Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Physicochemical Characterization
2.1. X-ray Scattering
2.2. Dynamic Light Scattering (DLS)
2.3. Static Light Scattering (SLS)
2.4. Zeta Potential (ZP) and Electrophoresis Light Scattering (ELS)
2.5. Scanning Electron Microscopy (SEM)
2.6. Transmission Electron Microscopy (TEM)
2.7. Scanning Probe Microscopies (SPM)
2.7.1. Atomic Force Microscopy (AFM)
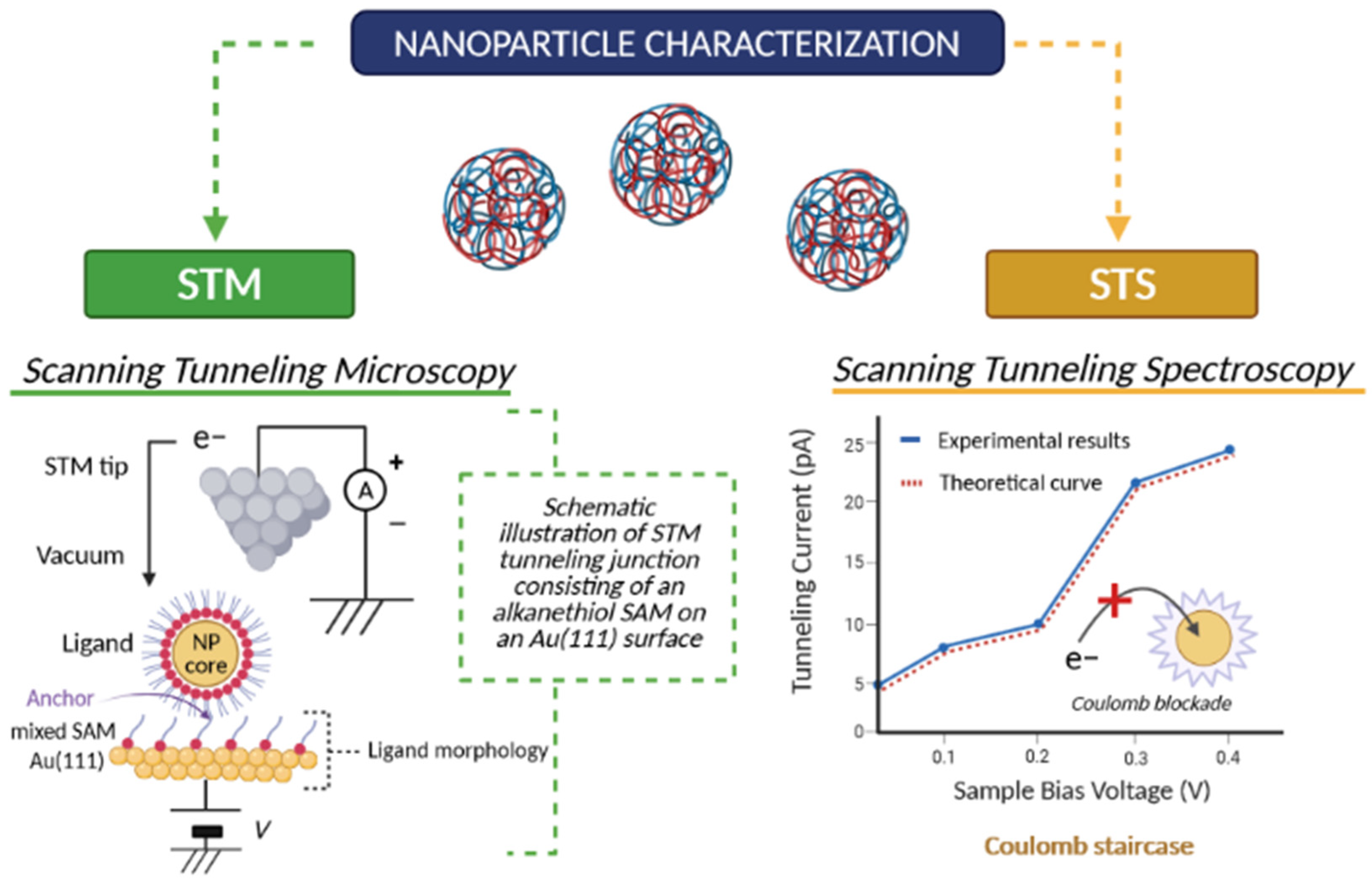
2.7.2. Scanning Tunneling Microscopy (STM) and Scanning Tunneling Spectroscopy (STS)
2.8. Porosimetry
2.9. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.10. Fluorescence Spectroscopy
2.11. Raman and Infrared Spectroscopy
2.12. Circular Dichroism (CD)
2.13. Nuclear Magnetic Resonance (NMR)
2.14. Mass Spectrometry (MS)
- (a)
- Ionization—the molecule is bombarded by a high-energy electron beam,
- (b)
- Fragmentation—occurs when excess vibrational energy is transferred to the molecular ion, with a process of scission of the bonds that hold the molecule together causing fragmentation. This technique can be used to determine the molecular weight, the atomic composition and the structural blocks observed through fragmentation [83].
2.15. Rheology
3. Effect of Physicochemical Properties on Nanopharmaceuticals Performance
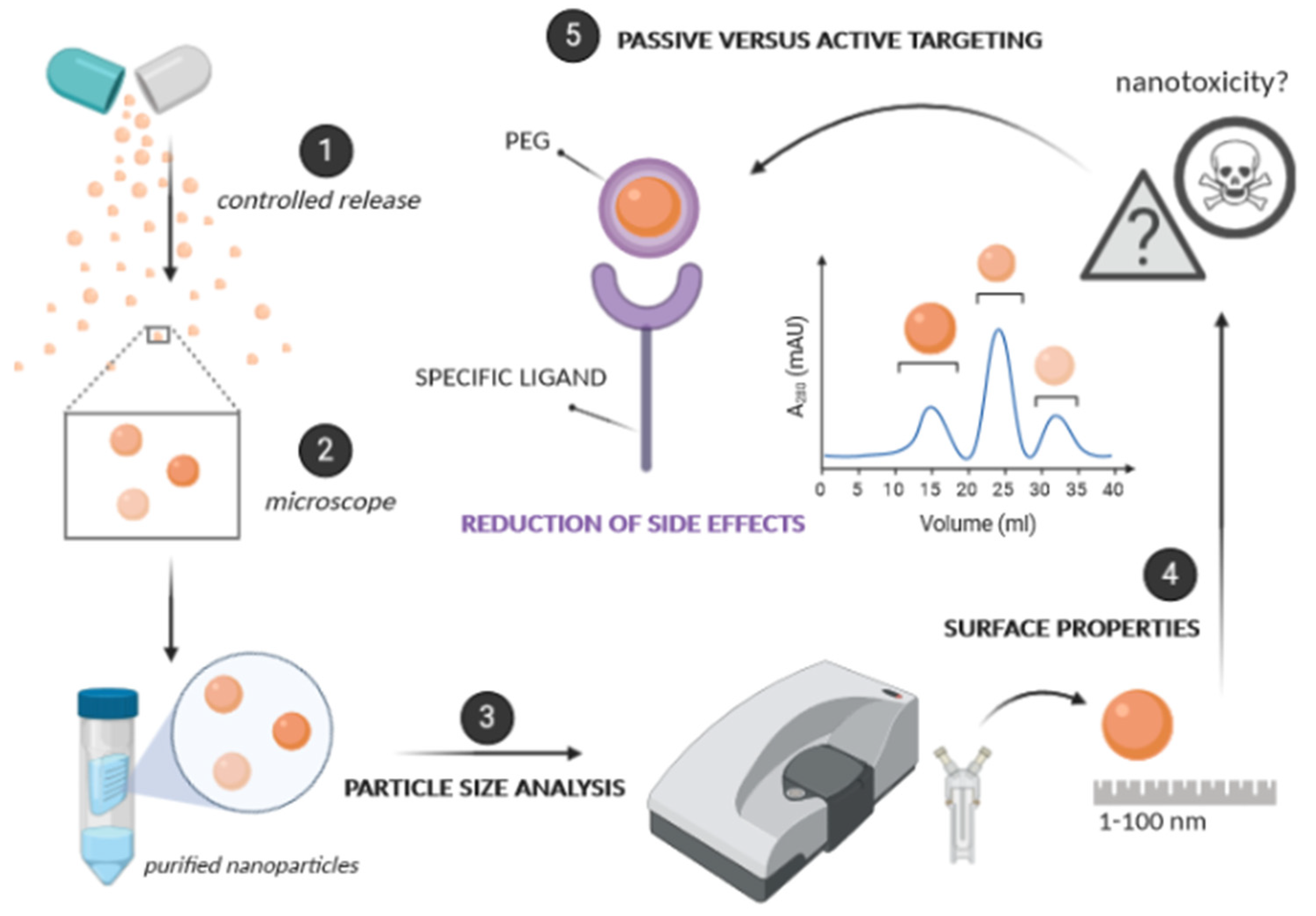
3.1. Size
3.2. Surface Properties
3.3. Passive versus Active Targeting
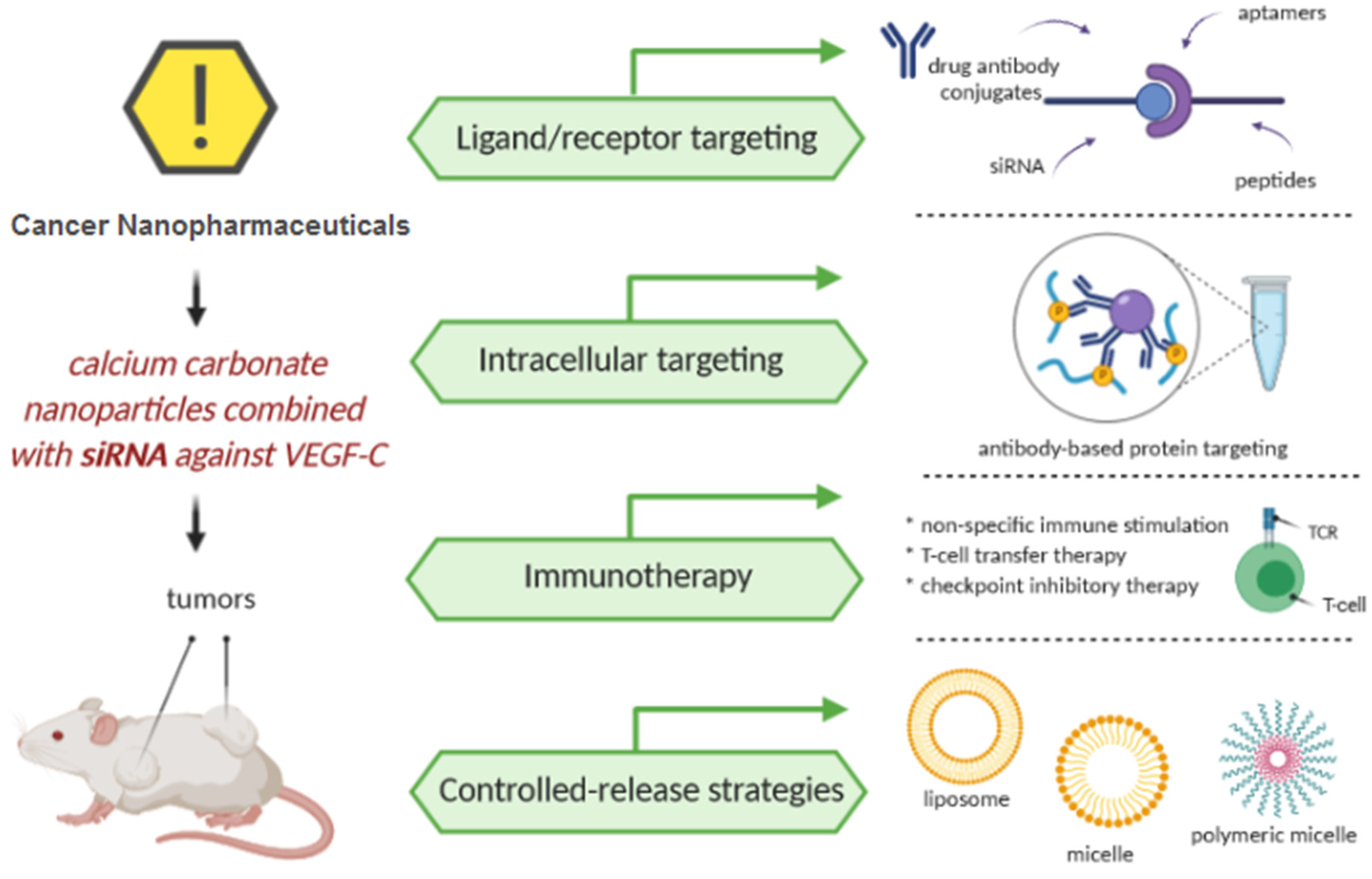
4. Cancer Nanopharmaceuticals
4.1. Ligand/Receptor Targeting
4.1.1. Small Molecule Receptors for Lectin and Foliates
4.1.2. Drug Antibody Conjugates
4.1.3. Aptamers
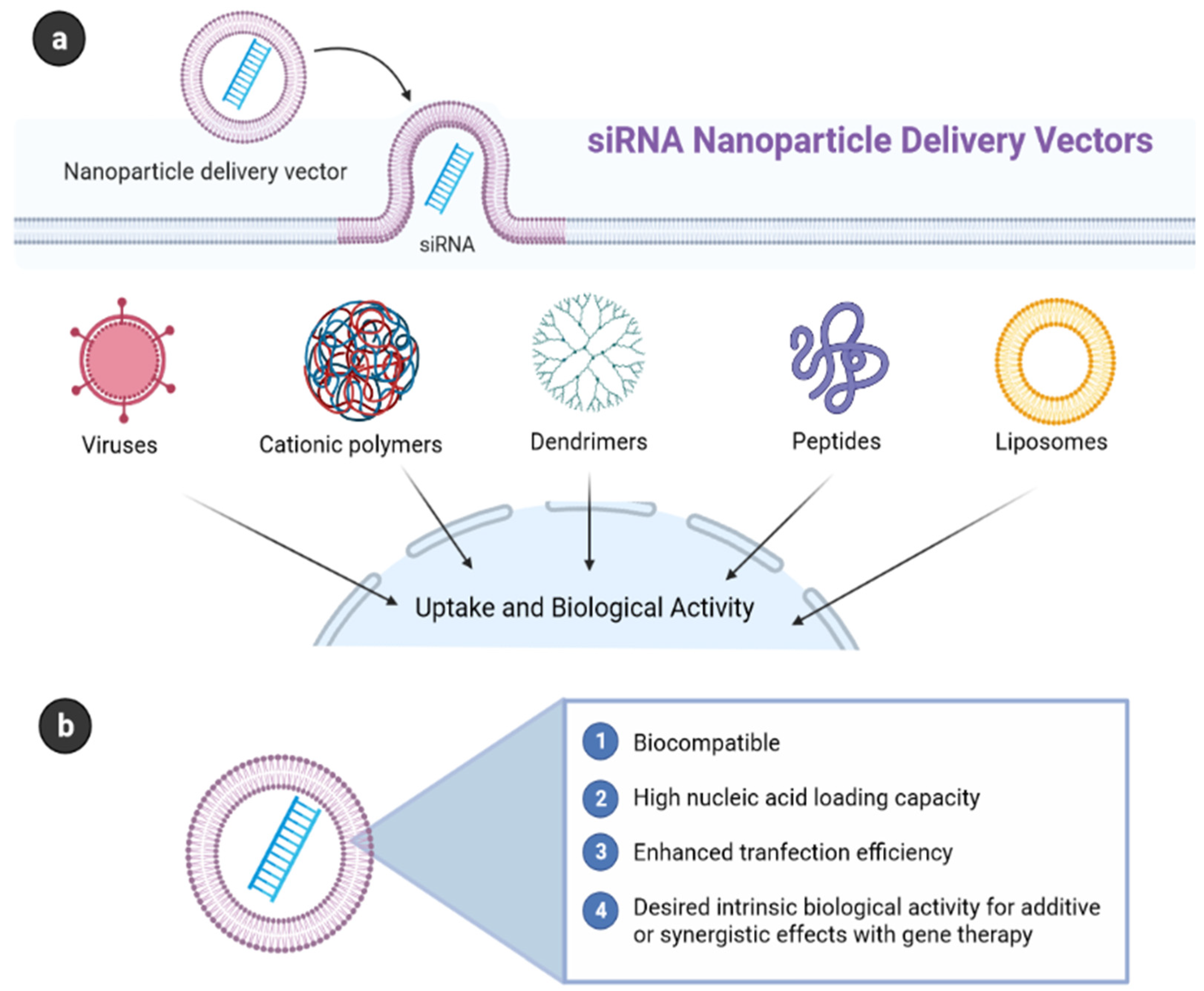
4.1.4. siRNA
4.1.5. Peptides
4.1.6. Cell-Penetrating Peptide (CPP) and Transferrin (Tf)
4.2. Intracellular Targeting
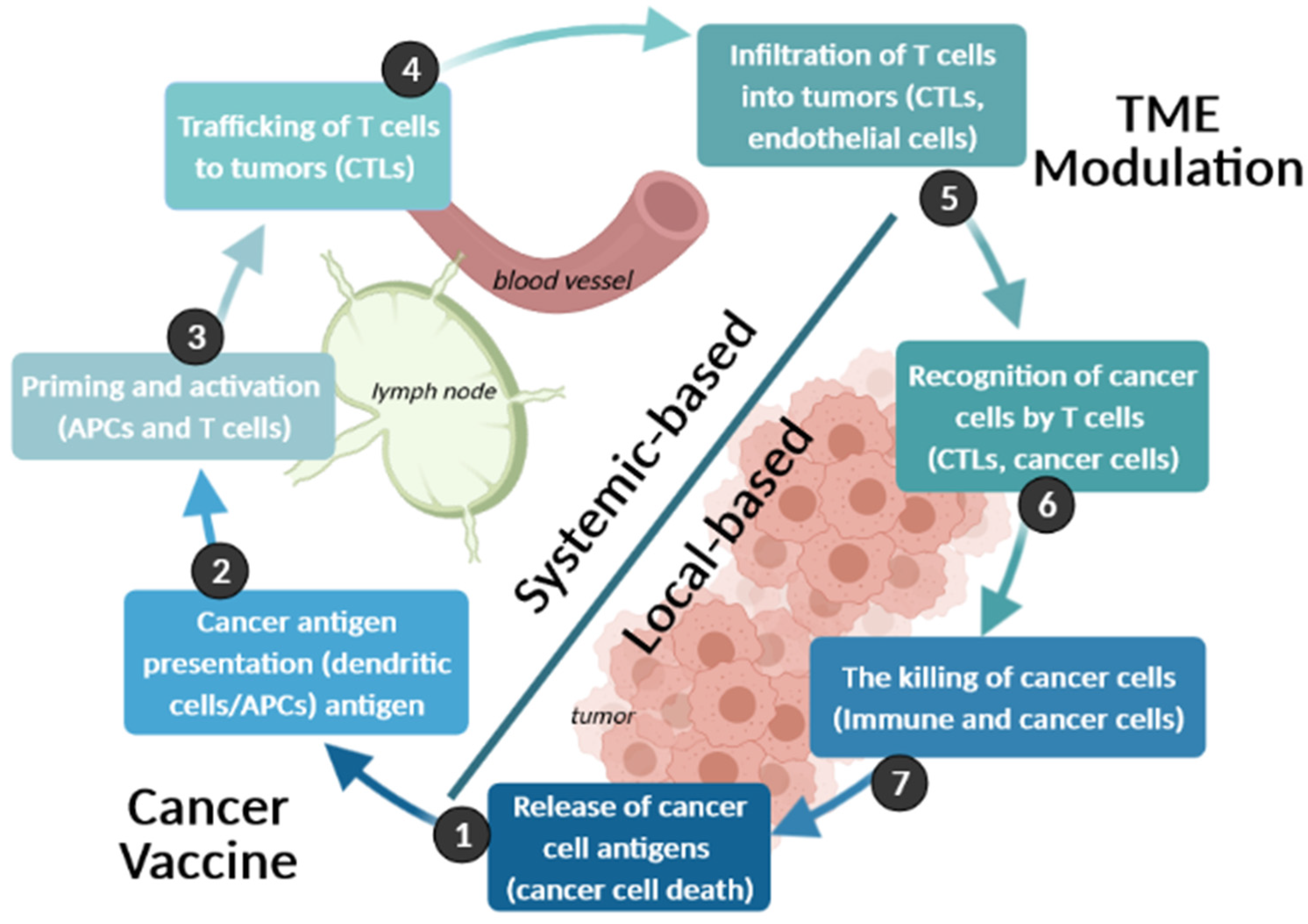
4.3. Immunotherapy
4.4. Controlled-Release Strategies
4.4.1. Inorganic Nanomaterials
4.4.2. Organic Nanomaterials
5. Nanopharmaceuticals-Based Cancer Treatments
6. Cancer Nanopharmaceuticals-Based Gene Delivery
7. The Impact of Cancer Nanopharmaceuticals on DNA Toxicity
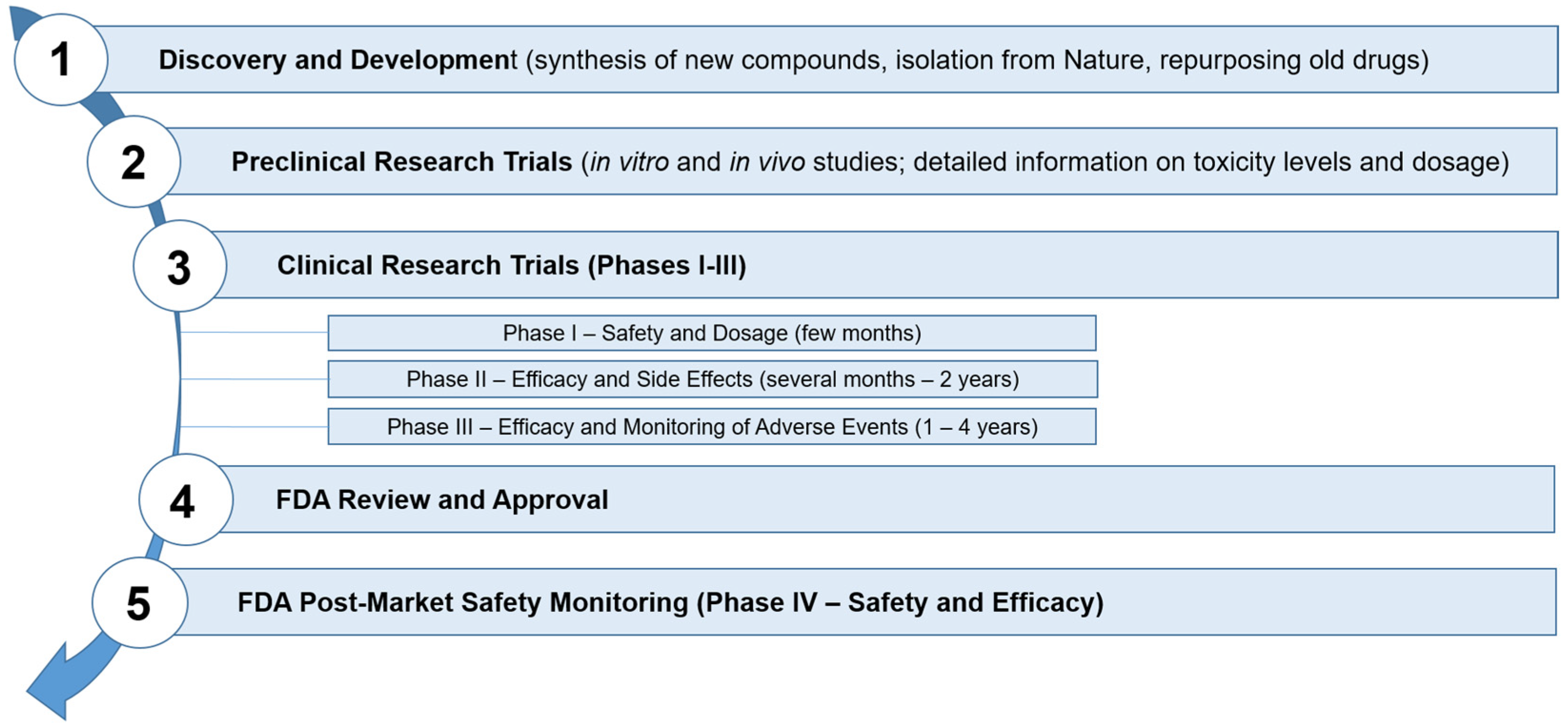
8. Regulatory Aspects
- How the physical-chemical property is measured;
- The way the property is reported;
- The measurement technique and the instrument used;
- The record in which the sample is collected and prepared for the examination.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.R.; George, S.K. Nanotherapeutics in cancer prevention, diagnosis and treatment. In Pharmacology and Therapeutics; BoD—Books on Demand: Norderstedt, Germany, 2014; p. 233. [Google Scholar]
- Parvanian, S.; Mostafavi, S.M.; Aghashiri, M. Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens. BioSens. Res. 2017, 13, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-Y.; Hu, H.-Z.; Qing, X.-C.; Zhang, Z.-C.; Shao, Z.-W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, G.; Rapalli, V.K.; Nagpal, S.; Dubey, S.K.; Saha, R.N. Nanocarriers as potential targeted drug delivery for cancer therapy. In Nanoscience in Medicine; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1, pp. 51–88. [Google Scholar]
- Souto, E.B.; Doktorovova, S.; Campos, J.R.; Martins-Lopes, P.; Silva, A.M. Surface-tailored anti-HER2/neu-solid lipid nanoparticles for site-specific targeting MCF-7 and BT-474 breast cancer cells. Eur. J. Pharm. Sci. 2019, 128, 27–35. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Zhang, N.; Liu, S.; Hu, W.; Nie, J.-J.; Zhang, K.; Yu, B.; Wang, Z.-G.; Xu, F.-J. Self-Assembled Nucleotide/Saccharide-Tethering Polycation-Based Nanoparticle for Targeted Tumor Therapy. ACS Mater. Lett. 2020, 2, 550–556. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.; Neves, A.; Pires, B.; Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-Conjugated Docetaxel-PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers 2019, 11, 1905. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control. Release 2016, 243, 303–322. [Google Scholar] [CrossRef]
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C 2020, 116, 111239. [Google Scholar] [CrossRef]
- Morais, R.P.; Novais, G.B.; Sangenito, L.S.; Santos, A.L.S.; Priefer, R.; Morsink, M.; Mendonça, M.C.; Souto, E.B.; Severino, P.; Cardoso, J.C. Naringenin-Functionalized Multi-Walled Carbon Nanotubes: A Potential Approach for Site-Specific Remote-Controlled Anticancer Delivery for the Treatment of Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4557. [Google Scholar] [CrossRef]
- Kuchur, O.; Tsymbal, S.; Shestovskaya, M.; Serov, N.; Dukhinova, M.; Shtil, A. Metal-derived nanoparticles in tumor theranostics: Potential and limitations. J. Inorg. Biochem. 2020, 209, 111117. [Google Scholar] [CrossRef]
