Graves’ Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review)
Abstract
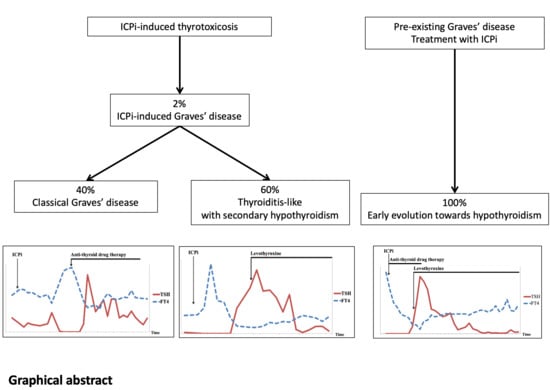
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. ICPi-Induced Graves’ Disease
3.3. Pre-Therapeutic Graves’ Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, P.A. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.-J.; You, B.; Dalle, S.; Péron, J. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Torino, F.; Corsello, S.M.; Salvatori, R. Endocrinological side-effects of immune checkpoint inhibitors. Curr. Opin. Oncol. 2016, 28, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Morganstein, D.; Lai, Z.; Spain, L.; Diem, S.; Levine, D.; Mace, C.; Gore, M.; Larkin, J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin. Endocrinol. 2017, 86, 614–620. [Google Scholar] [CrossRef]
- Shang, Y.H.; Zhang, Y.; Li, J.H.; Li, P.; Zhang, X. Risk of endocrine adverse events in cancer patients treated with PD-1 inhibitors: A systematic review and meta-analysis. Immunotherapy. 2017, 9, 261–272. [Google Scholar] [CrossRef]
- Anceau, C.C.; Abeillon, J.; Maillet, D.; Borson-Chazot, F.; Disse, E. Les dysthyroïdies sous immunothérapie anti-cancéreuse. Bull. Cancer 2020, 107, 262–271. [Google Scholar] [CrossRef]
- Chang, L.-S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.S.; Oury, A.; Daniels, G.A.; Bazhenova, L.; Patel, S.P. Incidence of Thyroid Function Test Abnormalities in Patients Receiving Immune-Checkpoint Inhibitors for Cancer Treatment. Oncologist. 2018, 23, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.N.; Whitelaw, B.C.; Palomar, M.T.P.; Wu, Y.; Carroll, P.V. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: Clinical review. Clin. Endocrinol. 2016, 85, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Sousa, R.; Ott, P.A.; Hodi, F.S.; Kaiser, U.B.; Tolaney, S.M.; Min, L. Endocrine dysfunction induced by immune checkpoint inhibitors: Practical recommendations for diagnosis and clinical management. Cancer 2018, 124, 1111–1121. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr.-Related Cancer 2018, 26, G1–G18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Buchbinder, E.I.; Haq, R.; Tolaney, S.; Barroso-Sousa, R.; Zhang, K.; Donahue, H.; et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol. Res. 2017, 5, 1133–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delivanis, D.A.; Gustafson, P.M.P.; Bornschlegl, S.; Merten, A.M.M.; Kottschade, A.L.; Withers, S.; Dietz, P.A.B.; Ryder, M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef] [PubMed]
- De Filette, J.; Jansen, Y.; Schreuer, M.; Everaert, H.; Velkeniers, B.; Neyns, B.; Bravenboer, B. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J. Clin. Endocrinol. Metab. 2016, 101, 4431–4439. [Google Scholar] [CrossRef]
- Levy, M.; Abeillon, J.; Dalle, S.; Assaad, S.; Borson-Chazot, F.; Disse, E.; Raverot, G.; Cugnet-Anceau, C. Anti-PD1 and Anti-PDL1-Induced Hypophysitis: A Cohort Study of 17 Patients with Longitudinal Follow-Up. J. Clin. Med. 2020, 9, 3280. [Google Scholar] [CrossRef] [PubMed]
