Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum Biomarkers
2.3. Digital Imaging Analysis
2.4. Mathematical Model
3. Results
3.1. Model Set-Up in Prototype Patiens
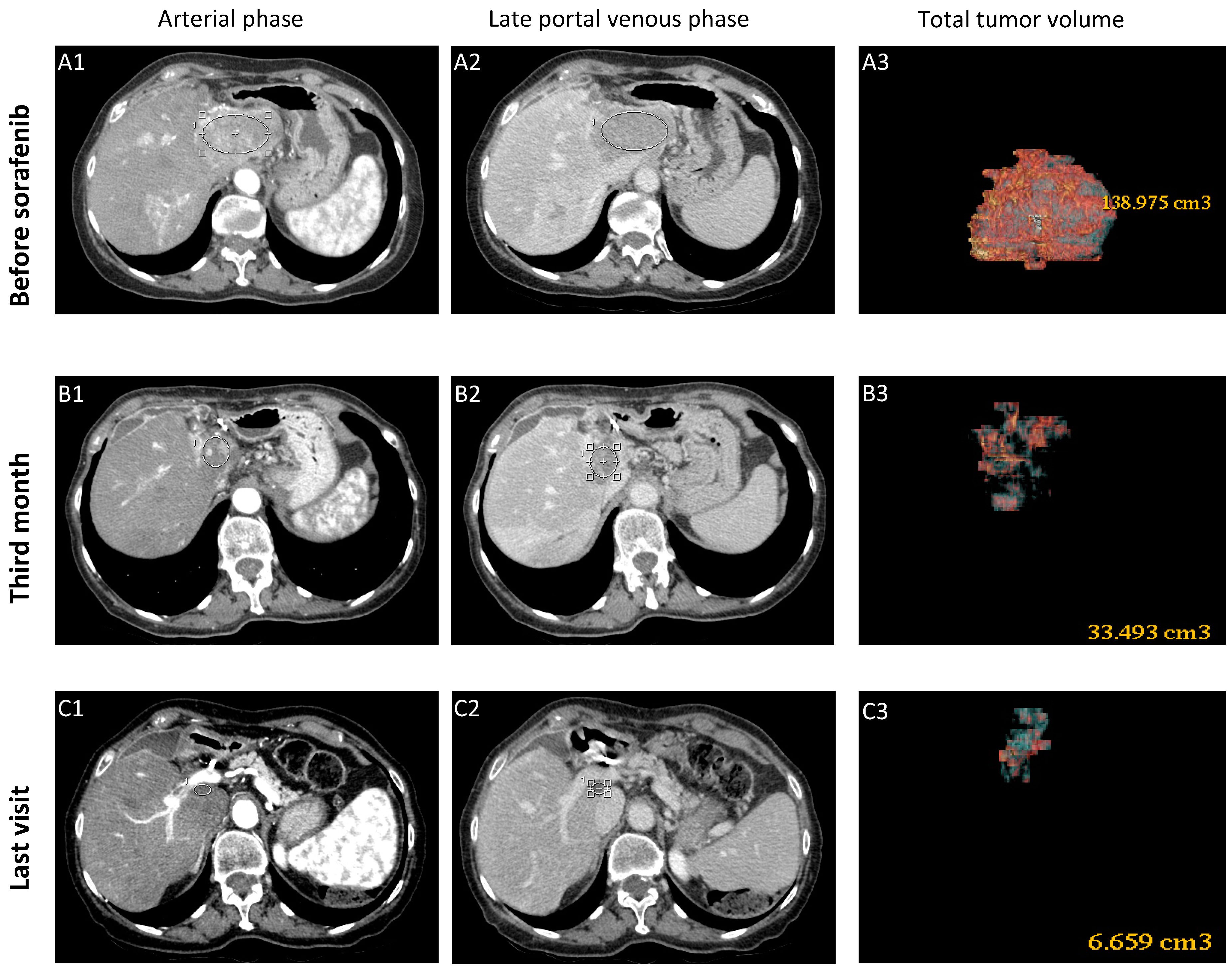
3.1.1. Case-1 (CR to Sorafenib)
