SENP Proteases as Potential Targets for Cancer Therapy
Abstract
:Simple Summary
Abstract
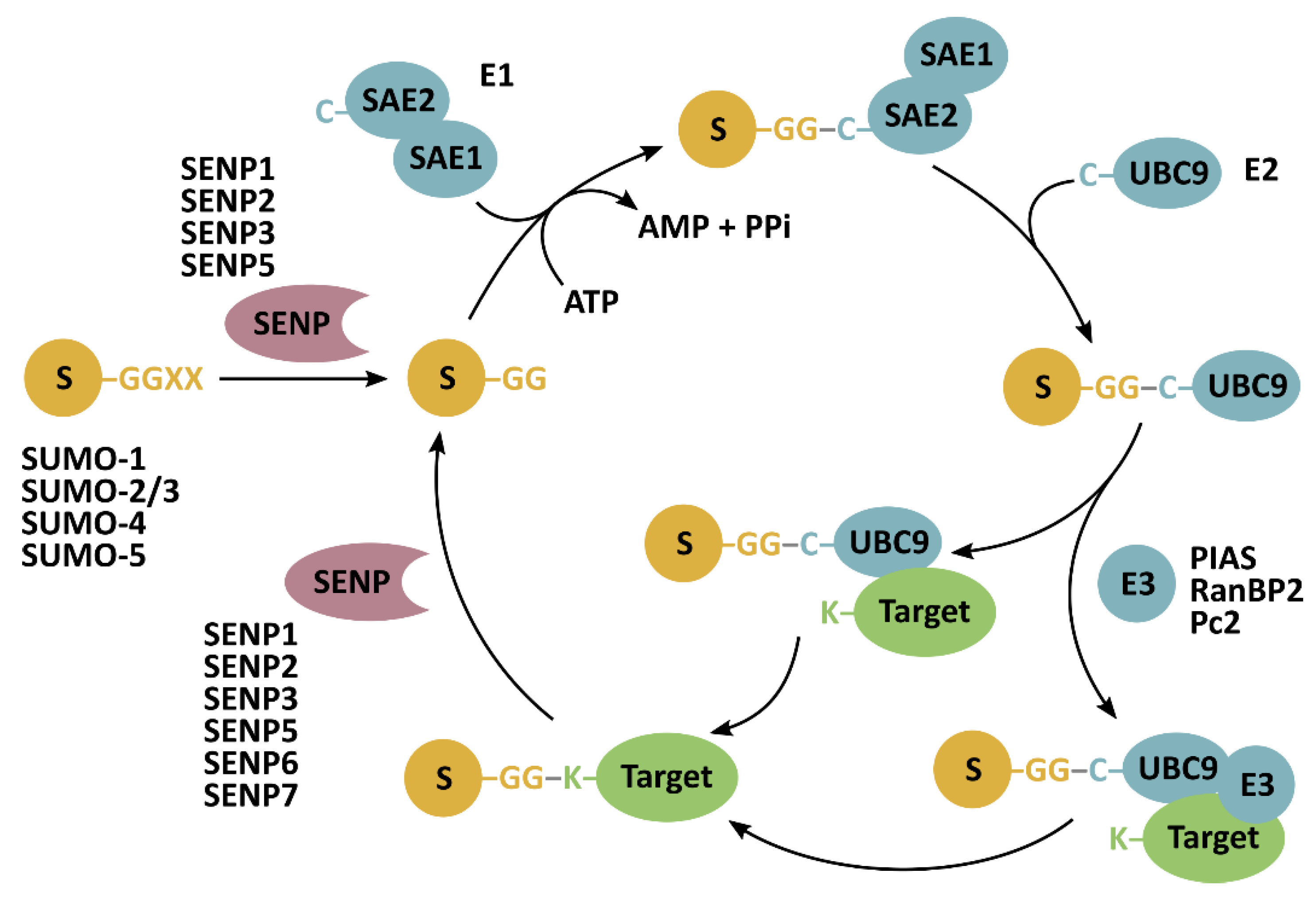
1. Introduction
2. SENP Proteases as Regulators of DNA Repair
3. The Role of SENP Proteases in the Cell Cycle
4. The Role of SENPs in Cancer Progression
5. SENP Proteases Inhibitors
5.1. Natural and Non-Natural Peptide-Based Inhibitors
5.2. Non-Peptidyl Small Molecular Weight SENP Inhibitors
5.3. The Use of Virtual Screening for the Identification of SENPs Inhibitors
5.4. Plant-Derived Inhibitors
5.5. The Quantitative High-Throughput Cell-Free Screen to Identify SENP Inhibitors (AlphaScreen)
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bawa-Khalfe, T.; Cheng, J.; Lin, S.-H.; Ittmann, M.M.; Yeh, E.T.H. SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J. Biol. Chem. 2010, 285, 25859–25866. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.; Huang, C.; Singh, M.; Carpenter, P.B.; Yeh, E.T.H. Regulation of DNA repair through deSUMOylation and SUMOylation of replication protein A complex. Mol. Cell 2010, 39, 333–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eifler, K.; Vertegaal, A.C.O. SUMOylation-Mediated Regulation of Cell Cycle Progression and Cancer. Trends Biochem. Sci. 2015, 40, 779–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tao, W.; Ni, S.; Chen, Q.; Zhao, Z.; Ma, L.; Fu, Y.; Jiao, Z. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci. 2015, 106, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Garvin, A.J.; Walker, A.K.; Densham, R.M.; Chauhan, A.S.; Stone, H.R.; Mackay, H.L.; Jamshad, M.; Starowicz, K.; Daza-Martin, M.; Ronson, G.E.; et al. The deSUMOylase SENP2 coordinates homologous recombination and nonhomologous end joining by independent mechanisms. Genes Dev. 2019, 33, 333–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, R.; Cohen, H.; Ben-Hamo, R.; Zilberberg, A.; Efroni, S. SENP5 mediates breast cancer invasion via a TGFβRI SUMOylation cascade. Oncotarget 2014, 5, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.P.; Wong, C.C.; Kai, A.K.; Ho, D.W.; Lau, E.Y.; Tsui, Y.M.; Chan, L.K.; Cheung, T.T.; Chok, K.S.H.; Chan, A.C.Y.; et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut 2017, 66, 2149–2159. [Google Scholar] [CrossRef]
- Ma, C.; Wu, B.; Huang, X.; Yuan, Z.; Nong, K.; Dong, B.; Bai, Y.; Zhu, H.; Wang, W.; Ai, K. SUMO-specific protease 1 regulates pancreatic cancer cell proliferation and invasion by targeting MMP-9. Tumour Biol. 2014, 35, 12729–12735. [Google Scholar] [CrossRef]
- Ren, Y.H.; Liu, K.J.; Wang, M.; Yu, Y.N.; Yang, K.; Chen, Q.; Yu, B.; Wang, W.; Li, Q.W.; Wang, J.; et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget 2014, 5, 7093–7104. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhang, X.C. Inhibition of SENP5 suppresses cell growth and promotes apoptosis in osteosarcoma cells. Exp. Ther. Med. 2014, 7, 1691–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Xia, N.; Li, T.; Xu, Y.; Zou, Y.; Zuo, Y.; Fan, Q.; Bawa-Khalfe, T.; Yeh, E.T.H.; Cheng, J. SUMO-specific protease 1 promotes prostate cancer progression and metastasis. Oncogene 2013, 32, 2493–2498. [Google Scholar] [CrossRef] [Green Version]
