Predictors of Survival in Atypical Meningiomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
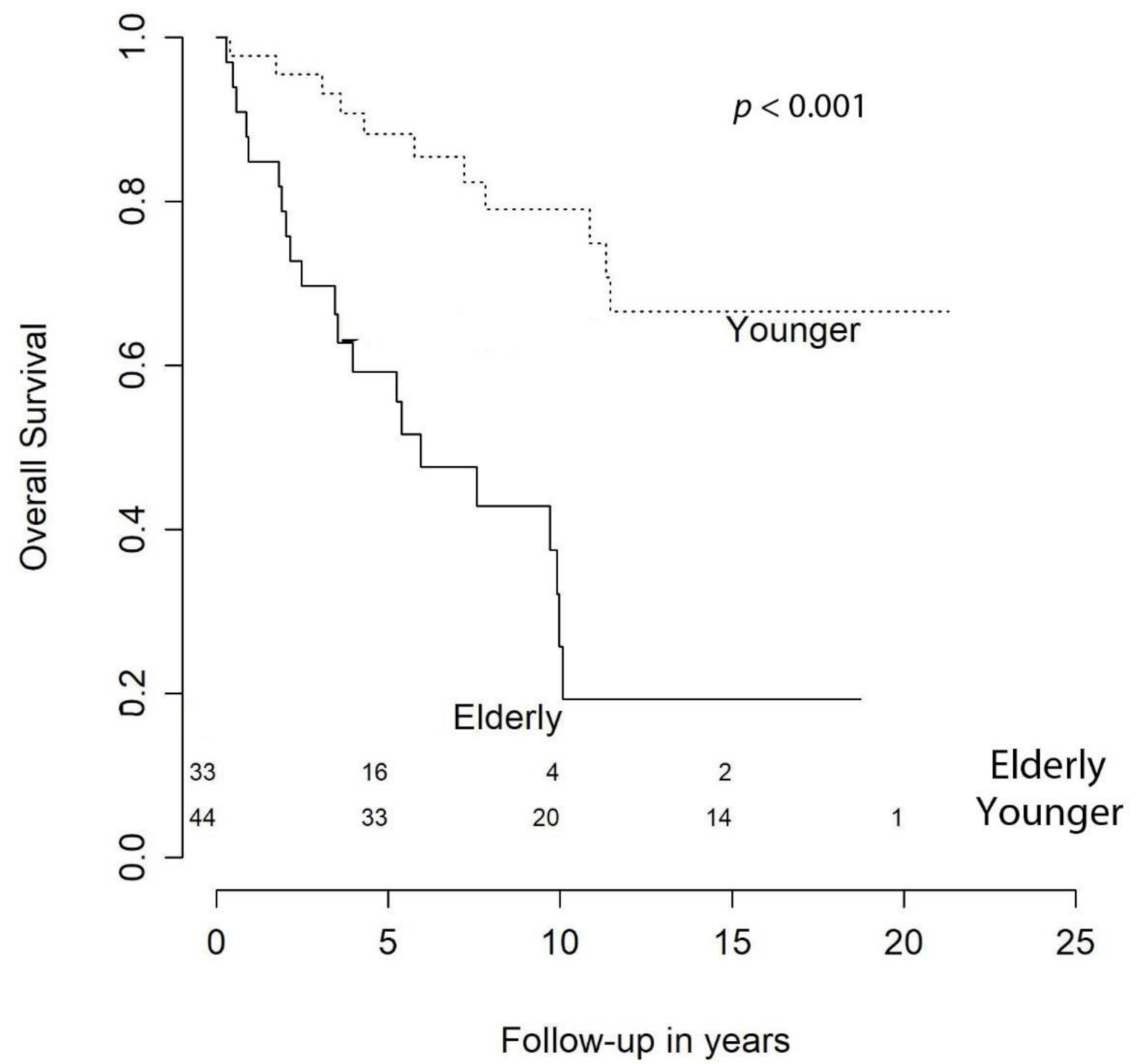
2.1 Overall Characteristics
2.2 Surgical and Neurological Outcomes
2.3 Predictors of Overall Survival and Retreatment-Free Survival
2.4 Predictors of Worsened Neurological Outcome
3. Discussion
4. Methods
4.1 Patient Cohort
4.2 Tumor Characteristics
4.3 Outcome
4.4 Ethics
4.5 Statistics
5. Conclusions
6. Strengths and Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Corniola, M.V.; Lemée, J.-M.; Da Broi, M.; Joswig, H.; Schaller, K.; Helseth, E.; Meling, T.R. Posterior fossa meningiomas: Perioperative predictors of extent of resection, overall survival and progression-free survival. Acta Neurochir. 2019, 161, 1003–1011. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar]
- Hasseleid, B.F.; Meling, T.R.; Ronning, P.; Scheie, D.; Helseth, E. Surgery for convexity meningioma: Simpson Grade I resection as the goal: Clinical article. J. Neurosurg. 2012, 117, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Konglund, A.; Rogne, S.G.; Lund-Johansen, M.; Scheie, E.; Helseth, E.; Meling, T.R. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurol. Scand. 2012, 127, 161–169. [Google Scholar]
- Lemée, J.-M.; Corniola, M.V.; Da Broi, M.; Joswig, H.; Scheie, D.; Schaller, K.; Helseth, E.; Meling, T.R. Extent of Resection in Meningioma: Predictive Factors and Clinical Implications. Sci. Rep. 2019, 9, 5944. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2018, 42, 163–173. [Google Scholar]
- Ødegaard, K.M.; Helseth, E.; Meling, T.R. Intraventricular meningiomas: A consecutive series of 22 patients and literature review. Neurosurg. Rev. 2013, 36, 57–64; discussion 64. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Bi, W.L.; Kandola, M.S.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; Beroukhim, R.; MelinReardong, D.A.; Wen, P.Y.; et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 2015, 121, 4376–4381. [Google Scholar] [CrossRef]
- Rydzewski, N.R.; Lesniak, M.S.; Chandler, J.P.; Kalapurakal, J.A.; Pollom, E.; Tate, M.C.; Bloch, O.; Kruser, T.; Bs, P.D.; Sachdev, S. Gross total resection and adjuvant radiotherapy most significant predictors of improved survival in patients with atypical meningioma. Cancer 2018, 124, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.K.; Suh, J.H.; Mohan, D.S.; A Prayson, R.; Lee, J.; Barnett, G.H. Local control and overall survival in atypical meningioma: A retrospective study. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 57–61. [Google Scholar] [CrossRef]