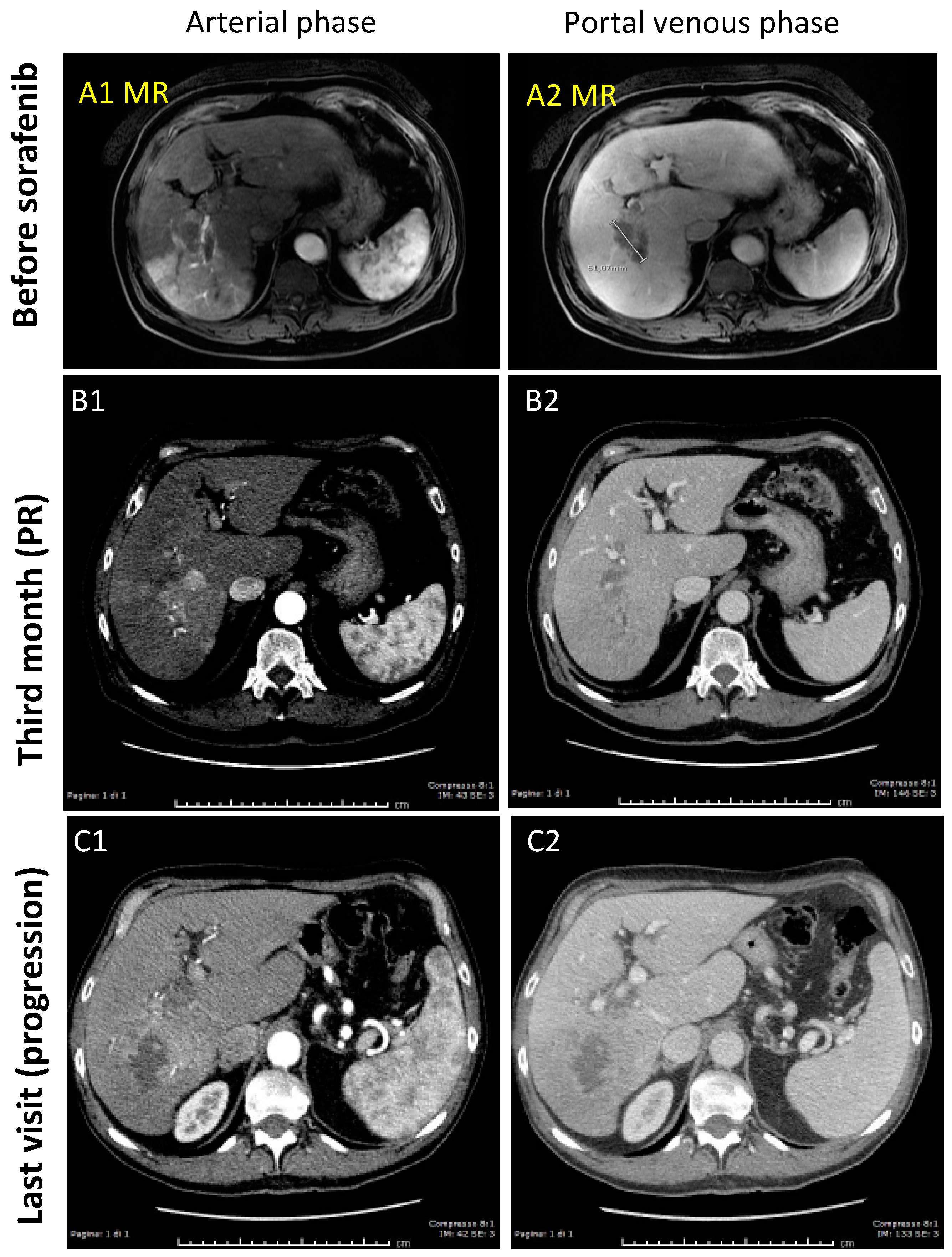
3.1.2. Case-2 (PR to Sorafenib)
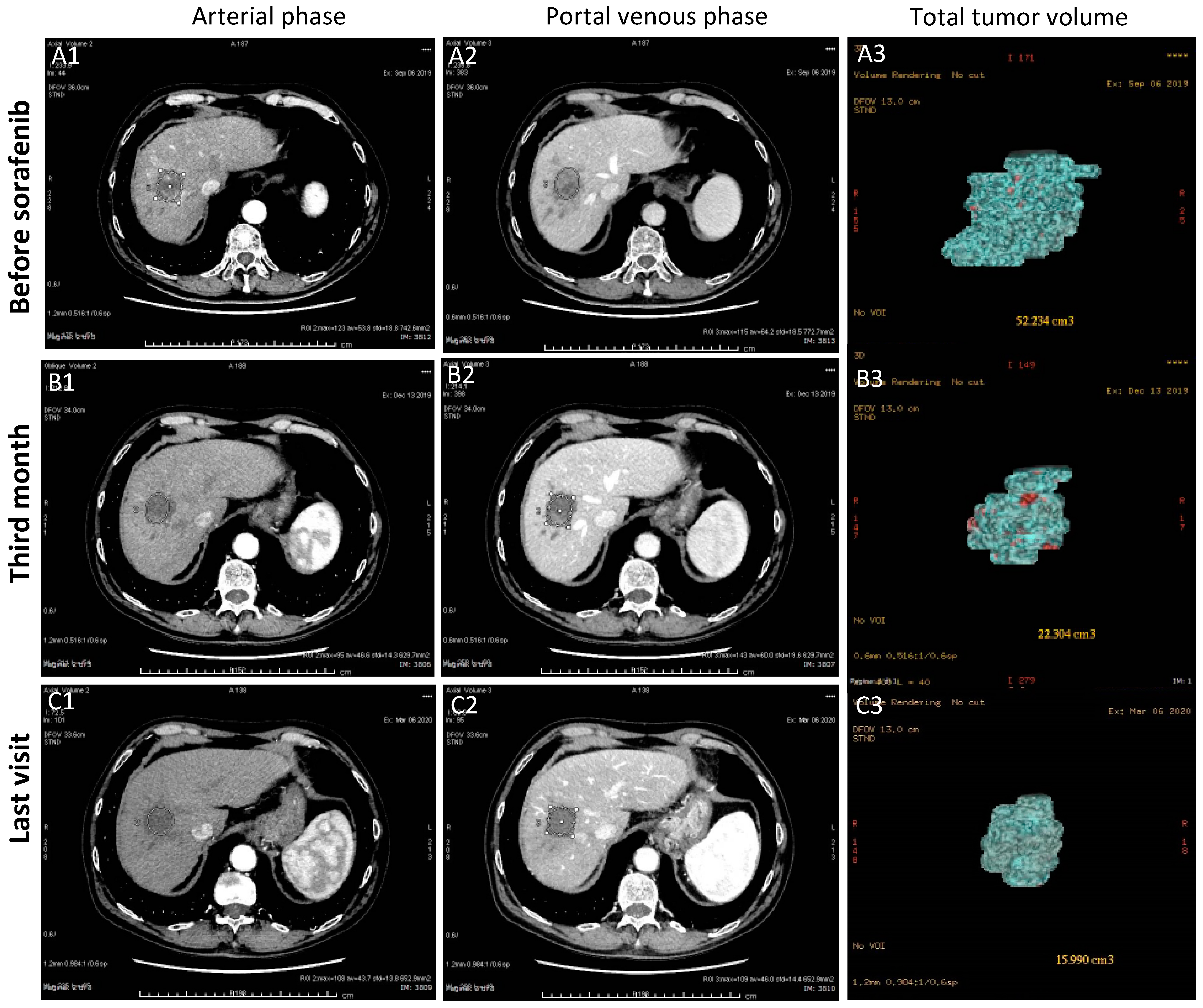
3.1.3. Case-3 (PR to Regorafenib)
