Evaluation of Survival, Recurrence Patterns and Adjuvant Therapy in Surgically Staged High-Grade Endometrial Cancer with Retroperitoneal Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics
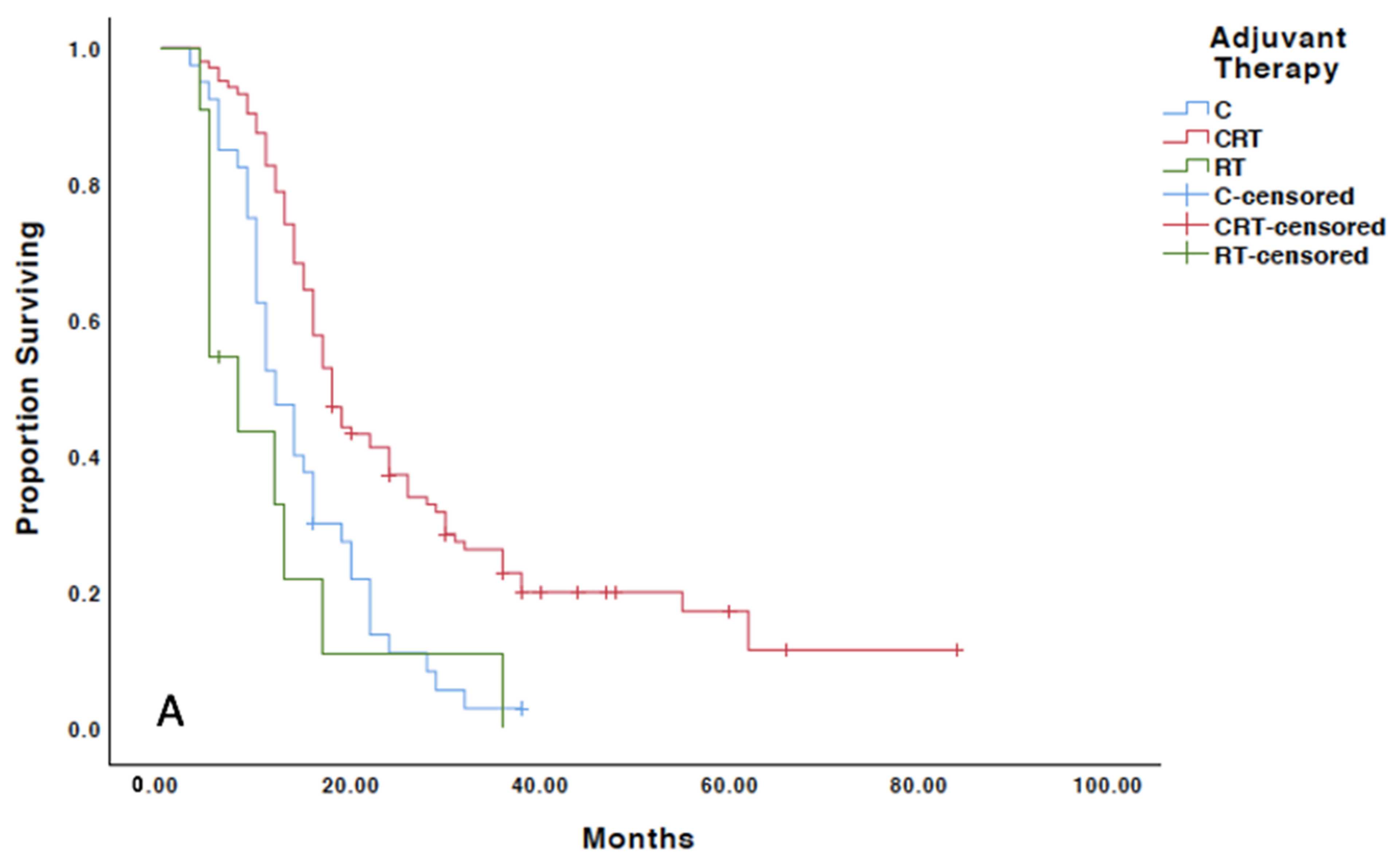
3.2. Adjuvant Therapy
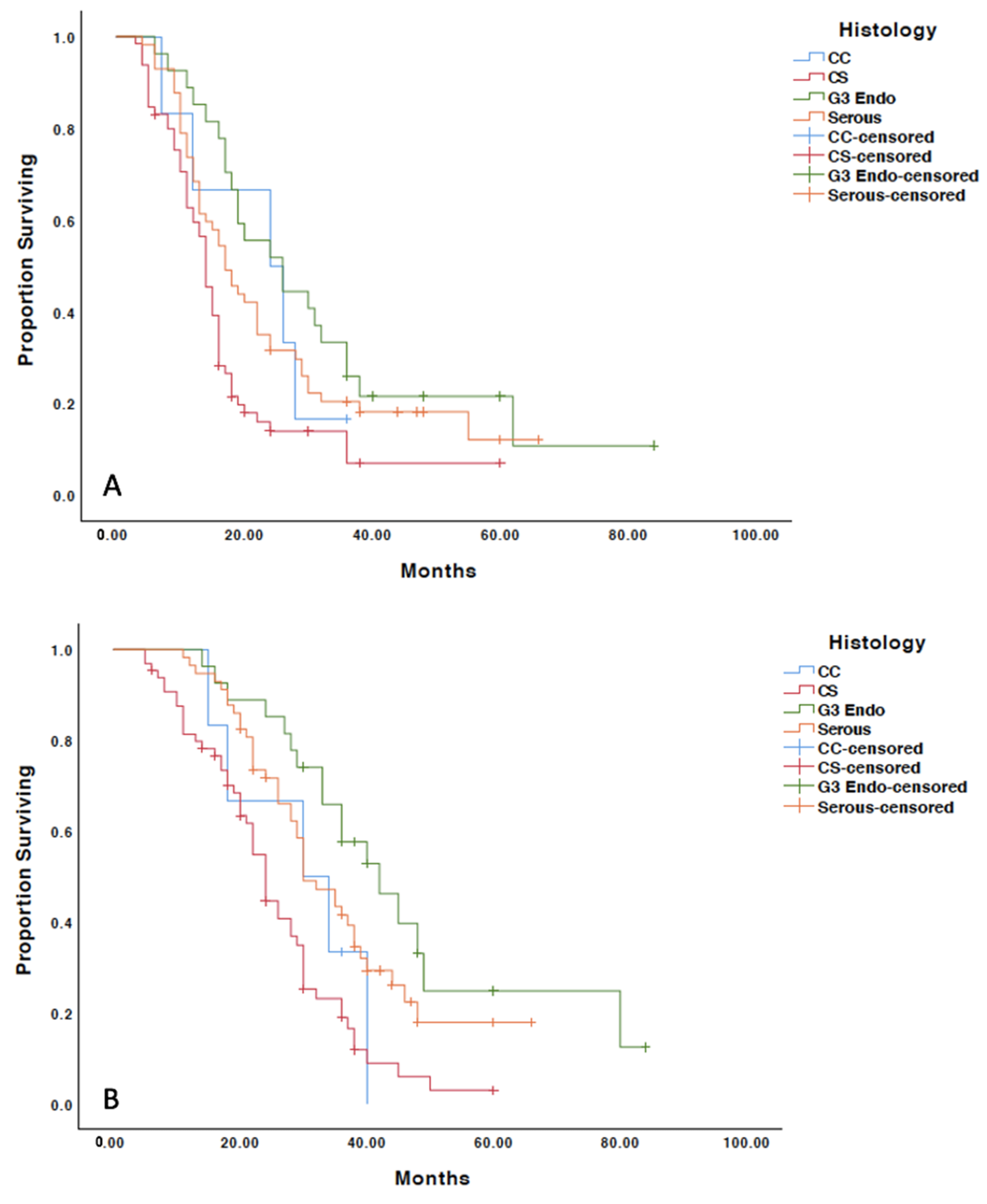
3.3. Recurrence Patterns
3.4. Treatment Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Endometrial carcinoma |
| Grade 3 | grade 3 endometrioid adenocarcinoma |
| HGEC | High-grade endometrial carcinoma |
| MMR | Mismatch repair |
| MSI | Microsatellite instability |
| PFS | Progression free survival |
| OS | Overall survival |
References
- National Institutes of Health. Cancer Facts: Uterine cancer. Surveillance, Epidemiology, and End Results Program. 2019. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 20 April 2021).