- Park, H.J.; Kang, H.-C.; Kim, I.H.; Park, S.-H.; Kim, D.G.; Park, C.-K.; Paek, S.H.; Jung, H.-W. The role of adjuvant radiotherapy in atypical meningioma. J. Neurooncology 2013, 115, 241–247. [Google Scholar]
- Zaher, A.; Mattar, M.A.; Zayed, D.H.; Ellatif, R.A.; Ashamallah, S.A. Atypical Meningioma: A Study of Prognostic Factors. World Neurosurg. 2013, 80, 549–553. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Clerkin, J.; Millward, C.P.; O’Halloran, P.J.; Waqar, M.; Looby, S.; Young, A.M.H.; Guilfoyle, M.R.; Fitzroll, D.; Devadass, A.; et al. Predictors of early progression of surgically treated atypical meningiomas. Acta Neurochir. 2018, 160, 1813–1822. [Google Scholar]
- Gabeau-Lacet, D.; Aghi, M.; Betensky, R.A.; Barker, F.G.; Loeffler, J.S.; Louis, D.N. Bone involvement predicts poor outcome in atypical meningioma. J. Neurosurg. 2009, 111, 464–471. [Google Scholar] [CrossRef]
- Streckert, E.M.S.; Hess, K.; Sporns, P.B.; Adeli, A.; Brokinkel, C.; Kriz, J.; Holling, M.; Eich, H.T.; Paulus, W.; Spille, D.C.; et al. Clinical, radiological, and histopathological predictors for long-term prognosis after surgery for atypical meningiomas. Acta Neurochir. 2019, 161, 1647–1656. [Google Scholar] [CrossRef]
- Aghi, M.K.; Carter, B.S.; Cosgrove, G.R.; Ojemann, R.G.; Amin-Hanjani, S.; Martuza, R.L.; Curry, W.T., Jr.; Barker, F.G. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009, 64, 56–60; discussion 60. [Google Scholar] [CrossRef]
- Hammouche, S.; Clark, S.; Wong, A.H.L.; Eldridge, P.; Farah, J.O. Long-term survival analysis of atypical meningiomas: Survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir. 2014, 156, 1475–1481. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef]
- DeMonte, F.; McDermott, M.W.; Al-Mefty, O. Al-Mefty’s Meningiomas, 2nd ed.; Thieme Medical: New York, NY, USA, 2011. [Google Scholar]
- Yang, S.-Y.; Park, C.-K.; Park, S.-H.; Kim, D.G.; Chung, Y.S.; Jung, H.-W. Atypical and anaplastic meningiomas: Prognostic implications of clinicopathological features. J. Neurol. Neurosurg. Psychiatry 2008, 79, 574–580. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar]
- Chen, W.C.; Magill, S.T.; Wu, A.; Vasudevan, H.N.; Morin, O.; Aghi, M.K.; Theodosopoulos, P.V.; Perry, A.; McDermott, M.W.; Sneed, P.K.; et al. Histopathological features predictive of local control of atypical meningioma after surgery and adjuvant radiotherapy. J. Neurosurg. 2018, 130, 443–450. [Google Scholar]