- Goichot, B.; Leenhardt, L.; Massart, C.; Raverot, V.; Tramalloni, J.; Iraqi, H. Diagnostic procedure in suspected Graves’ disease. Ann. d’Endocrinologie 2018, 79, 608–617. [Google Scholar] [CrossRef]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M.; on behalf of the European Group on Graves’’ Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, J.H. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best Pr. Res. Clin. Endocrinol. Metab. 2012, 26, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Gonnella, D.; Giusti, C.; Virili, C.; et al. Graves’ disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pr. Res. Clin. Endocrinol. Metab. 2020, 34, 101388. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S. Autoimmunity and Graves’ disease. Clin. Pharmacol. Ther. 2012, 91, 577–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torimoto, K.; Okada, Y.; Nakayamada, S.; Kubo, S.; Tanaka, Y. Anti-PD-1 Antibody Therapy Induces Hashimoto’s Disease with an Increase in Peripheral Blood Follicular Helper T Cells. Thyroid. 2017, 27, 1335–1336. [Google Scholar] [CrossRef]
- Angell, T.E.; Min, L.; Wieczorek, T.J.; Hodi, F.S. Unique cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes Dis. 2018, 5, 46–48. [Google Scholar] [CrossRef]
- Daniels, G.H.; Vladić, A.; Brinar, V.; Zavalishin, I.; Valente, W.; Oyuela, P.; Palmer, J.; Margolin, D.H. Alemtuzumab-Related Thyroid Dysfunction in a Phase 2 Trial of Patients With Relapsing-Remitting Multiple Sclerosis. J. Clin. Endocrinol. Metab. 2014, 99, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElnea, E.; Mhéalóid, Á.N.; Moran, S.; Kelly, R.; Fulcher, T. Thyroid-Like Ophthalmopathy in a Euthyroid Patient Receiving Ipilimumab. Orbit 2014, 33, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Vaidya, A.; Becker, C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 2011, 164, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Borodic, G.; Hinkle, D.M.; Cia, Y. Drug-Induced Graves Disease From CTLA-4 Receptor Suppression. Ophthalmic Plast. Reconstr. Surg. 2011, 27, e87–e88. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Qiu, L.-J.; Zhang, M.; Wen, P.-F.; Ye, X.-R.; Liang, Y.; Pan, H.-F.; Ye, N.-Q. CTLA-4 CT60(rs3087243) polymorphism and autoimmune thyroid diseases susceptibility: A comprehensive meta-analysis. Endocr. Res. 2014, 39, 180–188. [Google Scholar] [CrossRef]
- Vaidya, B.; Kendall-Taylor, P.; Pearce, S.H.S. The Genetics of Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2002, 87, 5385–5397. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Howson, J.M.; Esposito, L.; Heward, J.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.; Smith, A.N.; Di Genova, G.; Herr, M. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef]
- Azmat, U.; Liebner, D.; Joehlin-Price, A.; Agrawal, A.; Nabhan, F. Treatment of Ipilimumab Induced Graves’ Disease in a Patient with Metastatic Melanoma. Case Rep Endocrinol. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gan, E.H.; Mitchell, A.L.; Plummer, R.; Pearce, S.; Perros, P. Tremelimumab-Induced Graves Hyperthyroidism. Eur. Thyroid. J. 2017, 6, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Brancatella, A.; Viola, N.; Brogioni, S.; Montanelli, L.; Sardella, C.; Vitti, P.; Marcocci, C.; Lupi, I.; Latrofa, F. Graves’ Disease Induced by Immune Checkpoint Inhibitors: A Case Report and Review of the Literature. Eur. Thyroid. J. 2019, 8, 192–195. [Google Scholar] [CrossRef]
