With Squamous Cell Carcinoma Treated With Total Laryngectomy. JAMA Otolaryngol. Neck Surg. 2018, 144, 1030. [Google Scholar] [CrossRef]
- Zhu, X.; Heng, Y.; Zhou, L.; Tao, L.; Zhang, M. A prognostic nomogram for predicting risk of recurrence in laryngeal squamous cell carcinoma patients after tumor resection to assist decision making for postoperative adjuvant treatment. J. Surg. Oncol. 2019, 120, 698–706. [Google Scholar] [CrossRef]
- Saraniti, C.; Speciale, R.; Gallina, S.; Salvago, P. Prognostic role of resection margin in open oncologic laryngeal surgery: Survival analysis of a cohort of 139 patients affected by squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2019, 85, 603–610. [Google Scholar] [CrossRef]
- Sjögren, E. Transoral Laser Microsurgery in Early Glottic Lesions. Curr. Otorhinolaryngol. Rep. 2017, 5, 56–68. [Google Scholar] [CrossRef]
- Chiesa-Estomba, C.M.; González-García, J.A.; Larruscain, E.; Calvo-Henríquez, C.; Mayo-Yáñez, M.; A Sistiaga-Suarez, J.; Sistiaga-Suarez, J. CO2 Transoral Laser Microsurgery in Benign, Premalignant and Malignant (Tis, T1, T2) Lesion of the Glottis. A Literature Review. Medicines 2019, 6, 77. [Google Scholar] [CrossRef]
- Michel, J.; Fakhry, N.; Duflo, S.; Lagier, A.; Mancini, J.; Dessi, P.; Giovanni, A. Prognostic value of the status of resection margins after endoscopic laser cordectomy for T1a glottic carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 297–300. [Google Scholar] [CrossRef]
- Fiz, I.; Koelmel, J.C.; Sittel, C. Nature and role of surgical margins in transoral laser microsurgery for early and intermediate glottic cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, M.; Montagne, M.W.; Langeveld, T.P.M.; Veselic, M.; Van Benthem, P.P.G.; Sjögren, E.V. Evaluation of surgical margin status in patients with early glottic cancer (Tis-T2) treated with transoral CO2 laser microsurgery, on local control. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’Ara, S.; Leider-Trejo, L.; Akrish, S.; Cohen, J.T.; Billan, S.; Gil, Z. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: A prospective randomized controlled study. Head Neck 2016, 38, E1803–E1809. [Google Scholar] [CrossRef]
- Van Lanschot, C.G.; Mast, H.; Hardillo, J.A.; Monserez, D.; Hove, I.T.; Barroso, E.M.; Cals, F.L.; Smits, R.W.; Van Der Kamp, M.F.; Meeuwis, C.A.; et al. Relocation of inadequate resection margins in the wound bed during oral cavity oncological surgery: A feasibility study. Head Neck 2019, 41, 2159–2166. [Google Scholar] [CrossRef]
- Hughes, O.R.; Stone, N.; Kraft, M.; Arens, C.; Birchall, M.A.; Stone, N. Optical and molecular techniques to identify tumor margins within the larynx. Head Neck 2010, 32, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, G.; Cecconi, L.; Gallo, O. Laryngeal preneoplastic lesions and cancer: Challenging diagnosis. Qualitative literature review and meta-analysis. Crit. Rev. Oncol. 2016, 106, 64–90. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Garofolo, S.; Piazza, C.; Del Bon, F.; Mangili, S.; Guastini, L.; Mora, F.; Nicolai, P.; Peretti, G. Intraoperative Narrow Band Imaging Better Delineates Superficial Resection Margins During Transoral Laser Microsurgery for Early Glottic Cancer. Ann. Otol. Rhinol. Laryngol. 2014, 124, 294–298. [Google Scholar] [CrossRef]
- Hainăroșie, R.; Anghelina, F.; Hainăroșie, M.; Pietroșanu, C.; Ioniță, I.; Rusescu, A.; Paduraru, D.N.; Ene, B.; Badiu, D.C.; Zainea, V. The utility of Narrow Band Imaging in obtaining disease free resection margins during endo-scopic surgery of the larynx. Rom. Biotechnol. Lett. 2019, 24, 360–365. [Google Scholar] [CrossRef]
