The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Objectives
2.3. Evaluation of Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
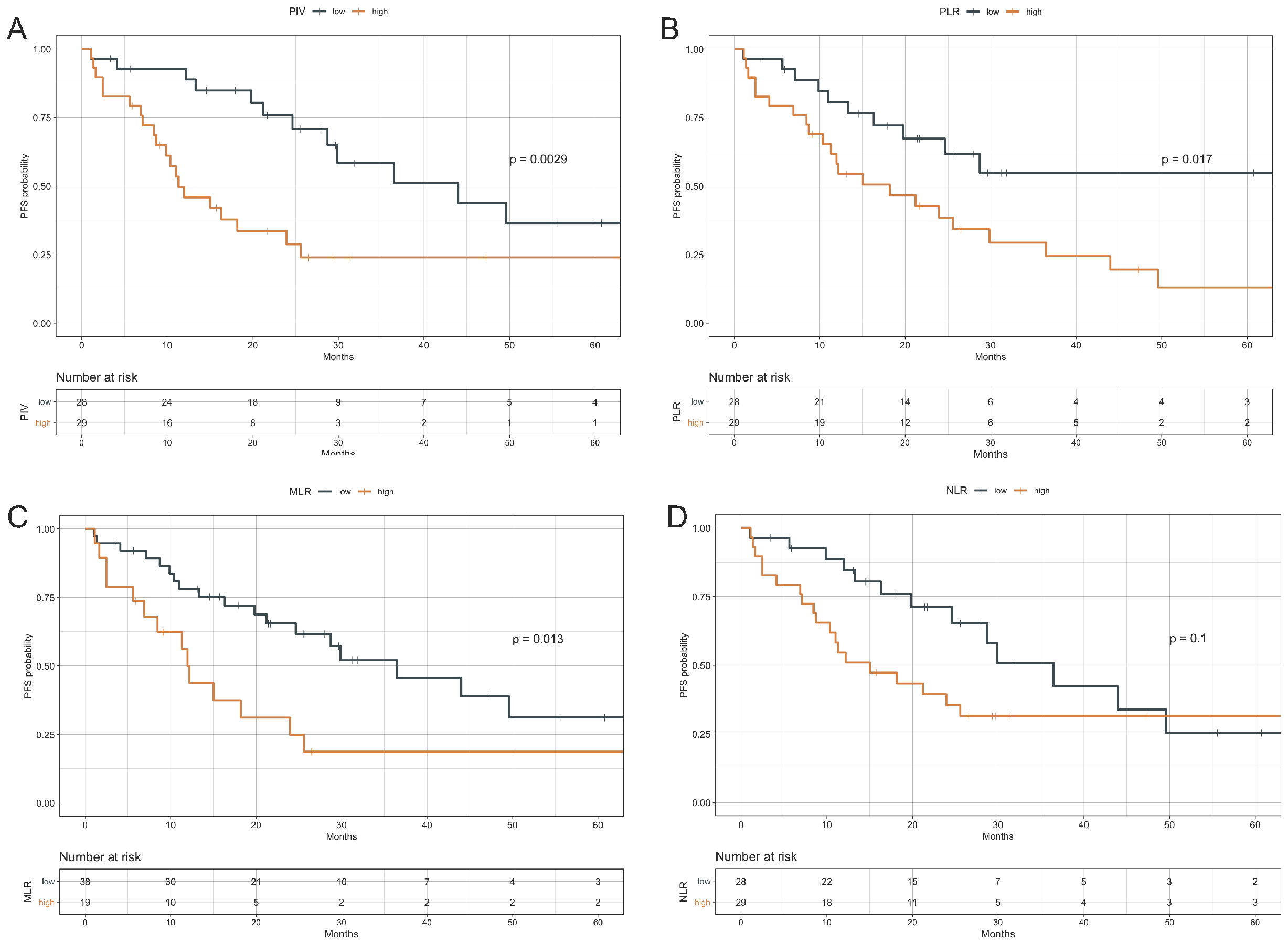
3.2. Impact of Peripheral Blood Parameters on PFS
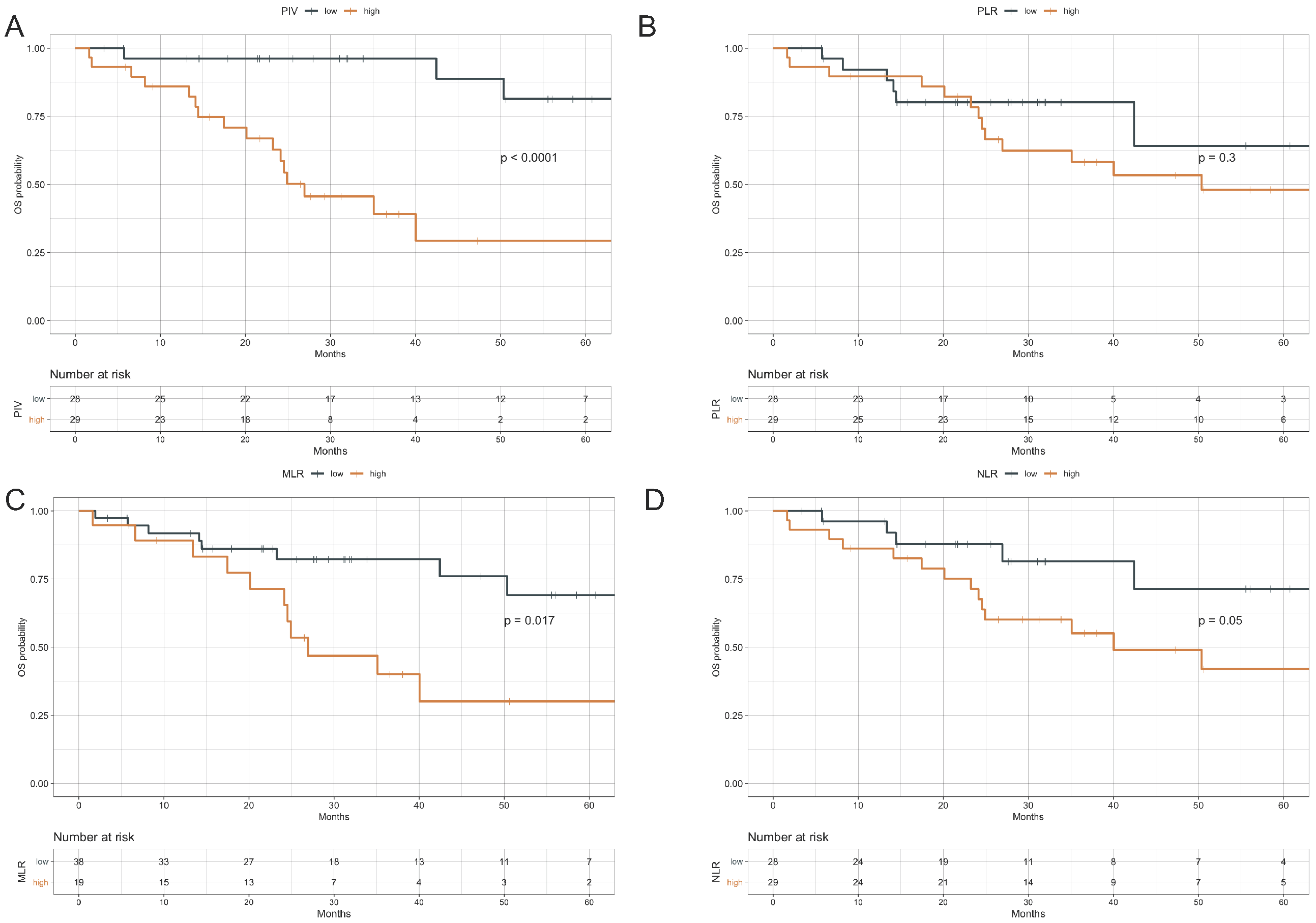
3.3. Impact of Peripheral Blood Parameters on OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Lewis Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A. Trastuzumab--mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; De Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Nahta, R.; Hung, M.C.; Esteva, F.J. The HER-2-Targeting Antibodies Trastuzumab and Pertuzumab Synergistically Inhibit the Survival of Breast Cancer Cells. Cancer Res. 2004, 64, 2343–2346. [Google Scholar] [CrossRef]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly. Enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Baselga, J.; Cortes, J.; Im, S.A.; Clark, E.; Ross, G.; Kiermaier, A.; Swain, S.M. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3753–3761. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; De Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Salgado, R.; Fox, S.; Savas, P.; Eng-Wong, J.; Clark, E.; Kiermaier, A.; Swain, S.M.; Baselga, J.; Michiels, S.; et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: A retrospective analysis of the CLEOPATRA study. Lancet. Oncol. 2017, 18, 52–62. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer. Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Cucurull, M.; Felip, E.; Garcia, J.J.; Erasun, C.; Angelats, L.; Teruel, I.; Martinez-Román, S.; Hernández, J.; Esteve, A.; España, S.; et al. Prognostic value of monocyte to lymphocyte ratio (MLR) in epithelial ovarian cancer (EOC). J. Clin. Oncol. 2019, 37, e17066. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef]