- Muller, R.H.; Runge, S.; Ravelli, V.; Mehnert, W.; Thunemann, A.F.; Souto, E.B. Oral bioavailability of cyclosporine: Solid lipid nanoparticles (SLN) versus drug nanocrystals. Int. J. Pharm. 2006, 317, 82–89. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Junyaprasert, V.B.; Souto, E.B.; Muller, R.H. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology. Int. J. Pharm. 2008, 354, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Arias, J.L. Advanced methodologies to formulate nanotheragnostic agents for combined drug delivery and imaging. Expert Opin. Drug Deliv. 2011, 8, 1589–1608. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Spiliopoulou, M.; Valmas, A.; Triandafillidis, D.-P.; Kosinas, C.; Fitch, A.; Karavassili, F.; Margiolaki, I. Applications of X-ray powder diffraction in protein crystallography and drug screening. Crystals 2020, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.R.; Li, T.; Zhao, H.; Liu, J.; Lei, Y.; Zhang, X.; Ren, Y.; Elam, J.W.; Meyer, R.J.; Winans, R.E. In situ diffraction of highly dispersed supported platinum nanoparticles. Catal. Sci. Technol. 2014, 4, 3053–3063. [Google Scholar] [CrossRef]
- Letzel, A.; Gökce, B.; Menzel, A.; Plech, A.; Barcikowski, S. Primary particle diameter differentiation and bimodality identification by five analytical methods using gold nanoparticle size distributions synthesized by pulsed laser ablation in liquids. Appl. Surf. Sci. 2018, 435, 743–751. [Google Scholar] [CrossRef]
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded Solid Lipid Nanoparticles (SLN). Pharm. Dev. Technol. 2020, 25, 832–844. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small angle X-ray scattering for nanoparticle research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, K.; Zhan, C.; Geng, L.; Chu, B.; Hsiao, B.S. Characterization of nanocellulose using small-angle neutron, X-ray, and dynamic light scattering techniques. J. Phys. Chem. B 2017, 121, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Hassan, P.A.; Rana, S.; Verma, G. Making sense of Brownian motion: Colloid characterization by dynamic light scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Pawłowska, S.; Kowalewski, T.; Pierini, F. Fibrous polymer nanomaterials for biomedical applications and their transport by fluids: An overview. Soft Matter 2018, 14, 8421–8444. [Google Scholar] [CrossRef]
- Kureha, T.; Minato, H.; Suzuki, D.; Urayama, K.; Shibayama, M. Concentration dependence of the dynamics of microgel suspensions investigated by dynamic light scattering. Soft Matter 2019, 15, 5390–5399. [Google Scholar] [CrossRef]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Sivakumaran, M.; Platt, M. Tunable resistive pulse sensing: Potential applications in nanomedicine. Nanomedicine 2016, 11, 2197–2214. [Google Scholar] [CrossRef] [Green Version]
- Temel, D.B.; Kinderman, F.; Eryilmaz, E. Developability in biophysical characterization. In Biophysical Characterization of Proteins in Developing Biopharmaceuticals, 2nd ed.; Houde, D.J., Berkowitz, S.A., Eds.; Elsevier: Cambridge, MA, USA, 2020; Chapter 17; pp. 505–526. [Google Scholar] [CrossRef]
- Minton, A.P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016, 501, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofail, S.A.; Bauer, J. Electrically polarized biomaterials. Adv. Mater. 2016, 28, 5470–5484. [Google Scholar] [CrossRef]
- Zielińska, A.; Ferreira, N.R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Mamouni, S.E.; Silva, A.M.; Nowak, I.; Santini, A.; Souto, E.B. Development and optimization of alpha-pinene-loaded solid lipid nanoparticles (SLN) using experimental factorial design and dispersion analysis. Molecules 2019, 24, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Corbett, J.C.; McNeil-Watson, F.; Jack, R.O.; Howarth, M. Measuring surface zeta potential using phase analysis light scattering in a simple dip cell arrangement. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 169–176. [Google Scholar] [CrossRef]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Keller, A.A.; Wang, T.; Li, F. The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths. Sci. Total Environ. 2014, 487, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Nellist, M.R.; Chen, Y.; Mark, A.; Gödrich, S.; Stelling, C.; Jiang, J.; Poddar, R.; Li, C.; Kumar, R.; Papastavrou, G. Atomic force microscopy with nanoelectrode tips for high resolution electrochemical, nanoadhesion and nanoelectrical imaging. Nanotechnology 2017, 28, 095711. [Google Scholar] [CrossRef] [PubMed]
- Spyratou, E.; Makropoulou, M.; Mourelatou, E.; Demetzos, C. Biophotonic techniques for manipulation and characterization of drug delivery nanosystems in cancer therapy. Cancer Lett. 2012, 327, 111–122. [Google Scholar] [CrossRef]
- Korolkov, V.V.; Summerfield, A.; Murphy, A.; Amabilino, D.B.; Watanabe, K.; Taniguchi, T.; Beton, P.H. Ultra-high resolution imaging of thin films and single strands of polythiophene using atomic force microscopy. Nat. Commun. 2019, 10, 1537. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. Handbook of Nano-Technology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Oliveira Brett, A.M.; Chiorcea, A.-M. Atomic force microscopy of DNA immobilized onto a highly oriented pyrolytic graphite electrode surface. Langmuir 2003, 19, 3830–3839. [Google Scholar] [CrossRef] [Green Version]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface studies by scanning tunneling microscopy. Phys. Rev. Lett. 1982, 49, 57. [Google Scholar] [CrossRef] [Green Version]