- Klimza, H.; Jackowska, J.; Piazza, C.; Banaszewski, J.; Wierzbicka, M. The role of intraoperative narrow-band imaging in transoral laser microsurgery for early and moderately advanced glottic cancer. Braz. J. Otorhinolaryngol. 2019, 85, 228–236. [Google Scholar] [CrossRef]
- Piersiala, K.; Klimza, H.; Jackowska, J.; Majewska, A.; Wierzbicka, M. Narrow band imaging in transoral laser microsurgery (TLM) in moderately advanced (T2, T3) glottic cancer. Otolaryngol. Polska 2018, 72, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Plaat, B.E.C.; Zwakenberg, M.A.; Van Zwol, J.G.; Wedman, J.; Van Der Laan, B.F.A.M.; Halmos, G.B.; Dikkers, F.G. Narrow-band imaging in transoral laser surgery for early glottic cancer in relation to clinical outcome. Head Neck 2017, 39, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Šifrer, R.; Urbančič, J.; Strojan, P.; Aničin, A.; Žargi, M. The assessment of mucosal surgical margins in head and neck cancer surgery with narrow band imaging. Laryngoscope 2017, 127, 1577–1582. [Google Scholar] [CrossRef]
- Srivastava, R. Narrow band imaging during transoral laser surgery for premalignant and early malignant glottic lesions. J. Head Neck Physicians Surg. 2016, 4, 35. [Google Scholar] [CrossRef]
- Vicini, C.; Montevecchi, F.; D’Agostino, G.; De Vito, A.; Meccariello, G. A novel approach emphasising intra-operative superficial margin enhancement of head-neck tumours with narrow-band imaging in transoral robotic surgery. Acta Otorhinolaryngol. Ital. 2015, 35, 157–161. [Google Scholar] [PubMed]
- Zwakenberg, M.A.; Halmos, G.B.; Wedman, J.; Laan, B.F.A.M.V.D.; Plaat, B.E.C. Evaluating Laryngopharyngeal Tumor Extension Using Narrow Band Imaging Versus Conventional White Light Imaging. Laryngoscope 2021. [Google Scholar] [CrossRef]
- Fielding, D.; Agnew, J.; Wright, D.; Hodge, R. DAFE autofluorescence assessment of oral cavity, larynx and bronchus in head and neck cancer patients. Photodiagnosis Photodyn. Ther. 2006, 3, 259–265. [Google Scholar] [CrossRef]
- Paczona, R.; Marandas, P.; Luboinski, B. Autofluorescence videoendoscopy for photodiagnosis of head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 544–548. [Google Scholar] [CrossRef]
- Succo, G.; Garofalo, P.; Fantini, M.; Monticone, V.; Abbona, G.; Crosetti, E. Direct autofluorescence during CO2 laser surgery of the larynx: Can it really help the surgeon? Acta Otorhinolaryngol. Ital. 2014, 34, 174–183. [Google Scholar]
- Czigner, J.; Csanády, M.; Kiss, J.G.; Ivan, L.; Jori, J. ALA (5-aminolevulinic acid)-induced protoporphyrin IX fluorescence in the endoscopic diagnostic and control of pharyngo-laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2003, 261, 262–266. [Google Scholar] [CrossRef]
- Digonnet, A.; Van Kerckhove, S.; Moreau, M.; Willemse, E.; Quiriny, M.; Ahmed, B.; Aubain, N.D.S.; Andry, G.; Bourgeois, P. Near infrared fluorescent imaging after intravenous injection of indocyanine green during neck dissection in patients with head and neck cancer: A feasibility study. Head Neck 2015, 38, E1833–E1837. [Google Scholar] [CrossRef] [PubMed]
- Dedivitis, R.A.; Pfuetzenreiter Jr, E.G.; Guimaraes, A.V. Contact endoscopy of the larynx as an auxiliary method to the surgical margins in frontolateral laryngectomy. Acta Otorhinolaryngol. Ital. 2009, 29, 16–20. [Google Scholar] [PubMed]
