Promising Role of Circulating Tumor Cells in the Management of SCLC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodological Approaches to CTC Studies in SCLC
2.1. CTC Count as Biomarker in SCLC
2.2. Molecular Characterization of CTCs in SCLC
2.3. Functional Studies of CTCs in Preclinical Models
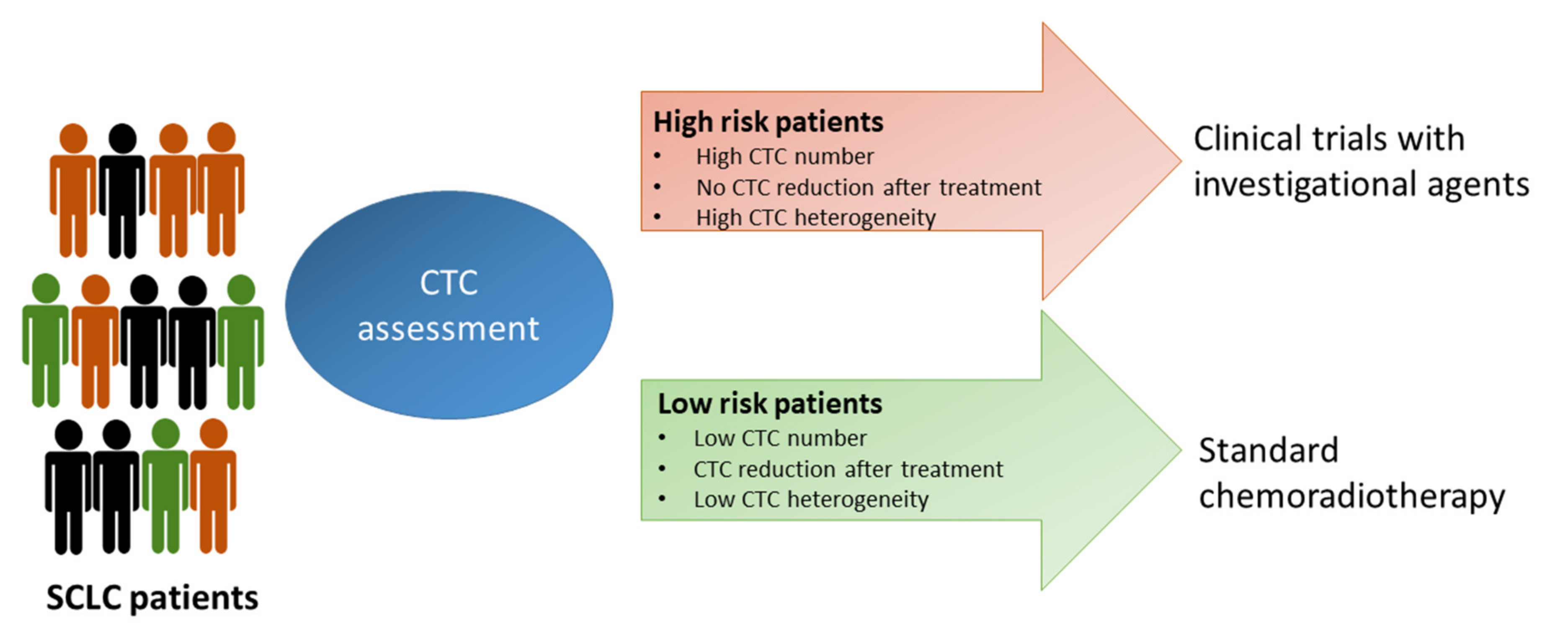
3. Open Questions and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Iams, W.T.; Porter, J.; Horn, L. Immunotherapeutic approaches for small-cell lung cancer. Nat. Rev. Clin. Oncol. 2020, 17, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Palumbo, G.; Carillio, G.; Manzo, A.; Montanino, A.; Sforza, V.; Costanzo, R.; Sandomenico, C.; La Manna, C.; Martucci, N.; et al. Immunotherapy in Small Cell Lung Cancer. Cancers 2020, 12, 2522. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, G.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Gallo, M.; Chicchinelli, N.; Rossi, A. The prognostic role of circulating tumor cells in lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 859–867. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, F.; Yoneda, K.; Kondo, N.; Hashimoto, M.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Rahman, S.; Tsubota, N.; Tsujimura, T.; et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin. Cancer Res 2009, 15, 6980–6986. [Google Scholar] [CrossRef] [Green Version]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.M.; Greystoke, A.; Lancashire, L.; Cummings, J.; Ward, T.; Board, R.; Amir, E.; Hughes, S.; Krebs, M.; Hughes, A.; et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am. J. Pathol. 2009, 175, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Naito, T.; Tanaka, F.; Ono, A.; Yoneda, K.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; Kenmotsu, H.; Shukuya, T.; et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 2012, 7, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltermann, T.J.N.; Pore, M.M.; van den Berg, A.; Timens, W.; Boezen, H.M.; Liesker, J.J.W.; Schouwink, J.H.; Wijnands, W.J.A.; Kerner, G.; Kruyt, F.A.E.; et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann. Oncol. 2012, 23, 2937–2942. [Google Scholar] [CrossRef]
- Gallo, M.; De Luca, A.; Frezzetti, D.; Passaro, V.; Maiello, M.R.; Normanno, N. The potential of monitoring treatment response in non-small cell lung cancer using circulating tumour cells. Expert Rev. Mol. Diagn. 2019, 19, 683–694. [Google Scholar] [CrossRef]
- Brezicka, T. Expression of epithelial-cell adhesion molecule (Ep-CAM) in small cell lung cancer as defined by monoclonal antibodies 17-1A and BerEP4. Acta Oncol. 2005, 44, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kularatne, B.Y.; Lorigan, P.; Browne, S.; Suvarna, S.K.; Smith, M.O.; Lawry, J. Monitoring tumour cells in the peripheral blood of small cell lung cancer patients. Cytometry 2002, 50, 160–167. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Gallo, M.; Franco, R.; Rossi, A.; De Luca, A.; Rocco, G.; Botti, G.; Gridelli, C.; Normanno, N. A “live” biopsy in a small-cell lung cancer patient by detection of circulating tumor cells. Lung Cancer 2009, 65, 123–125. [Google Scholar] [CrossRef]