- Wei, B.; Yao, M.; Xing, C.; Wang, W.; Yao, J.; Hong, Y.; Liu, Y.; Fu, P. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Onco. Targets. Ther. 2016, 9, 5567–5575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, X.Z.; Song, Y.X.; Gao, P.; Sun, J.X.; Wang, Z.N. High Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Breast Cancer: A Meta-Analysis. Biomed Res. Int. 2017, 2017, 9503025. [Google Scholar] [CrossRef]
- Geng, S.K.; Fu, S.M.; Fu, Y.P.; Zhang, H.W. Neutrophil to lymphocyte ratio is a prognostic factor for disease free survival in patients with breast cancer underwent curative resection. Medicine (Baltimore) 2018, 97, e11898. [Google Scholar] [CrossRef]
- Vernieri, C.; Mennitto, A.; Prisciandaro, M.; Huber, V.; Milano, M.; Rinaldi, L.; Cona, M.S.; Maggi, C.; Ferrari, B.; Manoukian, S.; et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict efficacy of platinum-based chemotherapy in patients with metastatic triple negative breast cancer. Sci. Rep. 2018, 8, 8703. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Onoda, N.; Noda, S.; Kawajiri, H.; Takashima, T.; Ohsawa, M.; Kitagawa, S.; Hirakawa, K. Predictive Value of Neutrophil/Lymphocyte Ratio for Efficacy of Preoperative Chemotherapy in Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2016, 23, 1104–1110. [Google Scholar] [CrossRef]
- Chae, S.; Kang, K.M.; Kim, H.J.; Kang, E.; Park, S.Y.; Kim, J.H.; Kim, S.H.; Kim, S.W.; Kim, E.K. Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr. Oncol. 2018, 25, e113–e119. [Google Scholar] [CrossRef]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef]
- Fuca, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Fulde, G.; Moulton, B.; Nadauld, L.D.; Rhodes, T. An elevated neutrophil-to-lymphocyte ratio associates with weight loss and cachexia in cancer. Sci. Rep. 2020, 10, 7535. [Google Scholar] [CrossRef]
- Gross, R.L.; Newberne, P.M. Role of nutrition in immunologic function. Physiol. Rev. 1980, 60, 188–302. [Google Scholar] [CrossRef] [PubMed]
- Dunki Jacobs, P.B.; Ruevekamp, M.; Hart, G.A.; de Graaf, P.W. Dietary influences on cell proliferation in bone marrow. Eur. J. Cancer. Clin. Oncol. 1989, 25, 953–957. [Google Scholar] [CrossRef]