- Stefanescu, D.C.; Ceachir, O.; Zainea, V.; Hainarosie, M.; Pietrosanu, C.; Ionita, I.G.; Hainarosie, R. The Use of Methylene Blue in Assessing Disease Free Margins during CO2 LASER Assisted Direct Laryngoscopy for Glottis Cancer. Rev. De Chim. 2016, 67, 1327–1328. [Google Scholar] [CrossRef]
- Shakhov, A.V.; Terentjeva, A.B.; Kamensky, V.A.; Snopova, L.B.; Gelikonov, V.M.; Feldchtein, F.I.; Sergeev, A.M. Optical coherence tomography monitoring for laser surgery of laryngeal carcinoma. J. Surg. Oncol. 2001, 77, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Arens, C.; Reußner, D.; Woenkhaus, J.; Leunig, A.; Betz, C.S.; Glanz, H. Indirect fluorescence laryngoscopy in the diagnosis of precancerous and cancerous laryngeal lesions. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 621–626. [Google Scholar] [CrossRef]
- Kraft, M.; Fostiropoulos, K.; Gürtler, N.; Arnoux, A.; Davaris, N.; Arens, C. Value of narrow band imaging in the early diagnosis of laryngeal cancer. Head Neck 2015, 38, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Popek, B.; Bojanowska-Poźniak, K.; Tomasik, B.; Fendler, W.; Jeruzal-Świątecka, J.; Pietruszewska, W. Clinical experience of narrow band imaging (NBI) usage in diagnosis of laryngeal lesions. Otolaryngol. Polska 2019, 73, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Cacciola, S.; Fernandes, W.B.; Fernandes, C.M.; Occhini, A.; Tinelli, C.; Benazzo, M. Effectiveness of narrow band imaging in the detection of premalignant and malignant lesions of the larynx: Validation of a new endoscopic clinical classification. Head Neck 2014, 37, 215–222. [Google Scholar] [CrossRef]
- Ni, X.-G.; Zhang, Q.-Q.; Wang, G.-Q. Narrow band imaging versus autofluorescence imaging for head and neck squamous cell carcinoma detection: A prospective study. J. Laryngol. Otol. 2016, 130, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Sikka, K.; Sakthivel, P.; Thakar, A.; A Singh, C.; Sharma, S.C.; Rajeshwari, M.; Kakkar, A. Role of narrow band imaging in the diagnosis of laryngeal lesions: Pilot study from India. Indian J. Cancer 2018, 55, 242–247. [Google Scholar] [CrossRef]
- Klimza, H.; Jackowska, J.; Wierzbicka, M. The usefulness of the NBI—Narrow band imaging for the larynx assessment. Otolaryngol. Polska 2018, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; D’Aguanno, V.; Greco, A.; Ralli, M.; De Vincentiis, M. The Prognostic Value of Adding Narrow-Band Imaging in Transoral Laser Microsurgery for Early Glottic Cancer: A Review. Lasers Surg. Med. 2020, 52, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Triantafyllou, A.; Suárez, C.; López, F.; Hunt, J.L.; Strojan, P.; Williams, M.D.; Braakhuis, B.J.; De Bree, R.; Hinni, M.L.; et al. Surgical margins in head and neck cancer: Intra- and postoperative considerations. Auris Nasus Larynx 2019, 46, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, K.M.; Tan, M. Role of Advanced Laryngeal Imaging in Glottic Cancer. Otolaryngol. Clin. N. Am. 2015, 48, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Betz, C.S.; Leunig, A.; Arens, C. Value of fluorescence endoscopy for the early diagnosis of laryngeal cancer and its precursor lesions. Head Neck 2010, 33, 941–948. [Google Scholar] [CrossRef]
- Saetti, R.; Derosas, F.; Silvestrini, M.; Narne, S. Efficacy of autofluoroscence videoendoscopy in the diagnosis of laryngeal lesions. Acta Otorhinolaryngol. Ital. 2007, 27, 181–185. [Google Scholar]