- Yajima, K.; Akise, Y. A Case Report of Graves’ Disease Induced by the Anti-Human Programmed Cell Death-1 Monoclonal Antibody Pembrolizumab in a Bladder Cancer Patient. Case Rep. Endocrinol. 2019, 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Iadarola, C.; Croce, L.; Quaquarini, E.; Teragni, C.; Pinto, S.; Bernardo, A.; Fonte, R.; Marinò, M.; Rotondi, M.; Chiovato, L. Nivolumab Induced Thyroid Dysfunction: Unusual Clinical Presentation and Challenging Diagnosis. Front. Endocrinol. 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Okajima, F.; Onda, T.; Fujimori, S.; Emoto, N.; Sugihara, H. New-onset graves’ disease after the initiation of nivolumab therapy for gastric cancer: A case report. BMC Endocr. Disord. 2020, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Oikawa, Y.; Nakajima, R.; Satomura, A.; Tanaka, R.; Kagamu, H.; Shimada, A. Simultaneous development of Graves’ disease and type 1 diabetes during anti-programmed cell death-1 therapy: A case report. J. Diabetes Investig. 2020, 11, 1006–1009. [Google Scholar] [CrossRef]
- Sagiv, O.; Kandl, T.J.; Thakar, S.D.; Thuro, B.A.; Busaidy, N.L.; Cabanillas, M.; Jimenez, C.; Dadu, R.; Graham, P.H.; Debnam, J.M.; et al. Extraocular Muscle Enlargement and Thyroid Eye Disease-like Orbital Inflammation Associated with Immune Checkpoint Inhibitor Therapy in Cancer Patients. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 50–52. [Google Scholar] [CrossRef]
- Park, E.S.; Rabinowits, G.; Hamnvik, O.-P.R.; Dagi, L.R. A case of Graves’ ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Campredon, P.; Imbert, P.; Mouly, C.; Grunenwald, S.; Mazières, J.; Caron, P. Severe Inflammatory Ophthalmopathy in a Euthyroid Patient during Nivolumab Treatment. Eur. Thyroid. J. 2018, 7, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. CHECKPOINT INHIBITOR IMMUNE THERAPY. Retin. 2018, 38, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Callahan, M.; Postow, M.A.; Wolchok, J.; Fagin, J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocrine-Related Cancer 2014, 21, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Orlov, S.; Salari, F.; Kashat, L.; Walfish, P.G. Induction of Painless Thyroiditis in Patients Receiving Programmed Death 1 Receptor Immunotherapy for Metastatic Malignancies. J. Clin. Endocrinol. Metab. 2015, 100, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Iwasaki, H.; Yamashita, T.; Yoshida, T.; Suganuma, N.; Yamanaka, T.; Masudo, K.; Nakayama, H.; Kohagura, K.; Rino, Y.; et al. Potential Risk Factors for Nivolumab-induced Thyroid Dysfunction. Vivo 2017, 31, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.; Wang, Y.; Menzies, A.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.; Obeid, M. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 2020, 31, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Danlos, F.-X.; Voisin, A.-L.; Dyevre, V.; Michot, J.-M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef]
- Maekura, T.; Naito, M.; Tahara, M.; Ikegami, N.; Kimura, Y.; Sonobe, S.; Kobayashi, T.; Tsuji, T.; Minomo, S.; Tamiya, A.; et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. Vivo 2018, 31, 1035–1039. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Kimbara, S.; Fujiwara, Y.; Iwama, S.; Ohashi, K.; Kuchiba, A.; Arima, H.; Yamazaki, N.; Kitano, S.; Yamamoto, N.; Ohe, Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018, 109, 3583–3590. [Google Scholar] [CrossRef] [Green Version]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.T.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid. 2017, 27, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients With Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Am. Soc. Clin. Oncol. J. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]