- Messaritakis, I.; Politaki, E.; Kotsakis, A.; Dermitzaki, E.K.; Koinis, F.; Lagoudaki, E.; Koutsopoulos, A.; Kallergi, G.; Souglakos, J.; Georgoulias, V. Phenotypic characterization of circulating tumor cells in the peripheral blood of patients with small cell lung cancer. PLoS ONE 2017, 12, e0181211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, C.; Wang, X.; Ranganathan, A.; Torigian, D.; Troxel, A.; Evans, T.; Cohen, R.B.; Vaidya, B.; Rao, C.; Connelly, M.; et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 2017, 112, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Gorges, T.M.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lucke, K.; Wikman, H.; Jackson, S.; Brychta, N.; et al. Enumeration and Molecular Characterization of Tumor Cells in Lung Cancer Patients Using a Novel in Vivo Device for Capturing Circulating Tumor Cells. Clin. Cancer Res. 2016, 22, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Drucker, A.; Teh, E.M.; Kostyleva, R.; Rayson, D.; Douglas, S.; Pinto, D.M. Comparative performance of different methods for circulating tumor cell enrichment in metastatic breast cancer patients. PLoS ONE 2020, 15, e0237308. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, B.; Ding, F.; Zhou, X.; Tu, P.; Yu, B.; He, Y.; Huang, P. Circulating tumor cell detection: A direct comparison between negative and unbiased enrichment in lung cancer. Oncol. Lett. 2017, 13, 4882–4886. [Google Scholar] [CrossRef] [Green Version]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schutze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Kallergi, G.; Politaki, E.; Alkahtani, S.; Stournaras, C.; Georgoulias, V. Evaluation of Isolation Methods for Circulating Tumor Cells (CTCs). Cell Physiol. Biochem. 2016, 40, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Guan, G.; Bhagat, A.A. ClearCell(R) FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytom. A 2018, 93, 1251–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.; Lim, W.T.; Han, J.; Bhagat, A.A.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.I.; et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694–710. [Google Scholar] [CrossRef] [Green Version]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.I.; Morgan, B.; Trautwein, J.; et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013, 5, 179ra147. [Google Scholar] [CrossRef] [Green Version]
- Chudziak, J.; Burt, D.J.; Mohan, S.; Rothwell, D.G.; Mesquita, B.; Antonello, J.; Dalby, S.; Ayub, M.; Priest, L.; Carter, L.; et al. Clinical evaluation of a novel microfluidic device for epitope-independent enrichment of circulating tumour cells in patients with small cell lung cancer. Analyst 2016, 141, 669–678. [Google Scholar] [CrossRef]
- Sollier-Christen, E.; Renier, C.; Kaplan, T.; Kfir, E.; Crouse, S.C. VTX-1 Liquid Biopsy System for Fully-Automated and Label-Free Isolation of Circulating Tumor Cells with Automated Enumeration by BioView Platform. Cytom. A 2018, 93, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, C.A.; Liu, S.Z.; Wilkerson, C.L.; Ramani, V.C.; Barzanian, N.A.; Huang, K.W.; Che, J.; Chiu, M.W.; Vuppalapaty, M.; Dimmick, A.M.; et al. Fast and Label-Free Isolation of Circulating Tumor Cells from Blood: From a Research Microfluidic Platform to an Automated Fluidic Instrument, VTX-1 Liquid Biopsy System. SLAS Technol. 2018, 23, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Di Trapani, M.; Manaresi, N.; Medoro, G. DEPArray system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytom. A 2018, 93, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, T.; Hashimoto, Y.; Watanabe, Y.; Kagawa, S.; Uno, F.; Kuroda, S.; Tazawa, H.; Kyo, S.; Mizuguchi, H.; Urata, Y.; et al. A simple biological imaging system for detecting viable human circulating tumor cells. J. Clin. Investig. 