- Wiesendanger, R.; Roland, W. Scanning Probe Microscopy and Spectroscopy: Methods and Applications; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Biscarini, F.; Ong, Q.K.; Albonetti, C.; Liscio, F.; Longobardi, M.; Mali, K.S.; Ciesielski, A.; Reguera, J.; Renner, C.; De Feyter, S. Quantitative analysis of scanning tunneling microscopy images of mixed-ligand-functionalized nanoparticles. Langmuir 2013, 29, 13723–13734. [Google Scholar] [CrossRef] [PubMed]
- Ong, Q.K.; Reguera, J.; Silva, P.J.; Moglianetti, M.; Harkness, K.; Longobardi, M.; Mali, K.S.; Renner, C.; De Feyter, S.; Stellacci, F. High-resolution scanning tunneling microscopy characterization of mixed monolayer protected gold nanoparticles. ACS Nano 2013, 7, 8529–8539. [Google Scholar] [CrossRef]
- Kano, S.; Tada, T.; Majima, Y. Nanoparticle characterization based on STM and STS. Chem. Soc. Rev. 2015, 44, 970–987. [Google Scholar] [CrossRef] [Green Version]
- Hansma, P.; Elings, V.; Marti, O.; Bracker, C. Scanning tunneling microscopy and atomic force microscopy: Application to biology and technology. Science 1988, 242, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Delbianco, M.; Anggara, K.; Michnowicz, T.; Pardo-Vargas, A.; Bharate, P.; Sen, S.; Pristl, M.; Rauschenbach, S.; Schlickum, U. Imaging single glycans. Nature 2020, 582, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Erdal, M.S.; Güngör, S. Electrospun Nanofibers as Carriers in Dermal Drug Delivery. In Nanopharmaceuticals: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2020; Volume 3, pp. 139–163. [Google Scholar]
- Astruc, D. Introduction: Nanoparticles in catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, E. 6—Recent advances in thermal analysis of nanoparticles: Methods, models and kinetics. In Modeling, Characterization, and Production of Nanomaterials; Tewary, V.K., Zhang, Y., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2015; pp. 167–178. [Google Scholar] [CrossRef]
- Cavendish, M.; Nalone, L.; Barbosa, T.; Barbosa, R.; Costa, S.; Nunes, R.; da Silva, C.F.; Chaud, M.V.; Souto, E.B.; Hollanda, L.; et al. Study of pre-formulation and development of solid lipid nanoparticles containing perillyl alcohol. J. Therm. Anal. Calorim. 2019, 1–8. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; Garcia, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, L.; Castelli, F.; Sarpietro, M.G. Differential scanning calorimetry analyses of idebenone-loaded solid lipid nanoparticles interactions with a model of bio-membrane: A comparison with in vitro skin permeation data. Pharmaceuticals 2018, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Kunc, F.; Balhara, V.; Sun, Y.; Daroszewska, M.; Jakubek, Z.J.; Hill, M.; Brinkmann, A.; Johnston, L.J. Quantification of surface functional groups on silica nanoparticles: Comparison of thermogravimetric analysis and quantitative NMR. Analyst 2019, 144, 5589–5599. [Google Scholar] [CrossRef]
- Biedunkiewicz, A.; Gabriel, U.; Figiel, P.; Grzesiak, D. Application of thermal analysis in nanotechnology. J. Therm. Anal. Calorim. 2010, 101, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.C.; Zeng, H.; Guo, C.; Cai, W. Nanomaterials: Processing and Characterization with Lasers; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jazani, S.; Sgouralis, I.; Shafraz, O.M.; Levitus, M.; Sivasankar, S.; Pressé, S. An alternative framework for fluorescence correlation spectroscopy. Nat. Commun. 2019, 10, 3662. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Dendisová, M.; Jeništová, A.; Parchaňská-Kokaislová, A.; Matějka, P.; Prokopec, V.; Švecová, M. The use of infrared spectroscopic techniques to characterize nanomaterials and nanostructures: A review. Anal. Chim. Acta 2018, 1031, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Church, J.S. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J. Food Drug Anal. 2014, 22, 29–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauffmann, T.H.; Kokanyan, N.; Fontana, M.D. Use of Stokes and anti-Stokes Raman scattering for new applications. J. Raman Spectrosc. 2019, 50, 418–424. [Google Scholar] [CrossRef]
- Geraldes, C.F. Introduction to Infrared and Raman-Based Biomedical Molecular Imaging and Comparison with Other Modalities. Molecules 2020, 25, 5547. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Neiva Correia, M.J.; Mateus, M.M.; Saraiva, J.A.; Vicente, A.A.; Moldão, M. Fourier transform infrared (FT-IR) spectroscopy as a possible rapid tool to evaluate abiotic stress effects on pineapple by-products. Appl. Sci. 2019, 9, 4141. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Wynendaele, E.; Xu, X.; Kosgei, A.; De Spiegeleer, B. Circular dichroism in functional quality evaluation of medicines. J. Pharm. Biomed. Anal. 2018, 147, 50–64. [Google Scholar] [CrossRef]