- Xiang-Ming, Y.; Zhi-Qiang, X.; Ting, Z.; Jian, W.; Jian, P.; Li-Qun, Y.; Ming-Cui, F.; Hong-Liang, X.; Xu, C.; Yun, Z. SENP1 regulates cell migration and invasion in neuroblastoma. Biotechnol. Appl. Biochem. 2016, 63, 435–440. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zuo, Y.; Deng, J.; Wang, L.S.; Chen, G.Q. SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett. 2011, 309, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zuo, Y.; Zhang, H.; Kang, X.; Yue, F.; Yi, Z.; Liu, M.; Yeh, E.T.H.; Chen, G.; Cheng, J. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J. Biol. Chem. 2010, 285, 36682–36688. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Luo, Y.; Gu, X.; Wang, X. Inhibition of SENP6-induced radiosensitization of human hepatocellular carcinoma cells by blocking radiation-induced NF-κB activation. Cancer Biother. Radiopharm. 2013, 28, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Mirecka, A.; Morawiec, Z.; Wozniak, K. Genetic Polymorphism of SUMO-Specific Cysteine Proteases-SENP1 and SENP2 in Breast Cancer. Pathol. Oncol. Res. 2016, 22, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, H.Y.; Xiao, F.J.; Wang, H.; Yang, Y.; Wang, L.; Gao, C.J.; Guo, Z.K.; Wu, C.T.; Wang, L.S. SENP1 inhibition induces apoptosis and growth arrest of multiple myeloma cells through modulation of NF-κB signaling. Biochem. Biophys. Res. Commun. 2015, 460, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Gong, H.; Wang, J.; Tao, L.; Xu, D.; Bao, E.; Liu, Z.; Qiu, J. SENP2 regulates MMP13 expression in a bladder cancer cell line through SUMOylation of TBL1/TBLR1. Sci. Rep. 2015, 5, 13996. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.J.; Zhu, H.Y.; Yang, C.; Ji, F. SENP2 regulates hepatocellular carcinoma cell growth by modulating the stability of β-catenin. Asian Pac. J. Cancer Prev. 2012, 13, 3583–3587. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Hu, S.; Luo, Q.; Ye, D.; Hu, D.; Chen, F. Overexpression of SENP3 in oral squamous cell carcinoma and its association with differentiation. Oncol. Rep. 2013, 29, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Guo, X.; Gong, Y.; Ding, X.; Yu, Y. Sentrin/small ubiquitin-like modifier-specific protease 5 protects oral cancer cells from oxidative stress-induced apoptosis. Mol. Med. Rep. 2015, 12, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Sun, J.; Wang, L.; Li, G.; Shen, Y.; Zhou, X.; Chen, W. Overexpression of SENP5 in oral squamous cell carcinoma and its association with differentiation. Oncol. Rep. 2008, 20, 1041–1045. [Google Scholar]
- Bialik, P.; Woźniak, K. SUMO proteases as potential targets for cancer therapy. Postepy Hig. Med. Dosw. 2017, 71, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Galanty, Y.; Belotserkovskaya, R.; Coates, J.; Polo, S.; Miller, K.M.; Jackson, S.P. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 2009, 462, 935–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maison, C.; Romeo, K.; Bailly, D.; Dubarry, M.; Quivy, J.P.; Almouzni, G. The SUMO protease SENP7 is a critical component to ensure HP1 enrichment at pericentric heterochromatin. Nat. Struct. Mol. Biol. 2012, 19, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Romeo, K.; Louault, Y.; Cantaloube, S.; Loiodice, I.; Almouzni, G.; Quivy, J.P. The SENP7 SUMO-Protease Presents a Module of Two HP1 Interaction Motifs that Locks HP1 Protein at Pericentric Heterochromatin. Cell Rep. 2015, 10, 771–782. [Google Scholar] [CrossRef]
