Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
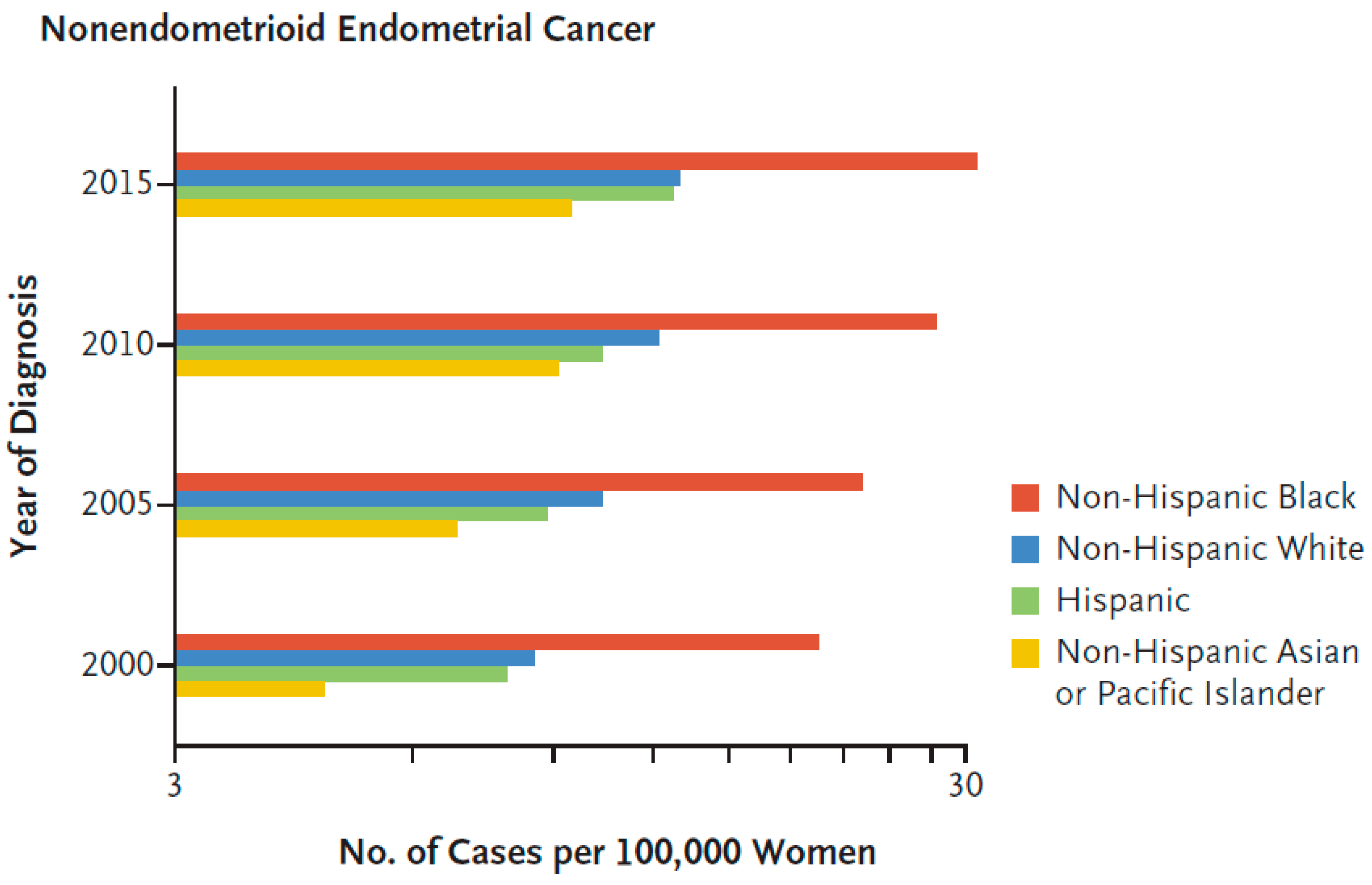
3.1. Histology, Pathogenic Types and Endometrial Disparity
3.2. Hereditary Cancer and Genetic Predisposition
3.3. Global Profiling to Identify Racial Differences in mRNA Transcripts and Proteins
3.4. Racial Differences in MicroRNAs
3.5. A Targeted Approach to Identifying Racial Differences in Specific Proteins
3.6. Pathway Analysis of Racial Differences in mRNA and Proteins
3.7. Race-Based Treatment and Outcome
3.8. Candidate Drugs for Race-Specific Treatment of Black Endometrial Cancer Patients
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, E. End Results (SEER) Program. Available online: https://seer.cancer.gov/ (accessed on 10 January 2021).