- Klinger, D.R.; Flores, B.C.; Lewis, J.J.; Hatanpaa, K.; Choe, K.; Mickey, B.; Barnett, S. Atypical Meningiomas: Recurrence, Reoperation, and Radiotherapy. World Neurosurg. 2015, 84, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamaridès, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fèvre-Montange, M.; Guyotat, J. WHO grade II and III meningiomas: A study of prognostic factors. J. Neuro-Oncol. 2009, 95, 367–375. [Google Scholar]
- Hardesty, D.A.; Wolf, A.B.; Brachman, D.G.; McBride, H.L.; Youssef, E.; Nakaji, P.; Porter, R.W.; Smith, K.A.; Spetzler, R.F.; Sanai, N. The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J. Neurosurg. 2013, 119, 475–481. [Google Scholar] [CrossRef]
- Jo, K.; Park, H.-J.; Nam, D.-H.; Lee, J.-I.; Kong, D.-S.; Park, K.; Kim, J.H. Treatment of atypical meningioma. J. Clin. Neurosci. 2010, 17, 1362–1366. [Google Scholar]
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The Use of the Nitrogen Mustards in the Palliative Treatment of Carcinoma—With Particular Reference to Bronchogenic Carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- Ibanez, L.F.A.; Hem, S.; Ajler, P.; Vecchi, E.; Ciraolo, C.; Baccanelli, M.; Tramontano, R.; Knezevich, F.; Carrizo, A. A new classification of complications in neurosurgery. World Neurosurg. 2011, 75, 709–715; discussion 604–711. [Google Scholar] [CrossRef]




| n | % | |
|---|---|---|
| 77 | 100 | |
| Sex | ||
| Male | 31 | 40.3% |
| Female | 46 | 59.7% |
| Age at Primary Surgery | ||
| Median [IQR] | 62.21 [22.87] years | |
| Preoperative KPS | ||
| 100 | 2 | 2.6% |
| 90 | 20 | 26.0% |
| 80 | 28 | 36.4% |
| 70 | 19 | 24.7% |
| <70 | 8 | 10.4% |
| Symptoms at Presentation | ||
| Neurological deficits | 51 | 66.2% |
| Raised ICP | 36 | 46.8% |
| Seizures | 21 | 27.3% |
| Asymptomatic | 1 | 1.3% |
| Location | ||
| Convexity | 22 | 28.6% |
| Falx | 12 | 15.6% |
| Parasagittal | 19 | 24.7% |
| CP angle | 4 | 5.2% |
| Lateral sphenoid wing | 5 | 6.5% |
| Medial sphenoid wing | 3 | 3.9% |
| Olfactory groove | 2 | 2.6% |
| Petroclival | 1 | 1.3% |
| Tentorium-intra | 3 | 3.9% |
| Tentorium-supra | 2 | 2.6% |
| Tuberculum sellae/suprasellar | 2 | 2.6% |
| Intraventricular | 2 | 2.6% |
| Bony Invasion | ||
| Yes | 17 | 22.1% |
| No | 60 | 77.9% |
| Multiple Meningiomas | ||
| Yes | 5 | 6.5% |
| No | 72 | 93.5% |
| n | % | |
|---|---|---|
| 77 | 100 | |
| Simpson Grade | ||
| Grade I | 35 | 45.5% |
| Grade II | 19 | 24.7% |
| Grade III | 6 | 7.8% |
| Grade IV | 16 | 20.8% |
| Grade V | 1 | 1.3% |
| 30-Day Mortality | ||
| 0 | 0.0% | |
| Early Postoperative Complications | ||