- Fasman, G.D. Circular Dichroism and the Conformational Analysis of Biomolecules; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kumar, M.; Jha, A. Pharmaceutical Applications of Circular Dichroism for Nanomaterial’s. Adv. Clin. Toxicol. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Spaeth, P.; Adhikari, S.; Le, L.; Jollans, T.; Pud, S.; Albrecht, W.; Bauer, T.; Caldarola, M.; Kuipers, L.; Orrit, M. Circular dichroism measurement of single metal nanoparticles using photothermal imaging. Nano Lett. 2019, 19, 8934–8940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogachev, Y.V.; Chernenco, J.S.; Gareev, K.; Kononova, I.; Matyushkin, L.; Moshnikov, V.; Nalimova, S. The study of aggregation processes in colloidal solutions of magnetite–silica nanoparticles by NMR relaxometry, AFM, and UV–vis-spectroscopy. Appl. Magn. Reson. 2014, 45, 329–337. [Google Scholar] [CrossRef]
- Marbella, L.E.; Millstone, J.E. NMR techniques for noble metal nanoparticles. Chem. Mater. 2015, 27, 2721–2739. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727. [Google Scholar]
- Shariatgorji, M.; Svenningsson, P.; Andrén, P.E. Mass spectrometry imaging, an emerging technology in neuropsychopharmacology. Neuropsychopharmacology 2014, 39, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siuzdak, G. An introduction to mass spectrometry ionization: An excerpt from the expanding role of mass spectrometry in biotechnology. JALA J. Assoc. Lab. Autom. 2004, 9, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Sharika, T.; Mishra, R.K.; Thomas, S. 14—Rheological characteristics of nanomaterials and nanocomposites. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2017; pp. 327–350. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; An, F.-F.; Zhang, X.; Chen, X.; Lee, C.-S. Preparation and size control of sub-100 nm pure nanodrugs. Nano Lett. 2015, 15, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Depp, V.; Alikhani, A.; Grammer, V.; Lele, B.S. Native protein-initiated ATRP: A viable and potentially superior alternative to PEGylation for stabilizing biologics. Acta Biomater. 2009, 5, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kuhne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J Control Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Koury, J.; Lucero, M.; Cato, C.; Chang, L.; Geiger, J.; Henry, D.; Hernandez, J.; Hung, F.; Kaur, P.; Teskey, G.; et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J. Immunol. Res. 2018, 2018, 9585614. [Google Scholar] [CrossRef]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509. [Google Scholar] [CrossRef]
- Jeanbart, L.; Swartz, M.A. Engineering opportunities in cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2015, 112, 14467–14472. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, D.; Zheng, L.; Lin, J.; Zhang, B.; Zhu, Y.; Li, N.; Xie, S.; Wang, Y.; Gao, N.; Huang, Z. Structural basis of assembly of the human T cell receptor–CD3 complex. Nature 2019, 573, 546–552. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A., 3rd; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, M.; Low, P.S. Ligand-targeted drug delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Soucy, J.R.; Hebert, J.; Auguste, D.T. Advances in receptor-mediated, tumor-targeted drug delivery. Adv. Ther. 2019, 2, 1800091. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Shi, Y.; Qi, T.; Qiu, S.; Huang, Y.; Zhao, X.; Sun, Q.; Lin, G. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct. Target. Ther. 2020, 5, 262. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Wang, X.; Nie, S.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Shukla, A.K.; Acharya, A. Lectin Nanoconjugates for Targeted Therapeutic Applications. In Nanomaterial-Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 103–127. [Google Scholar]
- David, A.; Kopecková, P.; Kopecek, J.; Rubinstein, A. The role of galactose, lactose, and galactose valency in the biorecognition of N-(2-hydroxypropyl) methacrylamide copolymers by human colon adenocarcinoma cells. Pharm. Res. 2002, 19, 1114–1122. [Google Scholar] [CrossRef]
- Beyer, V.P.; Monaco, A.; Napier, R.; Yilmaz, G.; Becer, C.R. Bottlebrush Glycopolymers from 2-Oxazolines and Acrylamides for Targeting Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin and Mannose-Binding Lectin. Biomacromolecules 2020, 21, 2298–2308. [Google Scholar] [CrossRef]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate Receptors and Transporters: Biological Role and Diagnostic/Therapeutic Targets in Cancer and Other Diseases; BioMed Central: London, UK, 2019. [Google Scholar]
- Ledermann, J.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, B.; Mohammadnia-Afrouzi, M.; Bakhshaei, P.; Yazdani, Y.; Ghalamfarsa, G.; Yousefi, M.; Sadreddini, S.; Jadidi-Niaragh, F.; Hojjat-Farsangi, M. Folate-conjugated nanoparticles as a potent therapeutic approach in targeted cancer therapy. Tumor Biol. 2015, 36, 5727–5742. [Google Scholar] [CrossRef]
- Kato, T.; Jin, C.S.; Ujiie, H.; Lee, D.; Fujino, K.; Wada, H.; Hu, H.-p.; Weersink, R.A.; Chen, J.; Kaji, M. Nanoparticle targeted folate receptor 1-enhanced photodynamic therapy for lung cancer. Lung Cancer 2017, 113, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milián, M.; Griebenow, K. Smart targeting to improve cancer therapeutics. Drug Des. Dev. Ther. 2019, 13, 3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30, 5737–5750. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.B.; Atar, K.O.; Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 2021, 596, 120268. [Google Scholar] [CrossRef]