- Doll, K.M.; Winn, A.N. Assessing endometrial cancer risk among US women: Long-term trends using hysterectomy-adjusted analysis. Am. J. Obstet. Gynecol. 2019, 221, 318.e1–318.e9. [Google Scholar] [CrossRef] [PubMed]
- Donkers, H.; Bekkers, R.; Massuger, L.; Galaal, K. Systematic review on socioeconomic deprivation and survival in endometrial cancer. Cancer Causes Control CCC 2019, 30, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donkers, H.; Bekkers, R.; Massuger, L.; Galaal, K. Socioeconomic deprivation and survival in endometrial cancer: The effect of BMI. Gynecol. Oncol. 2020, 156, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Meng, B.; Hoang, L.N.; McIntyre, J.B.; Duggan, M.A.; Nelson, G.S.; Lee, C.H.; Kobel, M. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol. Oncol. 2014, 134, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, C.C.; Cohn, D.E.; Mutch, D.G.; Stephens, J.A.; Suarez, A.A.; Goodfellow, P.J. Polymerase varepsilon (POLE) mutations in endometrial cancer: Clinical outcomes and implications for Lynch syndrome testing. Cancer 2015, 121, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttery, D.S.; Blighe, K.; Polymeros, K.; Symonds, R.P.; Macip, S.; Moss, E.L. Racial differences in endometrial cancer molecular portraits in The Cancer Genome Atlas. Oncotarget 2018, 9, 17093–17103. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Baskovic, M.; Lichtensztajn, D.Y.; Nguyen, T.; Karam, A.; English, D.P. Racial disparities in outcomes for high-grade uterine cancer: A California cancer registry study. Cancer Med. 2018, 7, 4485–4495. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.M.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Siegel, D.A.; Feuer, E.J.; Firth, A.U.; Kohler, B.A.; Scott, S.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J. Natl. Cancer Inst. 2019, 111, 1279–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Sherman, M.E.; Devesa, S.S. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 2003, 98, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.B.; Huang, Y.; Hur, C.; Tergas, A.I.; Khoury-Collado, F.; Melamed, A.; St Clair, C.M.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; et al. Impact of quality of care on racial disparities in survival for endometrial cancer. Am. J. Obstet. Gynecol. 2020, 223, 396.e1–396.e13. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Siegel, E.R.; Palmieri, M.; Thomas, M.; Cannon, M.J.; Kay, H.H.; Roman, J.J.; Burnett, A.; Pecorelli, S. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): A major prognostic indicator in uterine serous papillary cancer. Am. J. Obstet. Gynecol. 2005, 192, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, B.; Baptiste, C.; Chen, L.; Tergas, A.I.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Racial disparities in young women with endometrial cancer. Gynecol. Oncol. 2018, 148, 527–534. [Google Scholar] [CrossRef]
- Javadian, P.; Nezhat, F. Endometrial Carcinoma and its Precursors. Adv. Exp. Med. Biol. 2020, 1242, 59–72. [Google Scholar] [CrossRef]
- Johnson, A.L.; Medina, H.N.; Schlumbrecht, M.P.; Reis, I.; Kobetz, E.N.; Pinheiro, P.S. The role of histology on endometrial cancer survival disparities in diverse Florida. PLoS ONE 2020, 15, e0236402. [Google Scholar] [CrossRef]