2009, 119, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Narii, N.; Tomita, K.; Togo, S.; Takahashi, K.; Machitani, M.; Tachibana, M.; Ouchi, M.; Katagiri, N.; Urata, Y.; et al. Efficient detection of human circulating tumor cells without significant production of false-positive cells by a novel conditionally replicating adenovirus. Mol. Ther. Methods Clin. Dev. 2016, 3, 16001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foy, V.; Fernandez-Gutierrez, F.; Faivre-Finn, C.; Dive, C.; Blackhall, F. The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Igawa, S.; Gohda, K.; Fukui, T.; Ryuge, S.; Otani, S.; Masago, A.; Sato, J.; Murakami, K.; Maki, S.; Katono, K.; et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol. Lett. 2014, 7, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Liu, C.H.; Li, J.; Ma, X.P.; Gong, P. Clinical significance of circulating tumor cells in patients with small-cell lung cancer. Tumori 2017, 103, 242–248. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.Q.; Fan, Y.; Liu, Y.P.; Liu, Y.; Liu, Y.; Ma, L.X.; Liu, X.H.; Li, H.; Bao, H.Z.; et al. Circulating tumor cell counts/change for outcome prediction in patients with extensive-stage small-cell lung cancer. Future Oncol. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Rossi, A.; Morabito, A.; Signoriello, S.; Bevilacqua, S.; Di Maio, M.; Costanzo, R.; De Luca, A.; Montanino, A.; Gridelli, C.; et al. Prognostic value of circulating tumor cells’ reduction in patients with extensive small-cell lung cancer. Lung Cancer 2014, 85, 314–319. [Google Scholar] [CrossRef]
- Huang, C.H.; Wick, J.A.; Sittampalam, G.S.; Nirmalanandhan, V.S.; Ganti, A.K.; Neupane, P.C.; Williamson, S.K.; Godwin, A.K.; Schmitt, S.; Smart, N.J.; et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front. Oncol. 2014, 4, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, R.Y.; Fernandez-Gutierrez, F.; Foy, V.; Burns, K.; Pierce, J.; Morris, K.; Priest, L.; Tugwood, J.; Ashcroft, L.; Lindsay, C.R.; et al. Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: Analysis of the concurrent once-daily versus twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann. Oncol. 2019, 30, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Sabari, J.K.; Lok, B.H.; Laird, J.H.; Poirier, J.T.; Rudin, C.M. Unravelling the biology of SCLC: Implications for therapy. Nat. Rev. Clin. Oncol. 2017, 14, 549–561. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.T.; Li, B.G. Prognostic significance of circulating tumor cells in small-cell lung cancer patients: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8429–8433. [Google Scholar] [CrossRef] [Green Version]
- Messaritakis, I.; Politaki, E.; Plataki, M.; Karavassilis, V.; Kentepozidis, N.; Koinis, F.; Samantas, E.; Georgoulias, V.; Kotsakis, A. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer 2017, 104, 16–23. [Google Scholar] [CrossRef]