- Vahrmeijer, A.L.; Hutteman, M.; Van Der Vorst, J.R.; Van De Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Keereweer, S.; Van Driel, P.; Snoeks, T.; Kerrebijn, J.; de Jong, R.; Vahrmeijer, A.; Sterenborg, H.J.; Löwik, C. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.K.; Galagali, J.R.; Sethi, A.; Malik, A. Contact endoscopy for detection of residual or recurrent disease after radiotherapy for squamous cell carcinoma of the upper aerodigestive tract. J. Laryngol. Otol. 2020, 134, 344–349. [Google Scholar] [CrossRef]
- Mishra, A.; Sahai, K.; Singh, S.; Datta, R.; Nilakantan, A.; Sethi, A. Contact Endoscopy—A promising tool for evaluation of laryngeal mucosal lesions. J. Laryngol. Voice 2012, 2, 53. [Google Scholar] [CrossRef]
- Sergeev, A.M.; Gelikonov, V.M.; Gelikonov, G.V.; I Feldchtein, F.; Kuranov, R.V.; Gladkova, N.D.; Shakhova, N.M.; Snopova, L.B.; Shakhov, A.V.; Kuznetzova, I.N.; et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt. Express 1997, 1, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, J.; Klimza, H.; Piersiala, K.; Jackowska, J.; Wierzbicka, M. The usefulness of the narrow band imaging (NBI) in decision-making process regarding second look procedure (SL) in laryngeal cancer follow-up after transoral laser microsurgery. PLoS ONE 2020, 15, e0236623. [Google Scholar] [CrossRef] [PubMed]
- Zabrodsky, M.; Lukeš, P.; Lukesova, E.; Boucek, J.; Plzák, J. The Role of Narrow Band Imaging in the Detection of Recurrent Laryngeal and Hypopharyngeal Cancer after Curative Radiotherapy. BioMed Res. Int. 2014, 2014, 175398. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, Y.-C.; Chen, W.-C.; Chen, M.-F.; Chen, C.-C.; Lee, K.-F. Detection of Mucosal Recurrent Nasopharyngeal Carcinomas after Radiotherapy with Narrow-Band Imaging Endoscopy. Int. J. Radiat. Oncol. 2012, 83, 1213–1219. [Google Scholar] [CrossRef]
- Bibas, A.; Podoleanu, A.; Cucu, R.; Bonmarin, M.; Dobre, G.; Ward, V.; Odell, E.; Boxer, A.; Gleeson, M.; Jackson, D. 3-D optical coherence tomography of the laryngeal mucosa*. Clin. Otolaryngol. 2004, 29, 713–720. [Google Scholar] [CrossRef]
- Kraft, M.; Lüerssen, K.; Lubatschowski, H.; Glanz, H.; Arens, C. Technique of Optical Coherence Tomography of the Larynx During Microlaryngoscopy. Laryngoscope 2007, 117, 950–952. [Google Scholar] [CrossRef]
- Burns, J.A. Optical coherence tomography. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Englhard, A.S.; Betz, T.; Volgger, V.; Lankenau, E.; Ledderose, G.J.; Stepp, H.; Homann, C.; Betz, C.S. Intraoperative assessment of laryngeal pathologies with optical coherence tomography integrated into a surgical microscope. Lasers Surg. Med. 2017, 49, 490–497. [Google Scholar] [CrossRef]
- Odenthal, J.; Rijpkema, M.; Bos, D.; Wagena, E.; Croes, H.; Grenman, R.; Boerman, O.; Takes, R.; Friedl, P. Targeting CD44v6 for fluorescence-guided surgery in head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Schmidt, F.; Dittberner, A.; Koscielny, S.; Petersen, I.; Guntinas-Lichius, O. Feasibility of real-time near-infrared indocyanine green fluorescence endoscopy for the evaluation of mucosal head and neck lesions. Head Neck 2016, 39, 234–240. [Google Scholar] [CrossRef]
- Mehlmann, M.; Stepp, H.; Baumgartner, R.; Betz, C.; Grevers, G.; Leunig, A.; Arbogast, S. Fluorescence staining of laryngeal neoplasms following topical application of 5-aminolevulinic acid: Preliminary results #. Biomed. Opt. 1999, 25, 414–420. [Google Scholar] [CrossRef]