- Lara, O.D.; Wang, Y.; Asare, A.; Xu, T.; Chiu, H.S.; Liu, Y.; Hu, W.; Sumazin, P.; Uppal, S.; Zhang, L.; et al. Pan-cancer clinical and molecular analysis of racial disparities. Cancer 2020, 126, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Stinton, C.; Fraser, H.; Al-Khudairy, L.; Court, R.; Jordan, M.; Grammatopoulos, D.; Taylor-Phillips, S. Testing for lynch syndrome in people with endometrial cancer using immunohistochemistry and microsatellite instability-based testing strategies—A systematic review of test accuracy. Gynecol. Oncol. 2021, 160, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Schlotterer, C. Evolutionary dynamics of microsatellite DNA. Chromosoma 2000, 109, 365–371. [Google Scholar] [CrossRef]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Moreira, L.; Balaguer, F.; Lindor, N.; de la Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.; Burgart, L.J.; Thibodeau, S.N.; Rabe, K.G.; Petersen, G.M.; Goldberg, R.M.; Lindor, N.M. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer 2003, 97, 1421–1427. [Google Scholar] [CrossRef]
- Aarnio, M.; Sankila, R.; Pukkala, E.; Salovaara, R.; Aaltonen, L.A.; de la Chapelle, A.; Peltomäki, P.; Mecklin, J.P.; Järvinen, H.J. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int. J. Cancer 1999, 81, 214–218. [Google Scholar] [CrossRef]
- Lu, K.H.; Dinh, M.; Kohlmann, W.; Watson, P.; Green, J.; Syngal, S.; Bandipalliam, P.; Chen, L.-M.; Allen, B.; Conrad, P.; et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005, 105, 569–574. [Google Scholar] [CrossRef]
- Maxwell, G.L.; Risinger, J.I.; Hayes, K.A.; Alvarez, A.A.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. Racial disparity in the frequency of PTEN mutations, but not microsattelite instability, in advanced endometrial cancers. Clin. Cancer Res. 2000, 6, 2999–3005. [Google Scholar]
- Bateman, N.W.; Dubil, E.A.; Wang, G.; Hood, B.L.; Oliver, J.M.; Litzi, T.A.; Gist, G.D.; Mitchell, D.A.; Blanton, B.; Phippen, N.T.; et al. Race-specific molecular alterations correlate with differential outcomes for black and white endometrioid endometrial cancer patients. Cancer 2017, 123, 4004–4012. [Google Scholar] [CrossRef] [Green Version]
- Dubil, E.A.; Tian, C.; Wang, G.; Tarney, C.M.; Bateman, N.W.; Levine, D.A.; Conrads, T.P.; Hamilton, C.A.; Maxwell, G.L.; Darcy, K.M. Racial disparities in molecular subtypes of endometrial cancer. Gynecol. Oncol. 2018, 149, 106–116. [Google Scholar] [CrossRef]
- Carson, J.P.; Zhang, N.; Frampton, G.M.; Gerry, N.P.; Lenburg, M.E.; Christman, M.F. Pharmacogenomic identification of targets for adjuvant therapy with the topoisomerase poison camptothecin. Cancer Res. 2004, 64, 2096–2104. [Google Scholar] [CrossRef] [Green Version]
- Brachat, A.; Pierrat, B.; Xynos, A.; Brecht, K.; Simonen, M.; Brüngger, A.; Heim, J. A microarray-based, integrated approach to identify novel regulators of cancer drug response and apoptosis. Oncogene 2002, 21, 8361–8371. [Google Scholar] [CrossRef] [Green Version]
- Iglewski, B.H.; Liu, P.V.; Kabat, D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun. 1977, 15, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Caruso, J.A.; Duong, M.T.; Carey, J.P.W.; Hunt, K.K.; Keyomarsi, K. Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for Targeted Therapies. Cancer Res. 2018, 78, 5481–5491. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R., Jr. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol. Res. 2019, 139, 471–488. [Google Scholar] [CrossRef]