- Salgia, R.; Weaver, R.W.; McCleod, M.; Stille, J.R.; Yan, S.B.; Roberson, S.; Polzer, J.; Flynt, A.; Raddad, E.; Peek, V.L.; et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: Exploratory analysis of a phase II study. Investig. New Drugs 2017, 35, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Belani, C.P.; Dahlberg, S.E.; Rudin, C.M.; Fleisher, M.; Chen, H.X.; Takebe, N.; Velasco, M.R., Jr.; Tester, W.J.; Sturtz, K.; Hann, C.L.; et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 2016, 122, 2371–2378. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Litvak, A.M.; Varghese, A.M.; Krug, L.M.; Fleisher, M.; Teitcher, J.B.; Holodny, A.I.; Sima, C.S.; Woo, K.M.; Ng, K.K.; et al. A phase I trial of the Hedgehog inhibitor, sonidegib (LDE225), in combination with etoposide and cisplatin for the initial treatment of extensive stage small cell lung cancer. Lung Cancer 2016, 99, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Obermayr, E.; Agreiter, C.; Schuster, E.; Fabikan, H.; Weinlinger, C.; Baluchova, K.; Hamilton, G.; Hochmair, M.; Zeillinger, R. Molecular Characterization of Circulating Tumor Cells Enriched by A Microfluidic Platform in Patients with Small-Cell Lung Cancer. Cells 2019, 8, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.; Zhuo, M.; Su, Z.; Duan, J.; Gao, Y.; Wang, Z.; Zong, C.; Bai, H.; Chapman, A.R.; Zhao, J.; et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl. Acad. Sci. USA 2013, 110, 21083–21088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H.; et al. Inferring the Evolution and Progression of Small-Cell Lung Cancer by Single-Cell Sequencing of Circulating Tumor Cells. Clin. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyan, V.; Chalishazar, M.D.; et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Cayrefourcq, L.; Mazard, T.; Joosse, S.; Solassol, J.; Ramos, J.; Assenat, E.; Schumacher, U.; Costes, V.; Maudelonde, T.; Pantel, K.; et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015, 75, 892–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.; Burghuber, O.; Zeillinger, R. Circulating tumor cells in small cell lung cancer: Ex vivo expansion. Lung 2015, 193, 451–452. [Google Scholar] [CrossRef]
- Hamilton, G.; Hochmair, M.; Rath, B.; Klameth, L.; Zeillinger, R. Small cell lung cancer: Circulating tumor cells of extended stage patients express a mesenchymal-epithelial transition phenotype. Cell Adh. Migr. 2016, 10, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, G.; Rath, B.; Holzer, S.; Hochmair, M. Second-line therapy for small cell lung cancer: Exploring the potential role of circulating tumor cells. Transl. Lung Cancer Res. 2016, 5, 71–77. [Google Scholar] [CrossRef]
- Klameth, L.; Rath, B.; Hochmaier, M.; Moser, D.; Redl, M.; Mungenast, F.; Gelles, K.; Ulsperger, E.; Zeillinger, R.; Hamilton, G. Small cell lung cancer: Model of circulating tumor cell tumorospheres in chemoresistance. Sci. Rep. 2017, 7, 5337. [Google Scholar] [CrossRef] [Green Version]
- Lallo, A.; Gulati, S.; Schenk, M.W.; Khandelwal, G.; Berglund, U.W.; Pateras, I.S.; Chester, C.P.E.; Pham, T.M.; Kalderen, C.; Frese, K.K.; et al. Ex vivo culture of cells derived from circulating tumour cell xenograft to support small cell lung cancer research and experimental therapeutics. Br. J. Pharmacol. 2019, 176, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.; Simms, N.; Polanski, R.; et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef]