- Odenthal, J.; Friedl, P.; Takes, R.P. Compatibility of CO 2 laser surgery and fluorescence detection in head and neck cancer cells. Head Neck 2019, 41, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.S.; Rosenthal, E.L.; Chung, T.K.; De Boer, E.; Patel, N.; Prince, A.C.; Korb, M.L.; Walsh, E.M.; Young, E.S.; Stevens, T.M.; et al. Characterizing the Utility and Limitations of Repurposing an Open-Field Optical Imaging Device for Fluorescence-Guided Surgery in Head and Neck Cancer Patients. J. Nucl. Med. 2016, 58, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Berg, N.S.V.D.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Zhou, Q.; Lu, G.; Chirita, S.U.; Kaplan, M.J.; Divi, V.; et al. Optimal Dosing Strategy for Fluorescence-Guided Surgery with Panitumumab-IRDye800CW in Head and Neck Cancer. Mol. Imaging Biol. 2020, 22, 156–164. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef]
- Voskuil, F.J.; De Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; De Visscher, S.A.H.J.; Schepman, K.-P.; Elias, S.G.; Meersma, G.-J.; Jonker, P.K.C.; et al. Fluorescence-guided imaging for resection margin evaluation in head and neck cancer patients using cetuximab-800CW: A quantitative dose-escalation study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef]
- Barroso, E.M.; Smits, R.W.; Van Lanschot, C.G.; Caspers, P.J.; Hove, I.T.; Mast, H.; Sewnaik, A.; Hardillo, J.A.; Meeuwis, C.A.; Verdijk, R.; et al. Water Concentration Analysis by Raman Spectroscopy to Determine the Location of the Tumor Border in Oral Cancer Surgery. Cancer Res. 2016, 76, 5945–5953. [Google Scholar] [CrossRef]
- Lau, D.P.; Huang, Z.; Lui, H.; Anderson, D.W.; Berean, K.; Morrison, M.D.; Shen, L.; Zeng, H. Raman spectroscopy for optical diagnosis in the larynx: Preliminary findings. Lasers Surg. Med. 2005, 37, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Pujary, P.; Maheedhar, K.; C, M.K.; Pujary, K. Raman Spectroscopic Methods for Classification of Normal and Malignant Hypopharyngeal Tissues: An Exploratory Study. Pathol. Res. Int. 2011, 2011, 632493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teh, S.K.; Zheng, W.; Lau, D.P.; Huang, Z. Spectroscopic diagnosis of laryngeal carcinoma using near-infrared Raman spectroscopy and random recursive partitioning ensemble techniques. Analyst 2009, 134, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.; Stavroulaki, P.; Kendall, C.; Birchall, M.; Barr, H. Raman Spectroscopy for Early Detection of Laryngeal Malignancy: Preliminary Results. Laryngoscope 2000, 110, 1756–1763. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Lin, K.; Zheng, W.; Lau, D.P.; Huang, Z. In vivo, real-time, transnasal, image-guided Raman endoscopy: Defining spectral properties in the nasopharynx and larynx. J. Biomed. Opt. 2012, 17, 077002. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zheng, W.; Lim, C.M.; Huang, Z. Real-time in vivo diagnosis of laryngeal carcinoma with rapid fiber-optic Raman spectroscopy. Biomed. Opt. Express 2016, 7, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Zheng, B.; Su, L.; Chen, Y.; Ma, S.; Hu, Q.; Zou, X.; Yao, L.; Yang, Y.; et al. Rapid histology of laryngeal squamous cell carcinoma with deep-learning based stimulated Raman scattering microscopy. Theranostics 2019, 9, 2541–2554. [Google Scholar] [CrossRef]
| First Author | Year | Imaging Technique 4 | Control Group | Study Design | T-Stage | n = (Imaging) | n = (Control) | Surgical Procedure | Application of Imaging | Negative Margin Definition |
|---|---|---|---|---|---|---|---|---|---|---|
| Fiz [3] | 2017 | NBI | WL 2 | Retrospective | Tis-T2 | 311 | 323 | TLM | Pre-resection | ≥1 mm |
| Garofolo [18] | 2014 | NBI | WL 2 | Prospective | Tis-T1a | 82 | 152 | TLM | Pre-resection | ≥1 mm |
| Hainarosie [19] | 2019 | NBI | WL 1 | nr | nr | 23 | 23 1 | TLM | Post-resection | nr |