- Malavolta, M.; Bracci, M.; Santarelli, L.; Sayeed, M.A.; Pierpaoli, E.; Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Cardelli, M.; et al. Inducers of Senescence, Toxic Compounds, and Senolytics: The Multiple Faces of Nrf2-Activating Phytochemicals in Cancer Adjuvant Therapy. Mediat. Inflamm. 2018, 2018, 4159013. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Drullinsky, P.R.; Hurvitz, S.A. Mechanistic basis for PI3K inhibitor antitumor activity and adverse reactions in advanced breast cancer. Breast Cancer Res. Treat. 2020, 181, 233–248. [Google Scholar] [CrossRef]
- Pascual, J.; Turner, N.C. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, x12–x20. [Google Scholar] [CrossRef]
- Brandao, M.; Caparica, R.; Eiger, D.; de Azambuja, E. Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, x27–x42. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roubal, K.; Myint, Z.W.; Kolesar, J.M. Erdafitinib: A novel therapy for FGFR-mutated urothelial cancer. Am. J. Health-Syst. Pharm. 2020, 77, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Akbari, V.; Chou, C.P.; Abedi, D. New insights into affinity proteins for HER2-targeted therapy: Beyond trastuzumab. Biochim. Et Biophys. Acta Rev. Cancer 2020, 1874, 188448. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Lardon, F.; Deschoolmeester, V.; De Pauw, I.; Vermorken, J.B.; Specenier, P.; Pauwels, P.; Peeters, M.; Wouters, A. Spotlight on Volasertib: Preclinical and Clinical Evaluation of a Promising Plk1 Inhibitor. Med. Res. Rev. 2016, 36, 749–786. [Google Scholar] [CrossRef]
- Mross, K.; Frost, A.; Steinbild, S.; Hedbom, S.; Rentschler, J.; Kaiser, R.; Rouyrre, N.; Trommeshauser, D.; Hoesl, C.E.; Munzert, G. Phase I dose escalation and pharmacokinetic study of BI 2536, a novel Polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5511–5517. [Google Scholar] [CrossRef]
- Schoffski, P.; Blay, J.-Y.; De Greve, J.; Brain, E.; Machiels, J.-P.; Soria, J.-C.; Sleijfer, S.; Wolter, P.; Ray-Coquard, I.; Fontaine, C.; et al. Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network of Core Institutes (NOCI). Eur. J. Cancer 2010, 46, 2206–2215. [Google Scholar] [CrossRef]
- Hofheinz, R.-D.; Al-Batran, S.-E.; Hochhaus, A.; Jager, E.; Reichardt, V.L.; Fritsch, H.; Trommeshauser, D.; Munzert, G. An open-label, phase I study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4666–4674. [Google Scholar] [CrossRef] [Green Version]
- Olmos, D.; Barker, D.; Sharma, R.; Brunetto, A.T.; Yap, T.A.; Taegtmeyer, A.B.; Barriuso, J.; Medani, H.; Degenhardt, Y.Y.; Allred, A.J.; et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3420–3430. [Google Scholar] [CrossRef] [Green Version]
- Breitenbuecher, F.; von Pawel, J.; Sebastian, M.; Kortsik, C.; Ting, S.; Kasper, S.; Wohlschlager, J.; Worm, K.; Morresi-Hauf, A.; Schad, A.; et al. Comprehensive Biomarker Analyses in Patients with Advanced or Metastatic Non-Small Cell Lung Cancer Prospectively Treated with the Polo-Like Kinase 1 Inhibitor BI2536. Oncol. Res. Treat. 2017, 40, 435–439. [Google Scholar] [CrossRef]
- Weiss, G.J.; Jameson, G.; Von Hoff, D.D.; Valsasina, B.; Davite, C.; Di Giulio, C.; Fiorentini, F.; Alzani, R.; Carpinelli, P.; Di Sanzo, A.; et al. Phase I dose escalation study of NMS-1286937, an orally available Polo-Like Kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Investig. New Drugs 2018, 36, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.M.; Mi, Y.S.; Yu, F.D.; Han, Y.; Liu, X.S.; Lu, S.; Zhang, Y.; Zhao, S.L.; Ye, L.; Liu, T.T.; et al. SERPINA4 is a novel independent prognostic indicator and a potential therapeutic target for colorectal cancer. Am. J. Cancer Res. 2016, 6, 1636–1649. [Google Scholar]