- Cook, J.; Hagemann, T. Tumour-associated macrophages and cancer. Curr. Opin. Pharmacol. 2013, 13, 595–601. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, H.J.; Ha, S.J.; Lee, K.T.; Kwon, Y.G. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta 2013, 1835, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; McMillan, D.C. Cancer and systemic inflammation: Treat the tumour and treat the host. Br. J. Cancer. 2014, 110, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19673. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, E.Y.; Yun, J.S.; Park, Y.L.; Do, S.I.; Chae, S.W.; Park, C.H. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer 2018, 18, 938. [Google Scholar] [CrossRef]
- Gonda, K.; Shibata, M.; Ohtake, T.; Matsumoto, Y.; Tachibana, K.; Abe, N.; Ohto, H.; Sakurai, K.; Takenoshita, S. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol. Lett. 2017, 14, 1766–1774. [Google Scholar] [CrossRef]
- Ulas, A.; Avci, N.; Kos, T.; Cubukcu, E.; Fatih Olmez, O.; Bulut, N.; Degirmenci, M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2- positive early breast cancer receiving adjuvant trastuzumab? JBUON 2015, 20, 714–722. [Google Scholar]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 57) |
|---|---|
| AGE | |
| Median (Range) | 53 (26–78) |
| <50 | 19 (33.3%) |
| ≥50 | 38 (66.7%) |
| De novo metastatic disease | |
| no | 34 (59.6%) |
| yes | 23 (40.4%) |
| Number of metastatic sites | |
| ≤2 | 30 (52.6%) |
| >2 | 27 (47.4%) |
| Visceral metastases | |
| no | 27 (47.4%) |
| yes | 30 (52.6%) |
| Brain metastases | |
| no | 49 (86.0%) |
| yes | 8 (14.0%) |
| HR status * | |
| negative | 20 (35.1%) |
| positive | 37 (64.9%) |
| Previous trastuzumab | |
| no | 35 (61.4%) |
| yes | 22 (38.6%) |
| Previous anthracyclines | |
| no | 30 (52.6%) |
| yes | 27 (47.4%) |
| Previous taxanes | |
| no | 31 (54.4%) |
| yes | 26 (45.6%) |
| Type of chemotherapy regimen | |
| Docetaxel | 44 (77.2%) |
| Paclitaxel | 13 (22.8%) |
| PIV | |