- Arbuthnot, P. Antiviral Gene Therapy: Summary and Perspectives. Gene Ther. Viral Infect. 2015, 355–364. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Doktorovova, S.; Shegokar, R.; Rakovsky, E.; Gonzalez-Mira, E.; Lopes, C.M.; Silva, A.M.; Martins-Lopes, P.; Muller, R.H.; Souto, E.B. Cationic solid lipid nanoparticles (cSLN): Structure, stability and DNA binding capacity correlation studies. Int. J. Pharm. 2011, 420, 341–349. [Google Scholar] [CrossRef]
- Pangburn, T.O.; Petersen, M.A.; Waybrant, B.; Adil, M.M.; Kokkoli, E. Peptide-and aptamer-functionalized nanovectors for targeted delivery of therapeutics. J. Biomech. Eng. 2009, 131, 074005. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Y.; Duan, J.; Yuan, W.; Wang, C.; Xu, H.; Yang, X.-D. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS ONE 2011, 6, e24077. [Google Scholar] [CrossRef] [Green Version]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef]
- He, X.W.; Liu, T.; Chen, Y.X.; Cheng, D.J.; Li, X.R.; Xiao, Y.; Feng, Y.L. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008, 15, 193–202. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Liu, T.; Xiao, Y.; Feng, Y.L.; Cheng, D.J.; Tingting, G.; Zhang, L.; Zhang, Y.; Chen, Y.X.; Tingting, G.; et al. Vascular endothelial growth factor-C siRNA delivered via calcium carbonate nanoparticle effectively inhibits lymphangiogenesis and growth of colorectal cancer in vivo. Cancer Biother. Radiopharm. 2009, 24, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Peng, X.; Hou, J.; Wu, S.; Shen, J.; Wang, L. Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int. J. Nanomed. 2017, 12, 5331–5343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, Z.; Guo, S.; Zhang, L.; Sharma, A.; Robertson, G.P.; Huang, L. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol. Ther. 2013, 21, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Saw, P.E.; Song, E.-W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 2019, 10, 787–807. [Google Scholar] [CrossRef] [Green Version]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Agrahari, V.; Mandal, A.; Cholkar, K.; Natarajan, C.; Shah, S.; Joseph, M.; Trinh, H.M.; Vaishya, R.; Yang, X.; et al. Novel delivery approaches for cancer therapeutics. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 248–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Lopez, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J Nanobiotechnology 2018, 16, 32. [Google Scholar] [CrossRef]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252–37267. [Google Scholar] [CrossRef] [Green Version]
- Dufès, C.; Al Robaian, M.; Somani, S. Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Ther. Deliv. 2013, 4, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Qian, Z.M. Transferrin/transferrin receptor-mediated drug delivery. Med. Res. Rev. 2002, 22, 225–250. [Google Scholar] [CrossRef]
- Clark, A.J.; Davis, M.E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. USA 2015, 112, 12486–12491. [Google Scholar] [CrossRef] [Green Version]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–copper nanocluster–doxorubicin nanoparticles as targeted theranostic cancer Nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.-G.; Ku, S.K.; Yong, C.S. Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Yap, S.P.; Chua, K.L.; Itahana, K. The diverse and complex roles of radiation on cancer treatment: Therapeutic target and genome maintenance. Am. J. Cancer Res. 2012, 2, 372–382. [Google Scholar] [PubMed]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjug. Chem. 2019, 30, 273–283. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Liang, X.J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer 2012, 31, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Slastnikova, T.A.; Ulasov, A.V.; Rosenkranz, A.A.; Sobolev, A.S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharm. 2018, 9, 1208. [Google Scholar] [CrossRef] [Green Version]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Wente, S.R.; Rout, M.P. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol. 2010, 2, a000562. [Google Scholar] [CrossRef]
- Tran, E.J.; King, M.C.; Corbett, A.H. Macromolecular transport between the nucleus and the cytoplasm: Advances in mechanism and emerging links to disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Cross, D.; Burmester, J.K. Gene therapy for cancer treatment: Past, present and future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidai, C.; Kitano, H. Nonviral gene therapy for cancer: A review. Diseases 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I.; Doctor, D.; Chawla, K.; Chattopadhyay, S.; Chandra, D.; Chilukuri, N.; Betapudi, V. Gene therapy leaves a vicious cycle. Front. Oncol. 2019, 9, 297. [Google Scholar] [CrossRef]