- Sharma, S.; Javadekar, S.M.; Pandey, M.; Srivastava, M.; Kumari, R.; Raghavan, S.C. Homology and enzymatic requirements of microhomology-dependent alternative end joining. Cell Death Dis. 2015, 6, e1697. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.E.; Olshen, A.B.; Levine, D.A.; Viale, A.; Barakat, R.R.; Boyd, J. Molecular profiling of endometrial cancers from African–American and Caucasian women. Gynecol. Oncol. 2006, 101, 209–213. [Google Scholar] [CrossRef]
- Maxwell, G.L.; Allard, J.; Gadisetti, C.V.R.; Litzi, T.; Casablanca, Y.; Chandran, U.; Darcy, K.M.; Levine, D.A.; Berchuck, A.; Hamilton, C.A.; et al. Transcript expression in endometrial cancers from Black and White patients. Gynecol. Oncol. 2013, 130, 169–173. [Google Scholar] [CrossRef]
- Maxwell, G.L.; Shoji, Y.; Darcy, K.; Litzi, T.; Berchuck, A.; Hamilton, C.A.; Conrads, T.P.; Risinger, J.I. MicroRNAs in endometrial cancers from black and white patients. Am. J. Obs. Gynecol 2015, 212, 191.e1–191.e10. [Google Scholar] [CrossRef]
- Li, Q.; Shen, F.; Zhao, L. The relationship between lncRNA PCGEM1 and STAT3 during the occurrence and development of endometrial carcinoma. Biomed. Pharmacother. 2018, 107, 918–928. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Xu, D.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. Long noncoding RNA NEAT1 drives aggressive endometrial cancer progression via miR-361-regulated networks involving STAT3 and tumor microenvironment-related genes. J. Exp. Clin. Cancer Res. 2019, 38, 295. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chang, Y.; Cai, Y. Hsa_circ_0061140 promotes endometrial carcinoma progression via regulating miR-149-5p/STAT3. Gene 2020, 745, 144625. [Google Scholar] [CrossRef]
- Xu, J.B. MicroRNA-93-5p/IFNAR1 axis accelerates metastasis of endometrial carcinoma by activating the STAT3 pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5657–5666. [Google Scholar] [CrossRef]
- Dupont, J.; Wang, X.; Marshall, D.S.; Leitao, M.; Hedvat, C.V.; Hummer, A.; Thaler, H.; O’Reilly, R.J.; Soslow, R.A. Wilms Tumor Gene (WT1) and p53 expression in endometrial carcinomas: A study of 130 cases using a tissue microarray. Gynecol. Oncol. 2004, 94, 449–455. [Google Scholar] [CrossRef]
- Jeon, Y.T.; Kang, S.; Kang, D.H.; Yoo, K.Y.; Park, I.A.; Bang, Y.J.; Kim, J.W.; Park, N.H.; Kang, S.B.; Lee, H.P.; et al. Cyclooxygenase-2 and p53 expressions in endometrial cancer. Cancer Epidemiol. Prev. Biomark. 2004, 13, 1538–1542. [Google Scholar]
- Clifford, S.L.; Kaminetsky, C.P.; Cirisano, F.D.; Dodge, R.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I endometrial cancer. Am. J. Obs. Gynecol 1997, 176, s229–s232. [Google Scholar] [CrossRef]
- Schimp, V.L.; Ali-Fehmi, R.; Solomon, L.A.; Hammoud, A.; Pansare, V.; Morris, R.T.; Munkarah, A.R. The racial disparity in outcomes in endometrial cancer: Could this be explained on a molecular level? Gynecol. Oncol. 2006, 102, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Okayama, A.; Fujita, M.; Enomoto, T.; Sakata, M.; Tanizawa, O.; Ueshima, H. Clinicopathological characteristics of p53 overexpression in endometrial cancers. Int. J. Cancer 1994, 58, 14–19. [Google Scholar] [CrossRef]