- Garvin, A.J.; Densham, R.M.; Blair-Reid, S.A.; Pratt, K.M.; Stone, H.R.; Weekes, D.; Lawrence, K.J.; Morris, J.R. The deSUMOylase SENP7 promotes chromatin relaxation for homologous recombination DNA repair. EMBO Rep. 2013, 14, 975–983. [Google Scholar] [CrossRef]
- Zimmermann, M.; Lottersberger, F.; Buonomo, S.B.; Sfeir, A.; de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 2013, 339, 700–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duheron, V.; Nilles, N.; Pecenko, S.; Martinelli, V.; Fahrenkrog, B. Localisation of Nup153 and SENP1 to nuclear pore complexes is required for 53BP1-mediated DNA double-strand break repair. J. Cell Sci. 2017, 130, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Bologna, S.; Altmannova, V.; Valtorta, E.; Koenig, C.; Liberali, P.; Gentili, C.; Anrather, D.; Ammerer, G.; Pelkmans, L.; Krejci, L.; et al. Sumoylation regulates EXO1 stability and processing of DNA damage. Cell Cycle 2015, 14, 2439–2450. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.; Kunz, K.; Piller, T.; Tascher, G.; Hölper, S.; Stehmeier, P.; Keiten-Schmitz, J.; Schick, M.; Keller, U.; Müller, S. The SUMO Isopeptidase SENP6 Functions as a Rheostat of Chromatin Residency in Genome Maintenance and Chromosome Dynamics. Cell Rep. 2019, 29, 480–494. [Google Scholar] [CrossRef] [Green Version]
- Gibbs-Seymour, I.; Oka, Y.; Rajendra, E.; Weinert, B.T.; Passmore, L.A.; Patel, K.J.; Olsen, J.V.; Choudhary, C.; Bekker-Jensen, S.; Mailand, N.; et al. Ubiquitin-SUMO circuitry controls activated fanconi anemia ID complex dosage in response to DNA damage. Mol. Cell 2015, 57, 150–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guervilly, J.H.; Gaillard, P.H. SLX4: Multitasking to maintain genome stability. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 475–514. [Google Scholar] [CrossRef] [PubMed]
- Cubeñas-Potts, C.; Goeres, J.D.; Matunis, M.J. SENP1 and SENP2 affect spatial and temporal control of sumoylation in mitosis. Mol. Biol. Cell 2013, 24, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Di Bacco, A.; Ouyang, J.; Lee, H.Y.; Catic, A.; Ploegh, H.; Gill, G. The SUMO-specific protease SENP5 is required for cell division. Mol. Cell Biol. 2006, 26, 4489–4498. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, D.; Arnaoutov, A.; Dasso, M. The SUMO protease SENP6 is essential for inner kinetochore assembly. J. Cell Biol. 2010, 188, 681–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Huang, C.; Liu, B.; Wang, Y.; Xia, N.; Fan, Q.; Chen, G.Q.; Cheng, J. Mitotic Phosphorylation of SENP3 Regulates DeSUMOylation of Chromosome-Associated Proteins and Chromosome Stability. Cancer Res. 2018, 78, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tian, J.; Huang, C.; Ma, J.; Hu, G.; Chen, Y.; Wang, T.; Cai, R.; Zuo, Y.; Tan, H.; et al. P53 suppresses SENP3 phosphorylation to mediate G2 checkpoint. Cell Discov. 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Ao, Q.; Su, W.; Guo, S.; Cai, L.; Huang, L. SENP1 desensitizes hypoxic ovarian cancer cells to cisplatin by up-regulating HIF-1α. Sci. Rep. 2015, 5, 16396. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Kang, X.; Zhang, S.; Yeh, E.T.H. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 2007, 131, 584–595. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Han, Y.; Wang, Y.; Sun, X.; Yan, S.; Yeh, E.T.H.; Chen, Y.; Cang, H.; Li, H.; Shi, G.; et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009, 28, 2748–2762. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, J.; Yang, K.; Cang, H.; Huang, X.Z.; Li, H.; Yi, J. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol. Sin. 2012, 33, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.J.; Xu, Z.; Wang, Z.; Zhang, H.; Zhuang, Z.W.; Simons, M.; Min, W. SUMOylation of VEGFR2 regulates its intracellular trafficking and pathological angiogenesis. Nat. Commun. 2018, 9, 3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Bawa, T.; Lee, P.; Gong, L.; Yeh, E.T.H. Role of desumoylation in the development of prostate cancer. Neoplasia 2016, 8, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, D.; Wang, Z.; Yeh, E.T.H. SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol. Cell Biol. 2014, 24, 6021–6028. [Google Scholar] [CrossRef] [Green Version]
- Kaikkonen, S.; Jääskeläinen, T.; Karvonen, U.; Rytinki, M.M.; Makkonen, H.; Gioeli, D.; Paschal, B.M.; Palvimo, J.J. SUMO-specific protease 1 (SENP1) reverses the hormone-augmented SUMOylation of androgen receptor and modulates gene responses in prostate cancer cells. Mol. Endocrinol. 2009, 23, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.Y.; Asai, N.; Costantini, F.; Hsu, W. SUMO-specific protease 2 is essential for modulating p53-Mdm2 in development of trophoblast stem cell niches and lineages. PLoS Biol. 2018, 6, e310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chiu, S.Y.; Hsu, W. SUMO-specific protease 2 in Mdm2-mediated regulation of p53. Cell Death Differ. 2011, 18, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, S.F.; Liang, X.T.; Liang, H.X.; Wang, T.T.; Wu, S.Q.; Qiu, Z.J.; Zhan, R.; Xu, Z.S. SENP2 exerts an anti-tumor effect on chronic lymphocytic leukemia cells through the inhibition of the Notch and NF-κB signaling pathways. Int. J. Oncol. 2019, 54, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Achour, T.N.; Sentis, S.; Teyssier, C.; Philippat, A.; Lucas, A.; Corbo, L.; Cavaillès, V.; Jalaguier, S. Transcriptional Repression of Estrogen Receptor α Signaling by SENP2 in Breast Cancer Cells. Mol. Endocrinol. 2014, 28, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huang, Y.S.; Lin, Y.M.; Lin, C.J.; Jeng, J.C.; Liu, S.M.; Ho, T.L.; Chang, R.T.; Changou, C.A.; Ho, C.C.; et al. The role of sentrin-specific protease 2 substrate recognition in TGF-β-induced tumorigenesis. Sci. Rep. 2018, 8, 9786. [Google Scholar] [CrossRef]
- Hemelaar, J.; Borodovsky, A.; Kessler, B.M.; Reverter, D.; Cook, J.; Kolli, N.; Gan-Erdene, T.; Wilkinson, K.D.; Gill, G.; Lima, C.D.; et al. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol. Cell Biol. 2014, 24, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Albrow, V.E.; Ponder, E.L.; Fasci, D.; Békés, M.; Deu, E.; Salvesen, G.S.; Bogyo, M. Development of small molecule inhibitors and probes of human SUMO deconjugating proteases. Chem. Biol. 2011, 18, 722–732. [Google Scholar] [CrossRef] [Green Version]