| Klimza [20] | 2019 | NBI | na | Retrospective | T1-T2 | 44 | na | TLM | Pre-resection | ≥5 mm |
| Piersiala [21] | 2018 | NBI | na | Prospective | T2-T3 | 98 | na | TLM | Pre- and post-resection | ≥3 mm |
| Plaat [22] | 2017 | NBI | WL 2 | Retrospective | Tis-T2 | 42 | 51 | TLM | Pre- and post-resection | nr |
| Šifrer [23] | 2017 | NBI | WL 2 | Prospective | nr 3 | 14 | 8 | Laryngectomy | Pre- and post-resection | nr |
| Srivastava [24] | 2016 | NBI | na | Retrospective | Tis-T2 | 30 | na | TLM | Pre-resection | nr |
| Vicini [25] | 2015 | NBI | WL | Prospective | T1-T4 | 7 | 4 | Transoral surgery | Pre- and post-resection | ≥2 mm |
| Zwakenberg [26] | 2021 | NBI | WL 1 | Prospective | Tis-T4 | 89 | 89 1 | TLM, TLE | Pre-resection | nr |
| Fielding [27] | 2006 | AF | na | Prospective | nr | 48 | na | TLM | Pre-resection | nr |
| Paczona [28] | 2003 | AF | WL 1 | Prospective | nr | 10 | 10 1 | TLM | Pre- and post-resection | nr |
| Succo [29] | 2014 | AF | WL 1 | Prospective | T1-T2 | 73 | 73 1 | TLM | Pre-resection | ≥1 mm |
| Csanády [30] | 2004 | FLI (5-ALA) | na | nr | T1-T2 | 13 | na | TLM | Pre- and post-resection | nr |
| Digonnet [31] | 2016 | FLI (ICG) | na | Prospective | T1-T4 | 3 | na | Laryngectomy | Pre- and post-resection | nr |
| Dedivitis [32] | 2009 | CE | na | Prospective | T1b-T2 | 10 | na | Open surgery | Pre-resection | ≥2 mm |
| Stefanescu [33] | 2016 | CE | na | nr | T1-T2 | 43 | na | TLM | Pre-resection | nr |
| Shakhov [34] | 2001 | OCT | na | nr | Tis-T2 | 26 | na | TLM | Pre-resection | nr |
| Article | T-Stage | n = NBI 1/WL | Negative Margin Rate 2 NBI (vs. WL) | Patient-Related Outcome NBI (vs. WL) | Diagnostic Outcome NBI (vs. WL) | Other Findings Related to NBI |
|---|---|---|---|---|---|---|
| Fiz [3] | Tis-T2 | 311/323 | 50% (30%) | RFS3: 83.9% (78.9%) DSS4: 98.7% (98.8%) | nr | nr |
| Garofolo [18] | Tis-T1a | 82/152 | 96.4% (76.3%) | nr | nr | nr |
| Hainarosie [19] | nr | 23/23 | 98% (58.8%) | nr | nr | nr |
| Klimza [20] | T1-T2 | 44/na | 100% | Local recurrence: 3/44: 6.8% | nr | Additional lesions not seen with WL |
| Piersiala [21] | T2-T3 | 98/na | 100% | Local recurrence: 5/98: 5.10% | NBI + WL: Sens 5: 100% Spec 6: 98.88% PPV 7: 90% NPV 8: 100% Accuracy: 98.98% | Additional lesions not seen with WL in 10.2% of patients |
| Plaat [22] | Tis-T2 | 42/51 | nr | Local recurrence: 2% (24%) Two-year RFS: 98% (82%) | nr | nr |
| Šifrer [23] | T3-T4 | 14/8 | 88.9% (70.9%) | nr | NPV: 95.9% (88.4%) | nr |
| Srivastava [24] | Tis-T2 | 30/na | nr | nr | nr | Upstaging TNM class |
| Vicini [25] | T1-T4 | 7/4 | 87.9% (57.9%) | nr | Sens: 72.5% Spec: 66.7% NPV: 87.9% | nr |
| Zwakenberg [26] | Tis-T4 | 89/89 | nr | nr | Sens: 95% Spec: 82% PPV: 87% NPV: 92% Accuracy: 89% | 5.7% increase in identified tumor extent with NBI compared to WL (p = 0.02) |
| Imaging Technique | Working Principle | Clinical Usability for Intraoperative Margin Assessment: Pros (+) and Cons (−) |
|---|---|---|
| Narrow-Band Imaging (NBI) | NBI uses blue (415 nm) and green (540 nm) light corresponding with the main peak absorbance of hemoglobin, to enhance visibility of mucosal and submucosal capillaries, respectively. | + widely studied + provides real-time information + does not require exogenous agents + can be used to scan large surfaces for occult tumors + suitable for mucosal margin delineation (pre-resection) − not suitable for deep margin assessment |