| No. | Sex Age | Indication | ICPi (Name) and Dose | Cycle/Time Since ICPi Initiation | Total ICPi Cycles Number | Other irAE (Time) | Maximum FT4/FT3 (in ULN) | TRAb at Diagnosis (in ULN) | Iodine 99mTc Uptake/ Hyper-Vascular Doppler | TPOAb | ATD (Duration in Weeks) | Corticosteroids (Time of Introduction) | Status at Last Follow-Up | Treatment at Last Follow-Up (Time of Introduction) | Total Follow-Up (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F 69 | Serous ovarian carcinoma | anti-PD-1 (Pembrolizumab) 200 mg/3 weeks | IX/41 weeks | XIV | G3 (week 15) | 1.3/1.3 | 2.7 | High/na | Negative | Carbimazole (45) | Yes * (week 15) | Euthyroidism | 0 | 57 |
| 2 | F 60 | Endometrial adenocarcinoma | anti-CTLA-4 and anti-PD-L1 (Durvalumab and Tremelimumab) 1500 mg and 76 mg | I/2 weeks | I | G3 (week 2) | 2.5/1.8 | 5.2 | na/No | Positive | Carbimazole (>29 **) | Yes * (week 2) | Hyperthyroidism | Carbimazole 1.25 mg/day (week 1) | 30 |
| 3 | M 60 | Small cell bronchial carcinoma | anti-PD-1 (Pembrolizumab) 200 mg/3 weeks | I/3 weeks | VI | No | 3.4/2 | 3.5 | na/na | Positive | No | No | Hypothyroidism | Levothyroxine 150 µg/day (week 17) | 48 |
| 4 | F 44 | Melanoma | anti-CTLA-4 and anti-PD-1 (Ipilimumab and Nivolumab) 3 and 1 mg/kg/3 weeks | I/2 weeks | II | G3 (week 5) | 1.9/na | 4.1 | Low/No | na | No | No | DCD *** | 0 *** | 13 |
| 5 | M 76 | Epidermoid lung carcinoma | anti-PD-1 (Nivolumab) 3 mg/kg/2 weeks | II/4 weeks | XXXVIII | No | 2.4/1.3 | 48 | na/No | Negative | Carbimazole (10) | No | Hypothyroidism | Levothyroxine 150 µg/day (week 16) | 143 |
| No. | Sex Age | Indication | ICPi (Name) and Dose | Total Number of ICPi Cycles | TSH/FT4/FT3 at Time of ICPi Initiation (in ULN) | TRAb at GD’s Diagnosis (in ULN) | TPOAb | Iodine 99mTc Uptake/ Hypervascular Doppler | irAE | ATD Duration (Weeks) | Time to Normalize FT4 (Weeks) | Max TSH/ Min FT4 (in ULN) and (Time) | Time to Normalize TRAb (Weeks) | Status at Last Follow-Up | Treatment at Last Follow-Up (Time of Introduction) | Total Follow-Up (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | M 67 | Melanoma | anti-PD1 (Nivolumab) 240 mg/2 weeks | IX | <0.01/1/1 | 2.4 | Negative | High/Yes | No | 1 | 10 | 13/0.1 (week 16) | na** | Hypothyroidism | Levothyroxine 150 µg/day (week 19) | 34 |
| 7 | M 49 | Bronchial adenocarcinoma | anti-PD1 (Pembrolizumab) 200 mg/3 weeks | IX | <0.01/3.4/2.5 | 1.8 | Positive | High/Yes | No | 23 | 8 | 17/0.5 (week 18) | 30 | Hypothyroidism | Levothyroxine 250 µg/day (week 21) | 94 |
| 8 | M 74 | Renal clear cell carcinoma | anti-PD1 (Nivolumab) 240 mg/2 weeks | XIII | <0.01/1.5/na | 14* | Positive | na/Yes | No | 16 | 9 | 10/0.6 (week 20) | 46 | Hypothyroidism | Levothyroxine 150 µg/day (week 13) | 64 |
| First Author, Year | ICPi (Name) and Dose | Sex Age | Personal/Familial History of AID or TD | Hyper-Thyroidism | GO | Cycle/Time | FT4/FT3 (in ULN) | TRAb (ULN) | TPOAb | Iodine 99mTc Uptake | Doppler US Vascular Pattern | Other irAE | Treatment (Duration) | Hypothyroidism Evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graves’ hyperthyroidism | ||||||||||||||
| De Filette, 2016 [19] | anti-PD-1 (Pembrolizumab) 2 mg/kg/na | na | na/na | Yes | No | na | na/na | Positive (na) | Negative | na | na | na | No | Yes |
| Azmat, 2016 [35] | anti-CTLA-4 (Ipilimumab) 3 mg/kg/2 weeks | M 67 | No/na | Yes | No | II/6 weeks | 2.1/2.2 | Positive (na) | na | High | na | na | ATD (na) then Thyroid-ectomy (a) | Yes (b) |
| Gan, 2017 [36] | anti-CTLA-4 (Tremelimumab) 1 cycle/12–24 weeks | M 55 | No/No | Yes | No | XX/8 years | 1.6/1.9 | Positive (3.1) | Positive | na | na | na | ATD (na) | No |
| Brancatella, 2019 [37] | anti-PD-1 (Nivolumab) 3 mg/kg/ 2 weeks | M 51 | na/na | Yes | No | IV/8 weeks | 1.3/1.6 | Negative | Negative | High | Hyper-vascular | na | ATD (na) | No |