- Ponder, E.L.; Albrow, V.E.; Leader, B.A.; Békés, M.; Mikolajczyk, J.; Fonović, U.P.; Shen, A.; Drag, M.; Xiao, J.; Deu, E.; et al. Functional characterization of a SUMO deconjugating protease of Plasmodium falciparum using newly identified small molecule inhibitors. Chem. Biol. 2011, 18, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Dobrotă, C.; Fasci, D.; Hădade, N.D.; Roiban, G.D.; Pop, C.; Meier, V.M.; Dumitru, I.; Matache, M.; Salvesen, G.S.; Funeriu, D.P. Glycine fluoromethylketones as SENP-specific activity based probes. Chembiochem 2012, 13, 80–84. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, W.; Wang, L.; Wen, D.; Zhao, Y.; Wang, Q.; Meng, Q.; Chen, G.; Wu, Y.; Zhou, H. Design, synthesis, and biological evaluation of benzodiazepine-based SUMO-specific protease 1 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 6389–6392. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Huang, Z.; Huang, M.; Luo, Y.; Liu, B.; Lu, H.; Wu, Y.; Peng, Y.; Zhang, J. 2-(4-Chlorophenyl)-2-oxoethyl 4-benzamidobenzoate derivatives, a novel class of SENP1 inhibitors: Virtual screening, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 6867–6870. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, T.; Chai, C.; Yang, Y.; Zheng, Y.; Zhou, P.; Qiao, X.; Zhang, B.; Liu, Z.; Wang, J.; et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS ONE 2012, 7, e37693. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Koma, Y.; Ban, H.S.; Nakamura, H. Discovery of 1-[4-(N-benzylamino)phenyl]-3-phenylurea derivatives as non-peptidic selective SUMO-sentrin specific protease (SENP)1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5169–5173. [Google Scholar] [CrossRef]
- Madu, I.G.; Namanja, A.T.; Su, Y.; Wong, S.; Li, Y.J.; Chen, Y. Identification and characterization of a new chemotype of non-covalent SENP inhibitors. ACS Chem. Biol. 2013, 8, 1435–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Ito, A.; Takemoto, M.; Yoshida, M.; Zhang, K.Y. Identification of 1,2,5-oxadiazoles as a new class of SENP2 inhibitors using structure based virtual screening. J. Chem. Inf. Model. 2014, 54, 870–880. [Google Scholar] [CrossRef]
- Wen, D.; Xu, Z.; Xia, L.; Liu, X.; Tu, Y.; Lei, H.; Wang, W.; Wang, T.; Song, L.; Ma, C.; et al. Important role of SUMOylation of Spliceosome factors in prostate cancer cells. J. Proteome Res. 2014, 13, 3571–3582. [Google Scholar] [CrossRef]
- Wu, J.; Lei, H.; Zhang, J.; Chen, X.; Tang, C.; Wang, W.; Xu, H.; Xiao, W.; Gu, W.; Wu, Y. Momordin Ic, a new natural SENP1 inhibitor, inhibits prostate cancer cell proliferation. Oncotarget 2016, 7, 58995–59005. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Z.; Zhang, J.; Zhou, H. Identification of SENP1 inhibitors through in silico screening and rational drug design. Eur. J. Med. Chem. 2016, 122, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstock, J.D.; Ye, D.; Smith, J.A.; Lee, Y.J.; Gessler, F.A.; Yasgar, A.; Kouznetsova, J.; Jadhav, A.; Wang, Z.; Pluchino, S.; et al. Quantitative high-throughput screening identifies cytoprotective molecules that enhance SUMO conjugation via the inhibition of SUMO-specific protease (SENP)2. FASEB J. 2018, 32, 