- Ito, K.; Watanabe, K.; Nasim, S.; Sasano, H.; Sato, S.; Yajima, A.; Silverberg, S.G.; Garrett, C.T. Prognostic significance of p53 overexpression in endometrial cancer. Cancer Res. 1994, 54, 4667–4670. [Google Scholar] [PubMed]
- Mutter, G.L.; Lin, M.-C.; Fitzgerald, J.T.; Kum, J.B.; Baak, J.P.A.; Lees, J.A.; Weng, L.-P.; Eng, H. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl. Cancer Inst. 2002, 92, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Baak, J.P.A.; Van Diermen, B.; Steinbakk, A.; Janssen, E.; Skaland, I.; Mutter, G.L.; Fiane, B.; Lovslett, K. Lack of PTEN expression in endometrial intraepithelial neoplasia is correlated with cancer progression. Hum. Pathol. 2005, 36, 555–561. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Mutter, G.L.; Ronnett, B.M.; Ioffe, O.B.; Duggan, M.A.; Rush, B.B.; Glass, A.G.; Richesson, D.A.; Chatterjee, N.; Langholz, B.; et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008, 68, 6014–6020. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.D.; Jiang, L.H.; Sun, D.W.; Li, J.; Ji, Z.L. The role of miR-130a in cancer. Breast Cancer 2017, 24, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.; Orloff, M.S.; Michener, C.; Chiesa-Vottero, A.; Prayson, R.; Nowacki, A.S.; Eng, C. Association of specific PTEN/10q haplotypes with endometrial cancer phenotypes in African-American and European American women. Gynecol. Oncol. 2015, 138, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Stokoe, D.; Taketani, Y.; McCormick, F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005, 65, 10669–10673. [Google Scholar] [CrossRef] [Green Version]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Copeland, L.; Maxwell, G.L.; Fowler, J.M. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006, 24, 2376–2385. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Gokden, M.; Palmieri, M.; Dunn, D.; Agha, J.; Roman, J.J.; Hutchins, L.; Pecorelli, S.; O’Brien, T.; et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin. Cancer Res. 2002, 8, 1271–1279. [Google Scholar]
- Skalnikova, H.; Vodicka, P.; Pelech, S.; Motlik, J.; Gadher, S.J.; Kovarova, H. Protein signaling pathways in differentiation of neural stem cells. Proteomics 2008, 8, 4547–4559. [Google Scholar] [CrossRef]
- Shimomura, K.; Sakakura, C.; Takemura, M.; Takagi, T.; Fukuda, K.; Kin, S.; Nakase, Y.; Miyagawa, K.; Ohgaki, M.; Fujiyama, J.; et al. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res. 2004, 24, 1113–1120. [Google Scholar]
- Allard, J.E.; Chandramouli, G.V.; Stagliano, K.; Hood, B.L.; Litzi, T.; Shoji, Y.; Boyd, J.; Berchuck, A.; Conrads, T.P.; Maxwell, G.L.; et al. Analysis of PSPHL as a Candidate Gene Influencing the Racial Disparity in Endometrial Cancer. Front. Oncol. 2012, 2, 65. [Google Scholar] [CrossRef] [Green Version]
- Van Arsdale, A.R.; Arend, R.C.; Cossio, M.J.; Erickson, B.K.; Wang, Y.; Doo, D.W.; Leath, C.A.; Goldberg, G.L.; Huang, G.S. Insulin-like growth factor 2: A poor prognostic biomarker linked to racial disparity in women with uterine carcinosarcoma. Cancer Med. 2018, 7, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.L.; Tian, C.; Risinger, J.; Brown, C.L.; Rose, G.S.; Thigpen, J.T.; Fleming, G.F.; Gallion, H.H.; Brewster, W.R.; Gynecologic Oncology Group, s. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: A Gynecologic Oncology Group study. Cancer 2006, 107, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.; Paladugu, R.; Jones, N.; Pierce, J.Y.; Scalici, J.; Hamilton, C.A.; Darcy, K.M.; Maxwell, G.L.; Rocconi, R.P. Minority participation in phase 1 gynecologic oncology clinical trials: Three decades of inequity. Gynecol. Oncol. 2020, 157, 729–732. [Google Scholar] [CrossRef]