- Shue, Y.T.; Lim, J.S.; Sage, J. Tumor heterogeneity in small cell lung cancer defined and investigated in pre-clinical mouse models. Transl. Lung Cancer Res. 2018, 7, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Frese, K.; Galvin, M.; Carter, M.; Franklin, L.; Morris, K.; Pierce, J.; Descamps, T.; Blackhall, F.; Dive, C.; et al. Brief report on the clinical characteristics of patients whose samples generate small cell lung cancer circulating tumour cell derived explants. Lung Cancer 2020, 150, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Stoney, R.; Frese, K.K.; Simms, N.; Rowe, W.; Pearce, S.P.; Humphrey, S.; Booth, L.; Morgan, D.; Dynowski, M.; et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat. Cancer 2020, 1, 437–451. [Google Scholar] [CrossRef]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results from the Phase II TRINITY Study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360. [Google Scholar] [CrossRef] [PubMed]

| Technology [Refs] | CTC Enrichment | CTC Detection and Characterization | % of CTC Detection § | Comments |
|---|---|---|---|---|
| Protein marker-based devices | ||||
| CellSearch System [12,22] | EpCAM antibodies-coated ferromagnetic beads | IF for CK8, 18, 19, DAPI and CD45 | ≥85% | FDA-approved semi-automated system. Do not detect EpCAM-negative CTCs. Do not recover viable cells. |
| CellCollector [23] | Functionalized medical wire associated with EpCAM antibodies | IF for EpCAM, CK and DAPI | Not applicable | CE-approved as medical device for in vivo CTC isolation. Capacity to process large volumes of blood with a high CTC detection rate. |
| RosetteSep System [24,25] | Depletion of leukocytes and erythrocytes by specific antibodies followed by density gradient centrifugation | ICC | 46.9% | Fast and easy-to-use. Collection of live cells with high purity for many applications (cell cultures, DNA/RNA extraction, implantation in mice). |
| Physical properties-based devices | ||||
| ISET [26,27] | Size-based filtration for isolation of CTCs | IF; FISH | 95% | Isolation of clusters and viable cells of epithelial and non-epithelial origin. Low recovery and purity. |
| ClearCell FX [28,29] | Microfluidic technology for CTC enrichment based on drag and size-dependent lift forces | IF; FISH | 85% | Capacity to capture viable and intact CTCs for in vivo and in vitro experiments and for NGS analysis. Small CTCs may escape detection. |
| CTC-iChip [30,31] | Microfluidic platform for size-based isolation in combination with EpCAM-based positive selection or CD45 negative depletion | IF; RT-PCR for tumor associated transcripts | >77% | Detection of both epithelial and non-epithelial CTCs. Capture and in vitro culture of viable CTCs for functional studies. |
| Parsortix [32] | Microfluidic platform for cell size and deformability-based separation | IF for CK, DAPI and CD45 | 78% | CE-marked for use as in vitro diagnostic device. Collection of viable CTCs for molecular and functional analysis. |
| VTX-1 Liquid Biopsy System [33,34] | Microfluidic separation of CTCs based on cell size and deformability | IF; FISH, RT-PCR; NGS for tumor-associated transcripts | 69%-79.5% | High recovery and purity of intact CTCs. No red blood cell lysis required. Suitable for many applications (genomic and proteomic analyses, enumeration, IF staining). |
| DEPArray [35] | Requires a pre-enrichment step with other technologies (e.g., CellSearch or Parsortix) | IF for CK, CD45, DAPI or Hoechst staining | 99.7% | Recovery of single viable cells. |
| Other Assays | ||||
| TelomeScan [36,37] | Detection of GFP-positive CTCs following incubation with a telomerase-specific conditionally replicating adenovirus expressing the GFP gene | IF | >70% | Isolation of live CTCs, including EpCAM negative cells and cells undergoing EMT. A modified assay has been developed to reduce false-positive results, based on targeting miR-142-3p to inhibit GFP-expressing blood cells. |