- Shegokar, R.; Fernandes, A.R.; Souto, E.B. Nanopharmaceuticals in immunology: What’s new in research? In Emerging Nanotechnologies in Immunology; Shegokar, R., Souto, E.B., Eds.; Elsevier: Boston, MA, USA, 2018; Chapter 1; pp. 1–22. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Cruvinel, W.D.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.D.; Silva, N.P.D.; Andrade, L.E.C. Immune system: Part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Moore, T.V.; Lyons, G.E.; Brasic, N.; Roszkowski, J.J.; Voelkl, S.; Mackensen, A.; Kast, W.M.; Le Poole, I.C.; Nishimura, M.I. Relationship between CD8-dependent antigen recognition, T cell functional avidity, and tumor cell recognition. Cancer Immunol. Immunother. 2009, 58, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.F.; Brenner, M.K.; Dotti, G. Immunotherapy of human cancers using gene modified T lymphocytes. Curr. Gene 2009, 9, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 2018, 33, 581–598. [Google Scholar] [CrossRef] [Green Version]
- Barrueto, L.; Caminero, F.; Cash, L.; Makris, C.; Lamichhane, P.; Deshmukh, R.R. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl. Oncol. 2020, 13, 100738. [Google Scholar] [CrossRef]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Kara, H.E.Ş.; Ertaş, N. Quantum Dots for Pharmaceutical and Biomedical Analysis. In Spectroscopic Analyses-Developments and Applications; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D’Emanuele, A. Carbon nanotubes in cancer therapy and drug delivery. J. Drug Deliv. 2012, 2012, 837327. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wu, F.; Yu, L.; Cai, Y.; Chen, H.; Zhang, M. Chloride binding capacity of LDHs with various divalent cations and divalent to trivalent cation ratios in different solutions. CrystEngComm 2019, 21, 6790–6800. [Google Scholar] [CrossRef]
- Zielińska, A.; Pereira, I.; Antunes, S.; Veiga, F.J.; Santos, A.C.; Nowak, I.; Silva, A.M.; Souto, E.B. Mesoporous silica nanoparticles as drug delivery systems against melanoma. In Design of Nanostructures for Theranostics Applications; Grumezescu, A.M., Ed.; William Andrew Publishing, Applied Science Publishers: Norwich, NY, USA, 2018; Chapter 10; pp. 437–466. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Let’s talk about lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 99. [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharm. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging Nanopharmaceuticals and Nanonutraceuticals in Cancer Management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Ma, X.; Duan, Z.; Chen, X. Diagnosis, Therapy, and Prognosis for Hepatocellular Carcinoma. Anal. Cell. Pathol. 2020, 2020, 8157406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, R.; Zhang, X.-D.; Li, G.-Z.; Qin, K.-L.; Yan, X. Comparison of transcatheter arterial chemoembolization with raltitrexed plus liposomal doxorubicin vs. tegafur plus pirarubicin for unresectable hepatocellular carcinoma. J. Gastrointest. Oncol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Wei, M.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Xu, Y.; Wu, C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 5123. [Google Scholar]
- Li, X.; Diao, W.; Xue, H.; Wu, F.; Wang, W.; Jiang, B.; Bai, J.; Lian, B.; Feng, W.; Sun, T. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett. 2020, 489, 163–173. [Google Scholar] [CrossRef]
- Gong, C.; Hu, K.; Wang, X.; Wangyang, P.; Yan, C.; Chu, J.; Liao, M.; Dai, L.; Zhai, T.; Wang, C.; et al. 2D Nanomaterial Arrays for Electronics and Optoelectronics. Adv. Funct. Mater. 2018, 28, 1706559. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alqadhi, A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Szymanski, M.; Favaro, M.; Azzoni, A.R.; Chaud, M.V.; Santana, M.H.; Silva, A.M.; Souto, E.B. Development and characterization of a cationic lipid nanocarrier as non-viral vector for gene therapy. Eur J Pharm Sci 2015, 66, 78–82. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for cancer: Targeting and multifunctionality in the future of cancer therapies. ACS Biomater. Sci. Eng. 2015, 1, 64–78. [Google Scholar] [CrossRef]
- Song, W.; Das, M.; Chen, X. Nanotherapeutics for immuno-oncology: A crossroad for new paradigms. Trends Cancer 2020, 6, 288–298. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: A nanovaccine confer immuneadjuvanting effects to fight cervical cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, J.; Ignacio, R.M.C.; Sharbeen, G.; Boyer, C.; Gonzales-Aloy, E.; Goldstein, D.; McCarroll, J.A.; Phillips, P.A.; Initiative, A.P.C.G. Targeting the Undruggable in pancreatic Cancer using Nano-based gene silencing drugs. Biomaterials 2020, 240, 119742. [Google Scholar] [CrossRef]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Maynard, J.; Peyman, S.A.; McLaughlan, J.R.; Fairclough, M.; Marston, G.; Valleley, E.M.; Jimenez-Macias, J.L. Ultrasound-triggered therapeutic microbubbles enhance the efficacy of cytotoxic drugs by increasing circulation and tumor drug accumulation and limiting bioavailability and toxicity in normal tissues. Theranostics 2020, 10, 10973. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Yassemi, A.; Kashanian, S.; Zhaleh, H. Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm. Dev. Technol. 2020, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.H.; Wang, H.Y.; Li, J.; Huang, Y.Z. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol. Sin. 2019, 40, 1373–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xie, D.; Zhao, Q.; You, Z.H. MicroRNAs and complex diseases: From experimental results to computational models. Brief. Bioinform. 2019, 20, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, W.J.; Shi, J.B.; Liu, M.M.; Liu, X.H. Therapeutic strategies for targeting telomerase in cancer. Med. Res. Rev. 2020, 40, 532–585. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.N.; Villanueva, J. Targeting telomerase for cancer therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef]