1677–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Claessens, L.A.; Vertegaal, A.C.O.; Ovaa, H. Chemical Tools and Biochemical Assays for SUMO Specific Proteases (SENPs). ACS Chem. Biol. 2019, 14, 2389–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chau, S.F.; Lam, K.H.; Chan, H.Y.; Ng, T.B.; Au, S.W. Crystal structure of the SENP1 mutant C603S-SUMO complex reveals the hydrolytic mechanism of SUMO-specific protease. Biochem. J. 2016, 398, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.; Müller, S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. 2014, 15, 422. [Google Scholar] [CrossRef] [Green Version]
| SENP Protease | Main Localization | Enzymatic Activity | Cancer/RNA Expression * | Clinical Studies | Molecular Studies | References |
|---|---|---|---|---|---|---|
| SENP1 | Nucleoplasm | C-terminal hydrolase, isopeptidase | Breast | polymorphism c.1691 + 36C > T (rs12297820) was associated with risk of metastases | [16] | |
| Colorectal/↑ | not related to tumor invasion, lymph node involvement or tumor cell differentiation | regulates cell cycle via CDK inhibitors (p16, p19, p21, and p27) | [13] | |||
| Myeloma/↑ | regulates sensitivity to apoptosis, proliferation, and colony formation regulates NF-κB signaling | [17] | ||||
| Liver/↑ | prognostic marker | TCGA | ||||
| Neuroblastoma/↑ | overexpressed in metastatic tissues vs. primary tumor tissue | promotes cell invasion and migration regulates the expression of CDH1, MMP-9, and MMP-2 | [12] | |||
| Pancreatic/↑ | correlates with lymph node metastasis and TNM stage | up-regulates MMP9 | [8] | |||
| Prostate/↑ | correlates with cancer aggressiveness and recurrence | androgen receptor and hypoxia-induced stabilization of HIF1α and overexpression of downstream proteins (MMP2/MMP9) | [1,11] | |||
| Renal/↑ | prognostic marker | TCGA | ||||
| SENP2 | Nuclear pore complex | C-terminal hydrolase, isopeptidase | Bladder/↓ | decreases cell migration and invasion inhibits the expression of MMP-13 | [18] | |
| Breast | polymorphism c.902C > A, p.Thr301Lys (rs6762208) was associated with cancer occurrence | [16] | ||||
| Endometrial/↑ | prognostic marker | TCGA | ||||
| Liver/↓ | suppresses growth and colony formation modulates the stability of β-catenin | [19] | ||||
| SENP3 | Nucleolus | Isopeptidase | Gastric/↑ | promotes epithelial-mesenchymal transition, cell migration, and metastasis potentiates the transcriptional activity of FOXC2 | [9] | |
| Head and neck/↑ | correlates with tumor differentiation | correlates with ROS | [20] | |||
| Pancreatic/↑ | prognostic marker | TCGA | ||||
| SENP5 | Nucleolus | C-terminal hydrolase, isopeptidase | Bone/↑ | promotes cell growth, its inhibition results in cell cycle arrest and apoptosis via the regulation of cyclin B1 and caspase 3/7 | [10] | |
| Breast/↑ | negatively correlates with survival | associates with cell proliferation, migration, invasion, and colony formation regulation of phenotype through SENP5-TGFb-MMP9 cascade | [6] | |||
| Endometrial/↑ | prognostic marker | TCGA | ||||
| Head and neck/↑ | associates with tumor differentiation | protects cells from oxidative stress-induced apoptosis through the stabilisation of mitochondria | [21,22] | |||
| Liver/↑ | prognostic marker | TCGA | ||||