| Autofluorescence (AF) | AF detects changes in tissue morphology and optical properties as a result of neoplasia. Using blue light, AF can differentiate between healthy and neoplastic laryngeal mucosa. | + provides real-time information + does not require exogenous agents + can be used to scan large surfaces for occult tumors − not suitable for deep margin assessment |
| Fluorescence Imaging (FLI) | FLI uses a systemically administered fluorescent agent that ‘targets’ tumor cells over healthy cells. A dedicated camera system is required to detect these fluorescent agents, facilitating real-time image-guided surgery. | + suitable for deep margin assessment (i.e., wound bed inspection) + provides real-time information + can be used to scan large surfaces for occult tumors + Near-infrared (NIR) FLI has high penetration depth (up to 10 mm) − requires administration of fluorescent agents − tumor-specific fluorescent agents have not been studied in laryngeal cancer yet |
| Contact Endoscopy (CE) | After staining of superficial mucosal cells with methylene blue, a microscopic endoscope is placed in direct contact with the surface, distinguishing tumor from healthy cells in vivo. | + Suitable to detect residual or recurrent cancer after radiotherapy − not suitable for deep margin assessment − requires topical staining − requires direct tissue contact − steep learning curve for image interpretation |
| Optical Coherence Tomography (OCT) | OCT is based on changes in refractive index of tumor cells, by detecting light that is backscattered off tissue boundaries. It is thus capable of imaging cross-sectional anatomy at high resolution in living tissue. | + provides real-time information + does not require exogenous agents − maximum penetration depth of ~2 mm − requires direct tissue contact |
| Raman Spectroscopy (RS) | RS uses spectral differences between normal and malignant tissue, and is capable of near-instant, accurate and non-invasive analysis of a tissue’s molecular composition. | + able to characterize tissues other than mucosa + suitable for deep margin assessment + high specificity + does not require exogenous agents − limited to point measurements − requires direct tissue contact − does not provide real-time information (although near-instant) − Intraoperative RS for margin assessment in laryngeal cancer has not been studied yet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauwerends, L.J.; Galema, H.A.; Hardillo, J.A.U.; Sewnaik, A.; Monserez, D.; van Driel, P.B.A.A.; Verhoef, C.; Baatenburg de Jong, R.J.; Hilling, D.E.; Keereweer, S. Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review. Cancers 2021, 13, 1895. https://doi.org/10.3390/cancers13081895
Lauwerends LJ, Galema HA, Hardillo JAU, Sewnaik A, Monserez D, van Driel PBAA, Verhoef C, Baatenburg de Jong RJ, Hilling DE, Keereweer S. Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review. Cancers. 2021; 13(8):1895. https://doi.org/10.3390/cancers13081895
Chicago/Turabian StyleLauwerends, Lorraine J., Hidde A. Galema, José A. U. Hardillo, Aniel Sewnaik, Dominiek Monserez, Pieter B. A. A. van Driel, Cornelis Verhoef, Robert J. Baatenburg de Jong, Denise E. Hilling, and Stijn Keereweer. 2021. "Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review" Cancers 13, no. 8: 1895. https://doi.org/10.3390/cancers13081895
APA StyleLauwerends, L. J., Galema, H. A., Hardillo, J. A. U., Sewnaik, A., Monserez, D., van Driel, P. B. A. A., Verhoef, C., Baatenburg de Jong, R. J., Hilling, D. E., & Keereweer, S. (2021). Current Intraoperative Imaging Techniques to Improve Surgical Resection of Laryngeal Cancer: A Systematic Review. Cancers, 13(8), 1895. https://doi.org/10.3390/cancers13081895