| Study [Ref] | Disease Stage | Treatment | Blood Sample Collection | Number of Patients | CTC Detection Method | Optimal Cut-Off | Main Findings |
|---|---|---|---|---|---|---|---|
| Hou et al. [13] | LS- and ES-SCLC | Chemotherapy | Baseline, days 2 and 22 after the treatment | 50 | CellSearch | No cut-off | Patients with a high number of CTCs (> 300) had a shorter median OS than patients with a low number of CTCs (< 2) (134 vs. 443 days). A persistently elevated CTC number at day 22 after treatment was considered an adverse prognostic factor at univariate analysis. |
| Hou et al. [41] | LS- and ES-SCLC | Chemotherapy | Baseline, post cycle 1 | 97 | CellSearch | 50 CTCs/7.5 mL blood | Patients with a CTC number > 50 had a shorter median PFS (4.6 versus 8.8 months) and OS compared to those with a CTC number < 50 (5.4 versus 11.5 months) at baseline. A number of CTC < 50 after one cycle of chemotherapy was associated with longer PFS and OS. At multivariate analysis, the CTC number at baseline was an independent prognostic factor for PFS (HR = 2.01) and OS (HR = 2.45). |
| Naito et al. [14] | LS- and ES-SCLC | Chemotherapy or chemoradiotherapy | Baseline, post treatment, at relapse | 51 | CellSearch | 8 CTCs/7.5 mL blood | Patients with a CTC count < 8 at baseline had longer OS than patients with CTC ≥8. Patients with a CTC count ≥8 after treatment and at relapse had a worse OS as compared with those with <8 CTCs at the same time points. |
| Hiltermann et al. [15] | LS- and ES-SCLC | Chemotherapy | Baseline, post cycle 1 and 4 | 59 | CellSearch | 2 CTCs/7.5 mL blood | Patients with a CTC count < 2 had longer OS than patients with a CTC number > 215 (729 vs. 157 days). At multivariate analysis, CTC count was an independent prognostic factor for PFS and OS at all time points. No correlations were observed between the decrease in CTC number from baseline to after one cycle of chemotherapy, and/or the absolute number of CTCs after one cycle of chemotherapy and response to treatment. |
| Cheng et al. [42] | ES-SCLC | Chemotherapy | Baseline, post cycle 2 and at progression | 91 | CellSearch | 10 CTCs/7.5 mL blood | Patients with a CTC count ≥ 10 at baseline had significantly shorter OS as compared with patients with a CTC count < 10 (8.2 vs. 16.6 months); no difference in PFS between the groups was observed. |
| Aggarwal et al. [21] | LS- and ES-SCLC | Chemotherapy or chemoradiotherapy | Baseline, during cycles 1, 2 (days 2, 3), 3,4 (day 1) and at relapse | 50 | CellSearch | 5 CTCs/7.5 mL blood 50 CTCs/7.5 mL blood | Patients with a CTC count < 5 at baseline had better PFS than patients with CTCs ≥ 5 (11 vs. 6.7 months). Using a cut-off of 50 CTCs, for patients with <50 CTCs, PFS and OS were both significantly longer compared to patients with CTCs ≥ 50. At multivariate analysis, a higher CTC count at baseline was associated with a high hazard of death and progression. The decrease in CTCs during the course of therapy was not significantly associated with the response. |
| Messaritakis et al. [20] | LS- and ES-SCLC | Chemotherapy | Baseline, after 1 cycle and at progression | 83 | CellSearch | 5 CTCs/7.5 mL blood | Patients with a high number of CTCs had a significantly shorter median PFS and OS compared to patients with a low number of CTCs, irrespective of the time of CTC enumeration. At multivariate analysis, the detection of CTCs at baseline was considered as an independent factor associated with decreased PFS, whereas CTC count at progression was associated with a reduced OS. A significantly higher number of CTCs at baseline was observed in patients with PD compared to patients who experienced a CR/PR or SD. |