- Morice, P.; Leary, A.; Creutzbery, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Mukthinuthalapati, P.K.; Farooq, M.Z.; Gupta, S. Trends of endometrial cancer in the United States from 2000–2015. J. Clin. Oncol. 2019, 37, 5591. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines. Uterine Neoplasms. Version 2.2019; NCCN: Plymouth Meeting, PA, USA, 2019. [Google Scholar]
- Miller, D.; Filiaci, V.; Fleming, G.; Mannel, R.; Cohn, D.; Matsumoto, T.; Tewari, K.; DiSilvestro, P.; Pearl, M.; Zaino, R. Randomized phase II noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2012, 125, 771. [Google Scholar] [CrossRef]
- Fleming, G.; Brunetto, V.; Cella, D.; Look, K.Y.; Reid, G.C.; Munkarah, A.R.; Kline, R.; Burger, R.A.; Goodman, A.; Burks, R.T. Phase III Trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2004, 22, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Rungruang, B.; Olawaiye, A.B. Comprehensive surgical staging for endometrial cancer. Rev. Obstet. Gynecol. 2012, 5, 28–34. [Google Scholar] [PubMed]
- Fanning, J.; Evans, M.C.; Peters, A.J.; Samuel, M.; Harmon, E.R.; Bates, J.S. Endometrial adenocarcinoma histologic subtypes: Clinical and pathologic profile. Gynecol Oncol. 1989, 32, 288–291. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Burke, T.W.; Eifel, P.J.; Ramondetta, L.M.; Silva, E.G.; Jhingran, A.; Oh, J.C.; Atkinson, E.N.; Broaddus, R.R.; Gershenson, D.M.; et al. Uterine papillary serous carcinoma (UPSC): A single institution review of 129 cases. Gynecol. Oncol. 2003, 91, 463–469. [Google Scholar] [CrossRef]
- Kandoth, C.; Shultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed] [Green Version]
- Randall, M.E.; Spirtos, N.M.; Dvoretsky, P. Whole abdominal radiotherapy versus combination chemotherapy with doxorubicin and cisplatin in advanced endometrial carcinoma (phase III): Gynecologic Oncology Group Study No. 122. J. Natl. Cancer Inst. Monogram. 1995, 19, 13–15. [Google Scholar] [PubMed]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N. Engl. J. Med. 2019, 380, 2317–2325. [Google Scholar] [CrossRef]
- Mundt, A.J.; Ride, R.; Rotmensch, J.; Waggoner, S.E.; Yamada, S.D.; Conell, P.P. Significant pelvic recurrence in high-risk pathologic stage I–IV endometrial carcinoma after adjuvant chemotherapy alone: Implications for adjuvant radiation therapy. Int. J. Radiat. Oncol. Bio. Phys. 2001, 50, 1145–1153. [Google Scholar] [CrossRef]
- Greven, K.; Winter, K.; Underhill, K.; Fontenesci, J.; Cooper, J.; Burke, T. Final analysis of RTOG 9708: Adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy follow surgery for patients with high-risk endometrial cancer. Gynecol. Oncol. 2006, 103, 155–159. [Google Scholar] [CrossRef]
- Goodman, C.R.; Hautoum, S.; Seagle, B.L.L.; Donnelly, E.D.; Barber, E.L.; Shahabi, S.; Matei, D.A.; Strauss, J.B. Association of chemotherapy and radiotherapy sequence with overall survival in locoregionally advanced endometrial cancer. Gynecol. Oncol. 2019, 153, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Secord, A.A.; Havrilesky, L.J.; O’Malley, D.M.; Bae-Jump, V.; Fleming, N.D.; Broadwater, G.; Cohn, D.E.; Gehrig, P.A. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol. Oncol. 2009, 114, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Albeesh, R.; Turgeon, G.A.; Alfeirei, J.; Mansure, J.J.; Fu, L.; Arseneau, J.; Zeng, X.; Jardon, K.; Gilbert, L.; Souhami, L. Adjuvant therapy in stage III endometrial cancer confined to the pelvis. Gynecol. Oncol. 2019, 152, 26–30. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomized phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- McEachron, J.; Zhou, N.; Spencer, C.; Shanahan, L.; Chatterton, C.; Singhal, P.; Lee, Y.C. Evaluation of the optimal sequence of chemotherapy and radiation therapy in the treatment of advanced endometrial cancer. J. Gynecol. Oncol. 2020, 31, e90. [Google Scholar] [CrossRef]