| Median (range) | 285 (0–4249) |
| PLR | |
| Median (range) | 159 (24–1347) |
| MLR | |
| Median (range) | 0.3 (0.0–1.0) |
| NLR | |
| Median (range) | 3.0 (0.9–14.6) |
| Univariate Analysis | HR (95% CI) | p |
|---|---|---|
| AGE | ||
| per 1 year | 1.01 (0.98–1.04) | 0.550 |
| ≥50 vs. <50 | 1.49 (0.70–3.18) | 0.307 |
| HR status * | ||
| Positive vs. negative | 0.94 (0.45–1.96) | 0.873 |
| De novo advanced disease | ||
| yes vs. no | 0.73 (0.35–1.51) | 0.395 |
| Number of metastatic sites | ||
| ≥3 vs. <3 | 2.36 (1.13–4.93) | 0.022 |
| Visceral metastases | ||
| yes vs. no | 2.14 (1.03–4.44) | 0.041 |
| Brain metastases | ||
| yes vs. no | 1.96 (0.74–5.17) | 0.175 |
| Previous trastuzumab | ||
| yes vs. no | 1.92 (0.96–3.86) | 0.067 |
| Previous anthracyclines | ||
| yes vs. no | 1.05 (0.52–2.11) | 0.887 |
| Previous taxanes | ||
| yes vs. no | 1.69 (0.84–3.38) | 0.141 |
| Type of chemotherapy regimen | ||
| Paclitaxel vs. docetaxel | 1.48 (0.66–3.32) | 0.337 |
| PIV | ||
| high vs. low | 2.93 (1.40–6.10) | 0.004 |
| MLR | ||
| high vs. low | 2.39 (1.18–4.85) | 0.015 |
| PLR | ||
| high vs. low | 2.43 (1.15–5.13) | 0.020 |
| NLR | ||
| high vs. low | 1.79 (0.88–3.64) | 0.105 |
| Multivariable analysis | HR (95% CI) | p |
| Number of metastatic sites | ||
| ≥3 vs. <3 | 3.78 (1.50–9.52) | 0.005 |
| Visceral metastases | ||
| yes vs. no | 1.73 (0.72–4.15) | 0.217 |
| Previous trastuzumab | ||
| yes vs. no | 4.29 (1.85–9.96) | <0.001 |
| PIV | ||
| high vs. low | 2.34 (0.99–5.51) | 0.052 |
| Univariate analysis | HR (95% CI) | p |
|---|---|---|
| AGE | ||
| per 1 year | 1.02 (0.98–1.06) | 0.374 |
| ≥50 vs. <50 | 2.01 (0.73–5.59) | 0.179 |
| HR status * | ||
| Positive vs. negative | 0.93 (0.37–2.34) | 0.874 |
| De novo advanced disease | ||
| yes vs. no | 0.76 (0.30–1.91) | 0.561 |
| Number of metastatic sites | ||
| ≥3 vs. <3 | 2.57 (1.02–6.49) | 0.045 |
| Visceral metastases | ||
| yes vs. no | 2.46 (0.95–6.41) | 0.065 |
| Brain metastases | ||