| Normanno et al. [43] | ES-SCLC | Chemotherapy | Baseline, post cycle 1 | 60 | CellSearch | No cut-off | A CTC count reduction higher than 89% following chemotherapy was associated with a lower risk of death. |
| Huang et al. [44] | ES-SCLC | Chemotherapy | Baseline and within 4 weeks after chemotherapy | 26 | CellSearch | No cut-off | A trend toward significance was observed between baseline CTCs and the percentage of change from post-treatment to baseline and OS |
| Igawa et al. [39] | LS- and ES-SCLC | Chemotherapy or chemoradiotherapy | Baseline, at cycle 2 and 3, post cycle 4 and at progression | 30 | TelomeScan | 2 CTCs/7.5 mL blood | Patients with a baseline CTC count < 2 had a significantly longer OS than patients with a CTC count ≥ 2. |
| Wang et al. [40] | LS- and ES-SCLC | Chemotherapy | Baseline, post cycle 1 | 42 | Negative immunomagnetic enrichment | 2 CTCs/7.5 mL blood | A CTC number ≥2 at baseline and after the first cycle of chemotherapy was significantly associated with worse PFS. |
| Tay et al. [45] | LS-SCLC | Chemoradiotherapy | Baseline | 75 | CellSearch | 2 CTCs/7.5 mL blood 15 CTCs/7.5 mL blood 50 CTCs/7.5 mL blood | A number of 2 or 15 or 50 CTCs at baseline significantly correlated with PFS and OS. Patients with a CTC number < 15 had a better median PFS (19.0 months vs. 5.5 months) and OS (26.7 months vs. 5.9 months) than patients with a CTC number ≥15. At multivariate analysis only the 15 CTC cut-off emerged as an independent prognostic marker |
| Investigational Drug | Phase | Number of Patients | Blood Sample Collection | CTC Detection Method | Optimal Cut-Off | Ref |
|---|---|---|---|---|---|---|
| Pazopanib | Phase II | 56 | Baseline, after the 1st cycle and at progression | CellSearch | 5 CTCs | [48] |
| LY2510924 plus CE | Phase II | 78 | Baseline, cycle 1 (day 7), cycle 2 (day 1), and at 30-day follow-up after the last dose | CellSearch | 6 CTCs/7.5 mL blood | [49] |
| Vismodegib or cixutumumab plus CE | Phase II | 120 | Baseline | CellSearch | 100 CTCs/7.5 mL blood | [50] |
| Sonidegib plus CE | Phase I | 14 | Baseline, after cycles 1,2,4,6, every 3 cycles during maintenance therapy and at disease progression | CellSearch | No cut-off | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, A.; Gallo, M.; Esposito, C.; Morabito, A.; Normanno, N. Promising Role of Circulating Tumor Cells in the Management of SCLC. Cancers 2021, 13, 2029. https://doi.org/10.3390/cancers13092029
De Luca A, Gallo M, Esposito C, Morabito A, Normanno N. Promising Role of Circulating Tumor Cells in the Management of SCLC. Cancers. 2021; 13(9):2029. https://doi.org/10.3390/cancers13092029
Chicago/Turabian StyleDe Luca, Antonella, Marianna Gallo, Claudia Esposito, Alessandro Morabito, and Nicola Normanno. 2021. "Promising Role of Circulating Tumor Cells in the Management of SCLC" Cancers 13, no. 9: 2029. https://doi.org/10.3390/cancers13092029
APA StyleDe Luca, A., Gallo, M., Esposito, C., Morabito, A., & Normanno, N. (2021). Promising Role of Circulating Tumor Cells in the Management of SCLC. Cancers, 13(9), 2029. https://doi.org/10.3390/cancers13092029