| Hematomas | 2 | 2.6% |
| Infections | 4 | 5.2% |
| Neurological Outcome at 6–12 Months | ||
| Improved/stable | 39 | 50.6% |
| Worsened | 9 | 11.7% |
| No data | 29 | 37.7% |
| Retreatment | ||
| Any retreatment | 22 | 28.6% |
| Radiotherapy only | 1 | 1.3% |
| Surgery only | 1 | 1.3% |
| Surgery and radiotherapy | 20 | 26.0% |
| Early Postoperative Complications after Retreatment | ||
| Hematomas | 0 | 0.0% |
| Infections | 1 | 4.5% |
| Cox Model for Overall Survival | ||||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age at Primary Surgery | 1.07 (1.03–1.10) | <0.001 | 1.08 (1.04–1.12) | <0.001 |
| Preoperative KPS Poor vs. Good | 3.70 (1.51–9.09) | 0.005 | 4.00 (1.49–11.11) | 0.006 |
| Meningioma Requiring Retreatment Any retreatment vs. No Retreatment | 2.13 (1.06–4.28) | 0.033 | 3.30 (1.49–7.32) | 0.033 |
| Extent of Resection STR vs. GTR | 2.28 (1.12–4.60) | 0.022 | 1.36 (0.59–3.12) | 0.474 |
| Location Skull base vs. non skull base | 0.90 (0.42–1.95) | 0.790 | - | - |
| Bony Invasion Yes vs. No | 0.82 (0.35–1.90) | 0.635 | - | - |
| Sex Female vs. Male | 0.67 (0.34–1.35) | 0.262 | - | - |
| Multiple Meningiomas Single vs. multiple | 0.90 (0.21–3.82) | 0.890 | - | - |
| Cox Model for Retreatment-Free Survival | ||||
| Univariable Analysis | Multivariable Analysis | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age at Primary Surgery | 0.98 (0.95–1.01) | 0.270 | - | - |
| Preoperative KPS Poor vs. Good | 0.75 (0.18–3.23) | 0.704 | - | - |
| Extent of Resection STR vs. GTR | 4.73 (2.06–10.87) | <0.001 | 4.18 (1.79–9.78) | <0.001 |
| Location Skull base vs. non skull base | 2.67 (1.17–6.06) | 0.020 | 2.09 (0.90–4.84) | 0.09 |
| Bony Invasion Yes vs. No | 0.52 (0.21–1.27) | 0.152 | - | - |
| Multiple Meningiomas Single vs. multiple | 1.29 (0.17–9.60) | 0.804 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Broi, M.; Borrelli, P.; Meling, T.R. Predictors of Survival in Atypical Meningiomas. Cancers 2021, 13, 1970. https://doi.org/10.3390/cancers13081970
Da Broi M, Borrelli P, Meling TR. Predictors of Survival in Atypical Meningiomas. Cancers. 2021; 13(8):1970. https://doi.org/10.3390/cancers13081970
Chicago/Turabian StyleDa Broi, Michele, Paola Borrelli, and Torstein R. Meling. 2021. "Predictors of Survival in Atypical Meningiomas" Cancers 13, no. 8: 1970. https://doi.org/10.3390/cancers13081970
APA StyleDa Broi, M., Borrelli, P., & Meling, T. R. (2021). Predictors of Survival in Atypical Meningiomas. Cancers, 13(8), 1970. https://doi.org/10.3390/cancers13081970