- McEachron, J.; Zhou, N.; Spencer, C.; Chatterton, C.; Shanahan, L.; Katz, J.; Naegele, S.; Singhal, P.; Lee, Y.C. Multimodality therapy associated with improved outcomes in patients with MSI advanced endometrial carcinoma. Int. J. Gynecol. Cancer 2020, 135, 895. [Google Scholar]
- McEachron, J.; Heyman, T.; Shanahan, L.; Tran, V.; Friedman, M.; Economos, K.; Singhal, P.K.; Lee, Y.C.; Kanis, M.J. Multimodality adjuvant therapy and survival outcomes in stage I-IV uterine carcinosarcoma. Int. J. Gynecol. Cancer 2020, 30, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef]
- Chiang, A.J.; Yu, K.J.; Chao, K.C.; Teng, N.N.H. The incidence of isolated para-aortic nodal metastasis in completely staged endometrial cancer patients. Gynecol. Oncol. 2011, 121, 122–125. [Google Scholar] [CrossRef]
- Goff, B.A.; Kato, D.; Schmidt, R.A.; Ek, M.; Ferry, J.A.; Muntz, H.G.; Cain, J.M.; Tamimi, H.K.; Figge, D.C.; Greer, B.E. Uterine papillary serous carcinoma: Patterns of metastatic spread. Gynecol. Oncol. 1994, 54, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kong, T.W.; Kim, W.Y.; Yoo, S.C.; Yoon, J.H.; Chang, K.H.; Ru, H.S. Lymph-vascular space invasion as a significant risk factor for isolated paraaortic lymph node metastasis in endometrial cancer: A study of 203 consecutive patients. Ann. Surg. Oncol. 2001, 18, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Cheung, M.K.; Osann, K.; Chen, L.; Teng, N.N.; Longacre, T.A.; Powell, M.A.; Hendrickson, M.R.; Kapp, D.S.; Chan, J.K. Uterine papillary serous carcinoma and clear cell carcinoma predict poorer survival compared to grade 3 endometrioid corpus cancer. Br. J. Cancer 2006, 94, 642–646. [Google Scholar] [CrossRef]
- Boruta, D.M.; Gehrig, P.A.; Groben, P.A.; Bae-Jump, V.; Boggess, J.F.; Fowler, W.C., Jr.; Van Le, L. Uterine serous and grade 3 endometrioid carcinomas: Is there a survival difference? Cancer 2004, 101, 2214–2221. [Google Scholar] [CrossRef]
- Cirisano, F.D., Jr.; Robboy, S.J.; Dodge, R.K.; Bentley, R.C.; Krigman, H.R.; Synan, I.S.; Soper, J.T.; Clarke-Pearson, D.L. The outcome of stage I–II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol. Oncol. 2000, 77, 55–65. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Vermij, L.; Horeweg, N.; Leon-Castillo, A.; Rutten, T.A.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Powell, M.E.; Singh, N.; Crosbie, E.J.; et al. HER2 status in high-risk endometrial cancers (PORTEC-3): Relationship with histotype, molecular classification, and clinical outcomes. Cancers 2020, 13, 44. [Google Scholar] [CrossRef]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grilini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S.; et al. ARID1A and CTNNB1/β-catenin molecular status affects the clinicopathologic features and prognosis of endometrial carcinoma: Implications for an improved surrogate molecular classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef]
- Einstein, M.K.; Klobocista, M.; Hou, J.Y.; Lee, S.; Mutyala, S.; Mehta, K.; Reimers, L.L.; Kuo, D.Y.S.; Huang, G.; Goldberg, G.L. Phase II trial of adjuvant pelvic radiation “sandwiched” between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma. Gynecol. Oncol. 2012, 124, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.L.; Einstein, M.H.; Novetsky, A.P.; Gebb, J.; Goldberg, G.L. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma. Gynecol. Oncol. 2008, 108, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Chang-Halfpenny, C.; Hwang-Graziano, J. Sequential versus “sandwich” sequencing of adjuvant chemoradiation for the treatment of stage III uterine endometrioid adenocarcinoma. Gynecol. Oncol. 2015, 137, 28–33. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (n = 155) | Grade 3 Endometrioid (n = 27) | Serous (n = 57) | Carcinosarcoma (n = 65) | Clear Cell (n = 6) | p-Value |
|---|---|---|---|---|---|---|