| yes vs. no | 4.02 (1.40–11.51) | 0.010 |
| Type of chemotherapy regimen | ||
| Paclitaxel vs. docetaxel | 1.23 (0.41–3.73) | 0.714 |
| Previous trastuzumab | ||
| yes vs. no | 1.80 (0.75–4.33) | 0.190 |
| Previous anthracyclines | ||
| yes vs. no | 0.90 (0.37–2.17) | 0.811 |
| Previous taxanes | ||
| yes vs. no | 1.66 (0.69–4.00) | 0.263 |
| PIV | ||
| high vs. low | 9.48 (2.69–33.44) | <0.001 |
| MLR | ||
| high vs. low | 2.82 (1.16–6.85) | 0.022 |
| PLR | ||
| high vs. low | 1.65 (0.63–4.35) | 0.308 |
| NLR | ||
| high vs. low | 2.65 (0.96–7.30) | 0.060 |
| Multivariable analysis | HR (95% CI) | p |
| Number of metastatic sites | ||
| ≥3 vs. <3 | 1.86 (0.66–5.24) | 0.239 |
| Visceral metastases | ||
| yes vs. no | 1.37 (0.44–4.32) | 0.588 |
| Brain metastases | ||
| yes vs. no | 1.44 (0.46–4.53) | 0.536 |
| PIV | ||
| high vs. low | 7.96 (2.18–29.09) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligorio, F.; Fucà, G.; Zattarin, E.; Lobefaro, R.; Zambelli, L.; Leporati, R.; Rea, C.; Mariani, G.; Bianchi, G.V.; Capri, G.; et al. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers 2021, 13, 1964. https://doi.org/10.3390/cancers13081964
Ligorio F, Fucà G, Zattarin E, Lobefaro R, Zambelli L, Leporati R, Rea C, Mariani G, Bianchi GV, Capri G, et al. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers. 2021; 13(8):1964. https://doi.org/10.3390/cancers13081964
Chicago/Turabian StyleLigorio, Francesca, Giovanni Fucà, Emma Zattarin, Riccardo Lobefaro, Luca Zambelli, Rita Leporati, Carmen Rea, Gabriella Mariani, Giulia V. Bianchi, Giuseppe Capri, and et al. 2021. "The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab" Cancers 13, no. 8: 1964. https://doi.org/10.3390/cancers13081964
APA StyleLigorio, F., Fucà, G., Zattarin, E., Lobefaro, R., Zambelli, L., Leporati, R., Rea, C., Mariani, G., Bianchi, G. V., Capri, G., de Braud, F., & Vernieri, C. (2021). The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers, 13(8), 1964. https://doi.org/10.3390/cancers13081964