- Maurer, S.; Salih, H.R.; Smirnow, I.; Lauer, U.M.; Berchtold, S. Suicide gene-armed measles vaccine virus for the treatment of AML. Int. J. Oncol. 2019, 55, 347–358. [Google Scholar] [CrossRef]
- Hossain, J.A.; Riecken, K.; Miletic, H.; Fehse, B. Cancer Suicide Gene Therapy with TK.007. Methods Mol. Biol. 2019, 1895, 11–26. [Google Scholar] [CrossRef]
- Cáceres, B.; Ramirez, A.; Carrillo, E.; Jimenez, G.; Griñán-Lisón, C.; López-Ruiz, E.; Jiménez-Martínez, Y.; Marchal, J.A.; Boulaiz, H. Deciphering the Mechanism of Action Involved in Enhanced Suicide Gene Colon Cancer Cell Killer Effect Mediated by Gef and Apoptin. Cancers 2019, 11, 264. [Google Scholar] [CrossRef] [Green Version]
- Beg, S.; Alharbi, K.S.; Alruwaili, N.K.; Alotaibi, N.H.; Almalki, W.H.; Alenezi, S.K.; Altowayan, W.M.; Alshammari, M.S.; Rahman, M. Nanotherapeutic systems for delivering cancer vaccines: Recent advances. Nanomedicine 2020, 15, 1527–1537. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in-PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110294. [Google Scholar] [CrossRef]
- Gonçalves, M.; Mignani, S.; Rodrigues, J.; Tomás, H. A glance over doxorubicin based-nanotherapeutics: From proof-of-concept studies to solutions in the market. J. Control. Release Off. J. Control. Release Soc. 2020, 317, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xia, Q.; Wang, P. Rationally Designed DNA Nanostructures for Drug Delivery. Front. Chem. 2020, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, H.Y.; Chen, L.Q.; Du, H.H.; Cui, J.H.; Zhang, L.W.; Lee, B.J.; Cao, Q.R. Enhanced Lysosomal Escape of pH-Responsive Polyethylenimine-Betaine Functionalized Carbon Nanotube for the Codelivery of Survivin Small Interfering RNA and Doxorubicin. ACS Appl Mater Interfaces 2019, 11, 9763–9776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, X.; Cheng, Y.; Guo, X.; Yuan, W. Iron Oxide Nanoparticles-Based Vaccine Delivery for Cancer Treatment. Mol. Pharm. 2018, 15, 1791–1799. [Google Scholar] [CrossRef]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [Green Version]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Kumar, G.; Nandakumar, K.; Mutalik, S.; Rao, C.M. Biologicals to direct nanotherapeutics towards HER2-positive breast cancers. Nanomedicine 2020, 27, 102197. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.Y.; Lin, Y.; Tang, M.; Zhai, C.H.; Xia, H.F.; Wang, J.; Zhang, Z.L.; Xie, Z.X.; Chen, G.; et al. Transformation of Viral Light Particles into Near-Infrared Fluorescence Quantum Dot-Labeled Active Tumor-Targeting Nanovectors for Drug Delivery. Nano Lett. 2019, 19, 7035–7042. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, W.; Zhang, J.; Xia, X. Merits of the ‘good’ viruses: The potential of virus-based therapeutics. Expert Opin. Biol. Ther. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for Antimetastatic Treatment. Trends Cancer 2020, 6, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Young, S.W.; Stenzel, M.; Yang, J.L. Nanoparticle-siRNA: A potential cancer therapy? Crit. Rev. Oncol. Hematol. 2016, 98, 159–169. [Google Scholar] [CrossRef]
- Liu, T.I.; Lu, T.Y.; Yang, Y.C.; Chang, S.H.; Chen, H.H.; Lu, I.L.; Sabu, A.; Chiu, H.C. New combination treatment from ROS-Induced sensitized radiotherapy with nanophototherapeutics to fully eradicate orthotopic breast cancer and inhibit metastasis. Biomaterials 2020, 257, 120229. [Google Scholar] [CrossRef] [PubMed]
- Heuts, J.; Varypataki, E.M.; van der Maaden, K.; Romeijn, S.; Drijfhout, J.W.; van Scheltinga, A.T.; Ossendorp, F.; Jiskoot, W. Cationic Liposomes: A Flexible Vaccine Delivery System for Physicochemically Diverse Antigenic Peptides. Pharm. Res. 2018, 35, 207. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II—Production scales and clinically compliant production methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Tutar, R.; Motealleh, A.; Khademhosseini, A.; Kehr, N.S. Functional Nanomaterials on 2D Surfaces and in 3D Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1904344. [Google Scholar] [CrossRef]
- Lee, W.; Liu, Y.; Lee, Y.; Sharma, B.K.; Shinde, S.M.; Kim, S.D.; Nan, K.; Yan, Z.; Han, M.; Huang, Y.; et al. Two-dimensional materials in functional three-dimensional architectures with applications in photodetection and imaging. Nat. Commun. 2018, 9, 1417. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharm. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Box, H.; van Elk, M.; Gaitan, S.; Geertsma, R.; Gainza Lafuente, E.; Owen, A.; Del Pozo, A.; Roesslein, M.; Bremer-Hoffmann, S. Anticipation of Regulatory Needs for Nanotechnology-Enabled Health Products; EUR 29919 EN; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]