| Age at surgery | 0.273 | |||||
| Mean (range) | 66 (43–85) | 64 (49–80) | 66 (49–81) | 67 (43–85) | 71 (56–83) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Race | 0.463 | |||||
| Caucasian | 25 (16) | 5 (19) | 6 (11) | 13 (20) | 1 (17) | |
| African-American | 127 (82) | 21 (78) | 51 (89) | 50 (77) | 5 (83) | |
| Other | 3 (2) | 1 (3) | 0 (0) | 2 (3) | 0 (0) | |
| FIGO Stage * | 0.318 | |||||
| IIIC1 | 63 (41) | 13 (48) | 18 (32) | 30 (46) | 2 (33) | |
| IIIC2 | 92 (59) | 14 (52) | 39 (68) | 35 (54) | 4 (67) | |
| Presence of LVSI | 0.434 | |||||
| Positive | 114 (74) | 17 (63) | 41 (72) | 51 (78) | 5 (83) | |
| Negative | 41(26) | 10 (37) | 16 (28) | 14 (22) | 1 (17) | |
| Adjuvant | 0.043 | |||||
| therapy | 104 (67) | 23 (85) | 41 (72) | 36 (55) | 4 (67) | |
| CRT | 40 (26) | 3 (11) | 16 (28) | 20 (30) | 1 (16) | |
| Chemotherapy Radiation | 11 (7) | 1 (4) | 0 (0) | 9 (15) | 1 (16) | |
| Sequence of CRT | 0.193 | |||||
| CR | 38 (37) | 4 (17) | 19 (46) | 13 (36) | 2 (50) | |
| CRC | 36 (35) | 10 (44) | 15 (37) | 10 (28) | 1 (25) | |
| RC | 30 (28) | 9 (39) | 7 (17) | 13 (36) | 1 (25) | |
| MMR Status | 0.005 | |||||
| Deficient | 19 (12) | 9 (33) | 6 (10) | 3 (5) | 1 (17) | |
| Unknown | 63 (41) | 6 (22) | 21 (37) | 34 (52) | 2 (33) | |
| Proficient | 73 (47) | 12 (45) | 30 (53) | 28 (43) | 3 (50) |
| Patient Characteristics | PFS | OS |
|---|---|---|
| All histologies | ||
| All patients | 16 | 30 |
| Chemotherapy | 12 | 22 |
| CRT | 18 | 35 |
| RT | 8 | 13 |
| p-value | <0.001 | <0.001 |
| G3 Endometrioid | ||
| All patients | 26 | 42 |
| Chemotherapy | 12 | 18 |
| CRT | 26 | 42 |
| p-value | 0.027 | 0.003 |
| Serous | ||
| All patients | 17 | 30 |
| Chemotherapy | 12 | 29 |
| CRT | 18 | 37 |
| p-value | 0.022 | 0.004 |
| Carcinosarcoma | ||
| All patients | 14 | 24 |
| Chemotherapy | 11 | 21 |
| CRT | 16 | 28 |
| RT | 5 | 11 |
| p-value | 0.001 | <0.001 |
| Variable | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (per year) | 0.99 | 0.97–1.02 | 0.419 | 1.00 | 0.98–1.03 | 0.915 |
| Presence of LVSI | 0.69 | 0.47–1.02 | 0.06 | 0.71 | 0.47–1.09 | 0.115 |
| Stage (IIIC1 vs. IIIC2) | 0.79 | 0.55–1.61 | 0.242 | 0.86 | 0.57–1.28 | 0.451 |
| Histology | 0.012 | 0.019 | ||||
| Serous vs. G3 | 0.73 | 0.43–1.25 | 0.258 | 0.66 | 0.35–1.19 | 0.161 |
| Serous vs. CS | 1.62 | 1.08–2.45 | 0.019 | 1.64 | 1.04–2.57 | 0.032 |
| Adjuvant therapy | 0.001 | <0.001 | ||||
| Chemotherapy vs. CRT | 0.55 | 0.36–0.83 | 0.004 | 0.45 | 0.28–0.68 | <0.001 |
| Chemotherapy vs. RT | 1.65 | 0.74–3.56 | 0.244 | 1.94 | 0.85–4.44 | 0.115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McEachron, J.; Marshall, L.; Zhou, N.; Tran, V.; Kanis, M.J.; Gorelick, C.; Lee, Y.-C. Evaluation of Survival, Recurrence Patterns and Adjuvant Therapy in Surgically Staged High-Grade Endometrial Cancer with Retroperitoneal Metastases. Cancers 2021, 13, 2052. https://doi.org/10.3390/cancers13092052
McEachron J, Marshall L, Zhou N, Tran V, Kanis MJ, Gorelick C, Lee Y-C. Evaluation of Survival, Recurrence Patterns and Adjuvant Therapy in Surgically Staged High-Grade Endometrial Cancer with Retroperitoneal Metastases. Cancers. 2021; 13(9):2052. https://doi.org/10.3390/cancers13092052
Chicago/Turabian StyleMcEachron, Jennifer, Lila Marshall, Nancy Zhou, Van Tran, Margaux J. Kanis, Constantine Gorelick, and Yi-Chun Lee. 2021. "Evaluation of Survival, Recurrence Patterns and Adjuvant Therapy in Surgically Staged High-Grade Endometrial Cancer with Retroperitoneal Metastases" Cancers 13, no. 9: 2052. https://doi.org/10.3390/cancers13092052
APA StyleMcEachron, J., Marshall, L., Zhou, N., Tran, V., Kanis, M. J., Gorelick, C., & Lee, Y.-C. (2021). Evaluation of Survival, Recurrence Patterns and Adjuvant Therapy in Surgically Staged High-Grade Endometrial Cancer with Retroperitoneal Metastases. Cancers, 13(9), 2052. https://doi.org/10.3390/cancers13092052