| Renal/↑ | prognostic marker | TCGA | ||||
| SENP6 | Nucleoplasm | Isopeptidase, chain editing | Liver/↑ | silencing SENP6 causes sensitisation to radiation and inhibition of cell proliferation required for radiation-induced NF-κB activation | [15] | |
| Renal/↑ | prognostic marker | TCGA | ||||
| Thyroid/↑ | prognostic marker | TCGA | ||||
| SENP7 | Nucleoplasm | Isopeptidase, chain editing | Head and neck/↑ | prognostic marker | TCGA |
| Inhibitor Name | Target Protein | Compound Name/Source | IC50 (µM) | Biological Activity | References |
|---|---|---|---|---|---|
| SUMO-1-VS | SENP2 | SUMO-1-vinyl sulfone | Interacted directly with SENP2 in its catalytic site as verified with SDS-PAGE. | [52] | |
| JCP666 | SENP1 SENP2 | Electrophilic aza-peptide epoxide with non-natural peptide backbone | 13.8 7 | Virtual screening-aided design. Included aza-aspartic acid epoxide with the bulky di-naphthyl amide susceptible to ring opening in aqueous media. SENP inhibition evaluated with ProSUMO processing assay combined with SDS-PAGE and a cleavage assay with SUMO-conjugated fluorogenic substrate. | [53,54] |
| VEA260 | SENP1 SENP2 | JCP666 analogue without aspartic acid side-chain | 7.1 3.7 | SENP inhibition evaluated with ProSUMO processing assay combined with SDS-PAGE and a cleavage assay with SUMO-conjugated fluorogenic substrate. | [53,54] |
| VEA499 VEA561 | SENP1 SENP2 SENP2 SENP6 SENP7 | Acyloxymethyl ketone (AOMK)-based compounds which retained the overall structure of VEA260 and JCP666 | 3.6 0.25 5.7 4.2 4.3 | AOMKs equipped with a large O-acyl-anthracene group—mimetics of the peptide vinyl sulfone inhibitors. VEA499 and VEA561 based on natural peptide sequences. VEA499 with sequence of SUMO-1 (QTGG) was most potent for hSENP1 and hSENP2, and VEA561 with the ubiquitin sequence (LRGG) was the most potent against hSENP6 and hSENP7. Enzymatic activity evaluated with a cleavage assay with SUMO-conjugated fluorogenic substrate. Low cell permeability. | [53] |
| N-acetylglycine fluoromethylketone (Compound 1) | SENP1 SENP2 | Glycine fluoromethylketone (G-FMK) with peptide sequence | 5–10 5–10 | G-FMK equipped with peptide sequence (FQQQTGG) specific to SUMO-2/3. G-FMK acted as SENP-specific activity based probe. It shared binding site for SENP1 with SUMO-1. Direct interaction between G-FMK and SENP1/2 assayed with activity-based labeling combined with SDS-PAGE. G-FMK targeted SENP1 and SENP2 in HEK293A cell lysates. | [55] |
| Compound 36 Compound 38 | SENP1 | Benzodiazepines | 15.5 9.2 | Compounds screened for SENP1 inhibition with SUMO-ΔRanGAP cleavage assay combined with SDS-PAGE. Compounds 36 and 38 inhibited the growth of prostate cancer cells (PC-3) with IC50 values of 13.0 and 35.7 μM, respectively. | [56] |
| J5 Compound 8d Compound 8e | SENP1 | 2-(4-Chlorophenyl)-2-oxoethyl 4-benzamidobenzoate derivatives | 2.385 1.175 1.080 | Developed with virtual screening. Molecular docking showed that J5 fitted in the SENP1 binding site. The SENP1 inhibitory potency was evaluated with SUMO-ΔRanGAP cleavage assay combined with SDS-PAGE. | [57] |