| NCT Number | Title | Status | Interventions | Phases |
|---|---|---|---|---|
| NCT00009841 | Gene Therapy in Treating Patients With Advanced Head and Neck Cancer | Completed | Biological: EGFR antisense DNA Biological: growth factor antagonist therapy Drug: DC-cholesterol liposome | Phase 1 |
| NCT00006033 | Interleukin-2 Gene or Methotrexate in Treating Patients With Recurrent or Refractory Stage III or Stage IV Head and Neck Cancer | Completed | Biological: gene therapy Biological: interleukin-2 gene Drug: methotrexate | Phase 2 |
| NCT00059605 | Phase I Study of IV DOTAP: Cholesterol-Fus1 in Non-Small-Cell Lung Cancer | Completed | Genetic: DOTAP:Chol-fus1 | Phase 1 |
| NCT00044993 | Chemotherapy Combined With Gene Therapy in Treating Patients Who Have Stage III or Stage IV Breast Cancer | Completed | Biological: Ad5CMV-p53 gene Drug: docetaxel Drug: doxorubicin hydrochloride Procedure: conventional surgery Procedure: neoadjuvant therapy | Phase 2 |
| NCT04486833 | TUSC2-nanoparticles (GPX-001) and Osimertinib in Patients With Stage IV Lung Cancer Who Progressed on Osimertinib Alone | Not yet recruiting | Biological: Quaratusugene ozeplasmid—intravenous infusion Drug: Osimertinib Oral Tablet | Phase 1|Phase 2 |
| NCT02337985 | Gene Therapy and Combination Chemotherapy in Treating Patients With AIDS-Related Non-Hodgkin Lymphoma | Active, not recruiting | Drug: Prednisone Biological: Rituximab Drug: Etoposide Drug: Doxorubicin Hydrochloride Drug: Vincristine Sulfate Drug: Cyclophosphamide Biological: Filgrastim Biological: Lentivirus Vector rHIV7-shI-TAR-CCR5RZ-transduced Hematopoietic Stem/Progenitor Cells | Phase 1 |
| NCT01591356 | EphA2 siRNA in Treating Patients With Advanced or Recurrent Solid Tumors | Recruiting | Drug: EphA2-targeting DOPC-encapsulated siRNA Other: Laboratory Biomarker Analysis Other: Pharmacological Study | Phase 1 |
| NCT02528682 | MiHA-loaded PD-L-silenced DC Vaccination After Allogeneic SCT | Completed | Biological: MiHA-loaded PD-L-silenced DC Vaccination | Phase 1|Phase 2 |
| NCT03087591 | APN401 in Treating Patients With Recurrent or Metastatic Pancreatic Cancer, Colorectal Cancer, or Other Solid Tumors That Cannot Be Removed by Surgery | Active, not recruiting | Other: Laboratory Biomarker Analysis Biological: siRNA-transfected Peripheral Blood Mononuclear Cells APN401 | Phase 1 |
| NCT03608631 | iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation | Recruiting | Drug: Mesenchymal Stromal Cells-derived Exosomes with KRAS G12D siRNA | Phase 1 |
| NCT04278326 | Primary Organoid Models and Combined Nucleic Acids Therapeutics for Anti-HPV Treatments | Recruiting | Procedure: Vaginal Biopsy | Not Applicable |
| Particle Class | Materials | Application |
|---|---|---|
| Natural materials or derivatives | Liposomes Chitosan Gelatine Dextrane Starch Alginates Metal-based nanoparticles | Drug/gene delivery |
| Dendrimers | Branched polymers | Drug delivery/gene delivery |
| Polymer carriers | Block copolymers Polylactic acid Polycaprolactone Polyethyleneimine Poly(cyano)acrylates | Drug/gene delivery |
| Nucleic acids | Micro RNA (miRNA) Small interfering RNA (siRNA) Oligonucleotides CRISPR/Cas9 Short hairpin RNA (shRNA) | Gene therapy RNA interference therapy |
| Non-viral vectors | Physically mediated methods (microinjection, ultrasound-mediated microbubble, microparticle bombardment, and electroporation) Chemical vectors (cationic polymers and cationic liposomes, shell nanoparticles, and polymeric nanoparticles), Biological methods (bacteria and specific mammalian cells). | Gene delivery |
| Viral vectors | Adenovirus-associated virus Lentivirus | cell-based gene therapy |
| Vesicles | Exosomes Bilosomes Niosomes Archeosomes minicells | Drug/gene delivery |
| Various | Silica-nanoparticles Mixtures of above | Gene delivery Reversal of tumor acidity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Szalata, M.; Gorczyński, A.; Karczewski, J.; Eder, P.; Severino, P.; Cabeda, J.M.; Souto, E.B.; Słomski, R. Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications. Cancers 2021, 13, 1896. https://doi.org/10.3390/cancers13081896
Zielińska A, Szalata M, Gorczyński A, Karczewski J, Eder P, Severino P, Cabeda JM, Souto EB, Słomski R. Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications. Cancers. 2021; 13(8):1896. https://doi.org/10.3390/cancers13081896
Chicago/Turabian StyleZielińska, Aleksandra, Marlena Szalata, Adam Gorczyński, Jacek Karczewski, Piotr Eder, Patrícia Severino, José M. Cabeda, Eliana B. Souto, and Ryszard Słomski. 2021. "Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications" Cancers 13, no. 8: 1896. https://doi.org/10.3390/cancers13081896
APA StyleZielińska, A., Szalata, M., Gorczyński, A., Karczewski, J., Eder, P., Severino, P., Cabeda, J. M., Souto, E. B., & Słomski, R. (2021). Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications. Cancers, 13(8), 1896. https://doi.org/10.3390/cancers13081896