- Sanz, G.; Singh, M.; Peuget, S.; Selivanova, G. Inhibition of p53 inhibitors: Progress, challenges and perspectives. J. Mol. Cell Biol. 2019, 11, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Zanjirband, M.; Rahgozar, S. Targeting p53-MDM2 Interaction Using Small Molecule Inhibitors and the Challenges Needed to be Addressed. Curr. Drug Targets 2019, 20, 1091–1111. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020, 26, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.F.; Carney, P.; Dodge, R.; Soper, J.T.; Clarke-Pearson, D.L.; Marks, J.R.; Berchuck, A. p53 overexpression in advanced-stage endometrial adenocarcinoma. Am. J. Obstet. Gynecol. 1996, 175, 1246–1252. [Google Scholar] [CrossRef]
- Romagosa, C.; Simonetti, S.; Lopez-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramon y Cajal, S. p16(Ink4a) overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef] [Green Version]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, B.; Farajnia, S.; Ahdi Khosroshahi, S.; Safari, F.; Yousefi, M.; Dariushnejad, H.; Rahbarnia, L. Immunotoxins in cancer therapy: Review and update. Int. Rev. Immunol. 2017, 36, 207–219. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Difference | Name (Gene Symbol) | Survival Association | Reference |
|---|---|---|---|
| RNA Increased in Black Patients | DSN1 homolog (DSN1) a | PFS in Black and White | [31] |
| PBX homeobox 1 (PBX1) a,d | PFS in Black b | [31] | |
| EPM2A laforin glucan phosphatase (EPM2A)a | PFS and OS in White b | [31] | |
| DNA Ligase 3 (LIG3) a | PFS worse for Black and improved for White | [31] | |
| Bet1 Golgi vesicular membrane trafficking protein like (BET1L) a | PFS in White | [31] | |
| HEAT repeat containing 6 (HEATR6) a | PFS in Black | [31] | |
| Family with sequence similarity 228 member B (FAM228B) a | PFS in Black b | [31] | |
| Adaptor-related protein complex 4 r1 subunit (ARCA5) a | PFS in Black | [31] | |
| PSMD5 antisense RNA 1 (head to head) (PSMD5-AS1) a | PFS in Black b | [31] | |
| Polyribonucleotide nucleotidyltransferase 1 (PNPT1) a | PFS in Black | [31] | |
| Adaptor-related protein complex 4 r1 subunit (AP4S1) a | PFS in Black b | [31] | |
| Cyclin E1 (CCNE1) a,c,d | Not determined | [32] | |
| Cyclin dependent kinase inhibitor 2A/P16 (CDKN2A) c,d | Not determined | [32] | |
| Cell division cycle 25C (CDC25C) c | Not determined | [32] | |
| Cyclin B2 (CCNB2) c | Not determined | [32] | |
| Mitotic checkpoint serine/threonine kinase B (BUB1B) c | Not determined | [32] | |
| Minichromosome Maintenance complex component 7 (MCM7) c | Not determined | [32] | |
| Polo like kinase 1 (PLK1) c,d | Not determined | [32] | |
| Minichromosome maintenance complex component 2 (MCM2) c | Not determined | [32] | |
| Baculoviral inhibitor of apoptosis repeat containing 7 (BIRC7) c | Not determined | [32] | |
| Laminin subunit gamma 1 (LAMC1) c | Not determined | [32] | |
| CDC28 protein kinase regulatory subunit 1B (CKS1B) c | Not determined | [32] | |
| Laminin subunit 5 (LAMA5) c | Not determined | [32] | |
| Wnt family member 7A (WNT7A) c | Not determined | [32] | |
| Fibroblast growth factor 12 (FGF12) c | Not determined | [32] | |
| Fibroblast growth factor 5 (FGF5) c | Not determined | [32] | |