| Triptolide | SENP1 | Diterpene lactone extracted from the Chinese herb Tripterygium wilfordii Hook F | 0.0203 (PC-3) 0.009754 (LNCaP) | Inhibited proliferation and induced cell death in prostate cancer cells (LNCaP and PC-3). Suppressed xenografted PC-3 tumor growth in nude mice. Down-regulated SENP1 and c-Jun expression in PCa cells and androgen receptor expression in LNCaP cells. Down-regulation or over-expression of SENP1 inhibited triptolide anti-cancer efficacy. | [58] |
| Compound 4 (GN6958) | SENP1 | 1-[4-(N-benzylamino)phenyl]-3-phenylurea derivative | 29.6 | Directly interacted with SENP1 in cells as evaluated with the use of HP SpinTrap affinity column combined with SDS-PAGE. Inhibited SENP1 enzymatic activity as assayed with fluorogenic substrate SUMO-1-AMC. Specific inhibitor, did not inhibit SENP2. Suppressed HIF-1α accumulation in HeLa cells. | [59] |
| SPI-01 | SENP1 SENP2 SENP7 | sulfonyl-benzene non-natural amino acid | 5.9 2.9 3.5 | Virtual screening was used for the study. SPI-01 inhibited the isopeptidase activities in cells as demonstrated with DUB-Glo assay. Inhibitory mechanism is mainly non-competitive as demonstrated with DUB-Glo enzyme kinetic experiments and NMR binding analysis. | [60] |
| Compound 117 Compound 69 | SENP2 SENP1 SENP2 SENP1 | 1,2,5-oxadiazoles | 3.7 >30 5.9 9.7 | Compound development with virtual screening. FRET-based assay for quantification of endopeptidase activity. | [61] |
| SI2 | SENP1 SENP2 SENP3 | Biphenyl-4-carboxylic acid ester with chlorobenzene moiety | 1.29 | Compound selection with hierarchical virtual screen. Cell-permeable SENP specific inhibitor. Occupied a tunnel in the catalytic centre of SENP1. | [62] |
| Momordin Ic | SENP1 | Natural pentacyclic triterpenoid extracted from various sources such as Kochia scoparia (L.) | 15.37 | Inhibited SENP1 in cells as shown with SUMO-2-ΔRanGAP1 cleavage assay combined with SDS-PAGE. Direct interaction with SENP1 in cells determined with cellular thermal shift assay. Inhibited prostate cancer PC-3 cell proliferation. Suppressed cell proliferation and induced cell death in a xenograft PC-3 tumor mouse model. | [63] |
| Compound 13m | SENP1 | 4′-methoxy-biphenyl-3-carboxylic acid 3-(3-phenylpropionylamino)-benzylamide | 3.5 | Designed with virtual screening. SENP1 inhibition determined by SUMO-RanGAP cleavage assay combined with SDS-PAGE. | [64] |
| Ebselen and 6-thioguanine | SENP2 | synthetic organo-selenium compound | Virtual-screening-assisted strategy of drug identification. Molecular docking calculations demonstrated that ebselen occluded the entrance to the SENP2 tunnel. Both ebselen and 6-thioguanine were non-cytotoxic, increased SUMO conjugation in B35 neuroblastoma cells, and protected the cells from OGD (in vitro stroke model). Ebselen upregulated global SUMOylation within the brains of mice. Both compounds inhibited SENP1. | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz, P.; Woźniak, K. SENP Proteases as Potential Targets for Cancer Therapy. Cancers 2021, 13, 2059. https://doi.org/10.3390/cancers13092059
Tokarz P, Woźniak K. SENP Proteases as Potential Targets for Cancer Therapy. Cancers. 2021; 13(9):2059. https://doi.org/10.3390/cancers13092059
Chicago/Turabian StyleTokarz, Paulina, and Katarzyna Woźniak. 2021. "SENP Proteases as Potential Targets for Cancer Therapy" Cancers 13, no. 9: 2059. https://doi.org/10.3390/cancers13092059