| Iadarola, 2019 [39] | anti-PD-1 (Nivolumab) 3 mg/kg/ 2 weeks | F 66 | na/na | Yes | No | II/4 weeks | 1/1.3 (c) | Negative | Negative | High | Normal | na | ATD (na) | No |
| Yajima, 2019 [38] | anti-PD-1 (Pembrolizumab) 200 mg/3 weeks | M 61 | No/No | Yes | No | V/14 weeks | 2.3/1.7 | Positive (2.5) (d) | Negative | na | Hyper-vascular | Colitis | ATD and GC (>20 weeks) (e) | No |
| Yamada, 2020 [40] | anti-PD-1 (Nivolumab) 240 mg/3 weeks | M 66 | No/No | Yes | No | II/6 weeks | >2.9/2.8 | Positive (15) | Negative | High | Hyper-vascular | No | ATD (>18 weeks) | No |
| Kurihara, 2020 [41] | anti-PD-1 (Nivolumab) 240 mg/2–5 weeks | M 48 | No/No | Yes | No | VI/16 weeks | 1.1/1.2 | Positive (1.5) | Negative | Normal | Normal | T1D | ATD (>64 weeks) | No |
| Graves’ hyperthyroidism and orbitopathy | ||||||||||||||
| Sagiv, 2019 [42] | anti-CTLA-4 (Tremelimumab) 10 mg/kg/4 weeks | M 51 | na/na | Yes | Yes | V/27 weeks | >1 (na) | Negative | Positive | na | na | na | GC (na) | No |
| Graves’ orbitopathy | ||||||||||||||
| Borodic, 2011 [31] | anti-CTLA-4 (Ipilimumab) na | F 51 | na/na | No | Yes | II/6 weeks | 1/na | Positive (29) | Positive | na | na | na | GC (na) | No |
| Min, 2011 [30] | anti-CTLA-4 (Ipilimumab) 10 mg/kg/3 weeks | F 51 | No/na | No | Yes | IV/12 weeks | 1/na | Positive (1.1) (f) | Positive | na | na | na | GC (na) | No |
| McElnea, 2014 [29] | anti-CTLA-4 (Ipilimumab) 3 mg/kg/2 weeks | F 68 | No/No | No | Yes | III/6 weeks | 1/na | Negative | Negative | na | na | na | GC (na) | No |
| Park, 2018 [43] | anti-PD-1 (Pembrolizumab) 1 cycle/2 weeks | M 52 | No/Yes (g) | No | Yes | III/9 weeks | na/na | na | na | na | na | na | GC (na) | No |
| Campredon, 2018 [44] | anti-PD-1 (Nivolumab) na | M 61 | No/No | No | Yes | III/6 weeks | 1/1 | Negative | Negative | na | na | na | GC (na) | No |
| Sagiv, 2019 [42] | anti-CTLA-4 and anti-PD-1 (Ipilimumab and Nivolumab) 3 mg/kg/2 weeks | M 73 | No | No | Yes | II/6 weeks | 1/1 | Negative | Negative | na | na | na | GC (na) | No |
| Graves’ disease before ICPis | ||||||||||||||
| Sagiv, 2019 [42] | anti-PD-1 (Nivolumab) 3 mg/kg/2 weeks | M 42 | No | Yes (h) | Yes (i) | III/8 weeks | >1 (na) | Positive (na) | Neg/na | na | na | na | No (j) | Yes (k) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peiffert, M.; Cugnet-Anceau, C.; Dalle, S.; Chikh, K.; Assaad, S.; Disse, E.; Raverot, G.; Borson-Chazot, F.; Abeillon-du Payrat, J. Graves’ Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review). Cancers 2021, 13, 1944. https://doi.org/10.3390/cancers13081944
Peiffert M, Cugnet-Anceau C, Dalle S, Chikh K, Assaad S, Disse E, Raverot G, Borson-Chazot F, Abeillon-du Payrat J. Graves’ Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review). Cancers. 2021; 13(8):1944. https://doi.org/10.3390/cancers13081944
Chicago/Turabian StylePeiffert, Mathilde, Christine Cugnet-Anceau, Stephane Dalle, Karim Chikh, Souad Assaad, Emmanuel Disse, Gérald Raverot, Françoise Borson-Chazot, and Juliette Abeillon-du Payrat. 2021. "Graves’ Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review)" Cancers 13, no. 8: 1944. https://doi.org/10.3390/cancers13081944
APA StylePeiffert, M., Cugnet-Anceau, C., Dalle, S., Chikh, K., Assaad, S., Disse, E., Raverot, G., Borson-Chazot, F., & Abeillon-du Payrat, J. (2021). Graves’ Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review). Cancers, 13(8), 1944. https://doi.org/10.3390/cancers13081944