| FGF receptor 3 (FGFR3) c,d | Not determined | [32] | |
| Erb-B2 receptor tyrosine kinase 2 (ERBB2) c,d | Not determined | [32] | |
| Breast cancer 2 DNA repair associated (BRCA2) c | Not determined | [32] | |
| Phosphatidylinositol-4, 5-bisphosphate3-kinase catalytic subunit α (PIK3CA) c,d | Not determined | [32] | |
| RNA Decreased in Black Race | Wilms tumor 1 associated protein (WTAP) a | PFS in White | [31] |
| Death-associated protein (DAP) a | PFS and OS in White b | [31] | |
| Mal T-cell differentiation protein like (MALL) a | PFS in White b | [31] | |
| Integrin subunit a3 (ITGA3) a | PFS and OS in White | [31] | |
| Uncoupling protein 2 (UCP2) a | PFS in White b | [31] | |
| Serpin family A member 4 (SERPINA4) a | PFS and OS in White b | [31] | |
| Calpain 6 (CAPN6) a | PFS in White b | [31] | |
| Solute carrier family 24 member 1 (SLC24A1) a | PFS in Black | [31] | |
| Chromosome 19 open reading frame 25 (C19orf25) a | PFS in Black | [31] | |
| WD repeat domain 97 (WDR97) a | PFS in Black | [32] | |
| Cyclin A2 (CCNA2) c | Not determined | [32] | |
| Mitotic arrest deficient 2 like 1 (MAD2L1) c | Not determined | [32] | |
| Cyclin E1 (CCNE2) c | Not determined | [32] | |
| Cell division cycle 25C (CDC25A) c | Not determined | [32] | |
| Cell division cycle 7 (CDC7) c,d | Not determined | [32] | |
| Structural maintenance of chromosomes 1B (SMC1B) c | Not determined | [32] | |
| RNA and Protein Decreased in Blacks | CCR4-NOT transcription complex subunit 1 (CNOT1) | Not determined | [31] |
| Peptidylprolyl isomerase B (PPIB) | Not determined | [31] | |
| Cell cycle and apoptosis regulator 2 (CCAR2) | Not determined | [31] | |
| Eukaryotic translation elongation factor 2 (EEF2)d | Not determined | [31] | |
| Junction plakoglobin (JUP) | Not determined | [31] | |
| PDZ and LIM domain 5 (PDLIM5) | Not determined | [31] | |
| Myosin IC (MYO1C) | Not determined | [31] | |
| Desmoglein 2 (DSG2) | Not determined | [31] | |
| Plakophilin 3 (PKP3) | Not determined | [31] | |
| DnaJ heat shock protein family (Hsp40) member C10 (DNAJC10) | Not determined | [31] |
| Protein | Method | Racial Difference | Result |
|---|---|---|---|
| p53 [90] | Immunohistochemistry in FFPE of all histology types | Increased in Blacks | Higher expression independently associated with worse survival in all patients |
| PTEN [30] | DNA mutations that decrease expression | Decreased in Blacks | Mutations are associated with more favorable outcomes |
| HER2/Neu [18] | Immunohistochemistry in FFPE of USC | Increased in Blacks | Higher expression associated with worse disease-related survival in all races |
| IGF2 [81] | Immunohistochemistry | Increased in Blacks | Higher expression associated with worse survival in all races and doubling of risk in Blacks with USC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadian, P.; Washington, C.; Mukasa, S.; Benbrook, D.M. Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity. Cancers 2021, 13, 1900. https://doi.org/10.3390/cancers13081900
Javadian P, Washington C, Mukasa S, Benbrook DM. Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity. Cancers. 2021; 13(8):1900. https://doi.org/10.3390/cancers13081900
Chicago/Turabian StyleJavadian, Pouya, Christina Washington, Shylet Mukasa, and Doris Mangiaracina Benbrook. 2021. "Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity" Cancers 13, no. 8: 1900. https://doi.org/10.3390/cancers13081900