3.2. Model Validation Cohort
3.2.1. Clinical Characteristics of the Patients
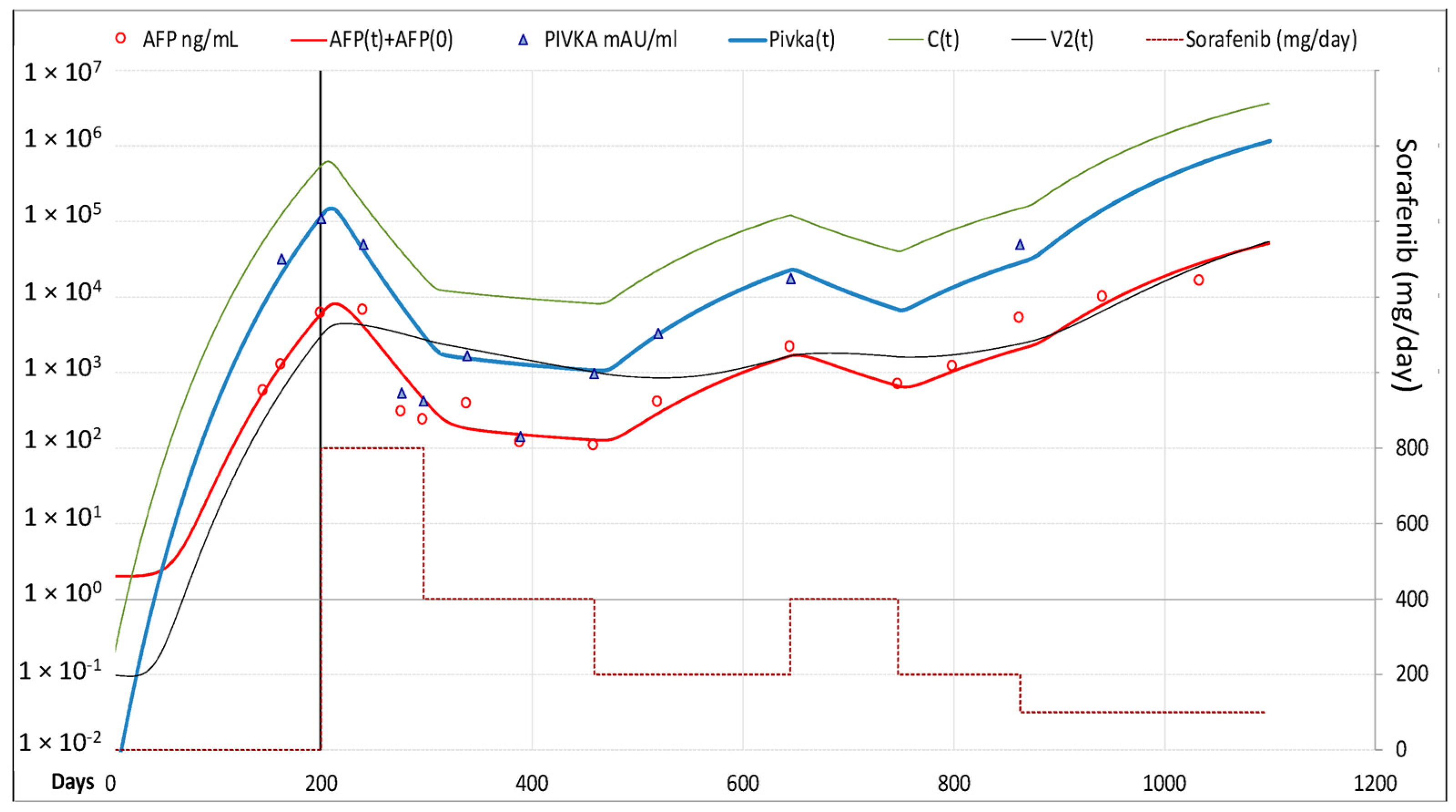
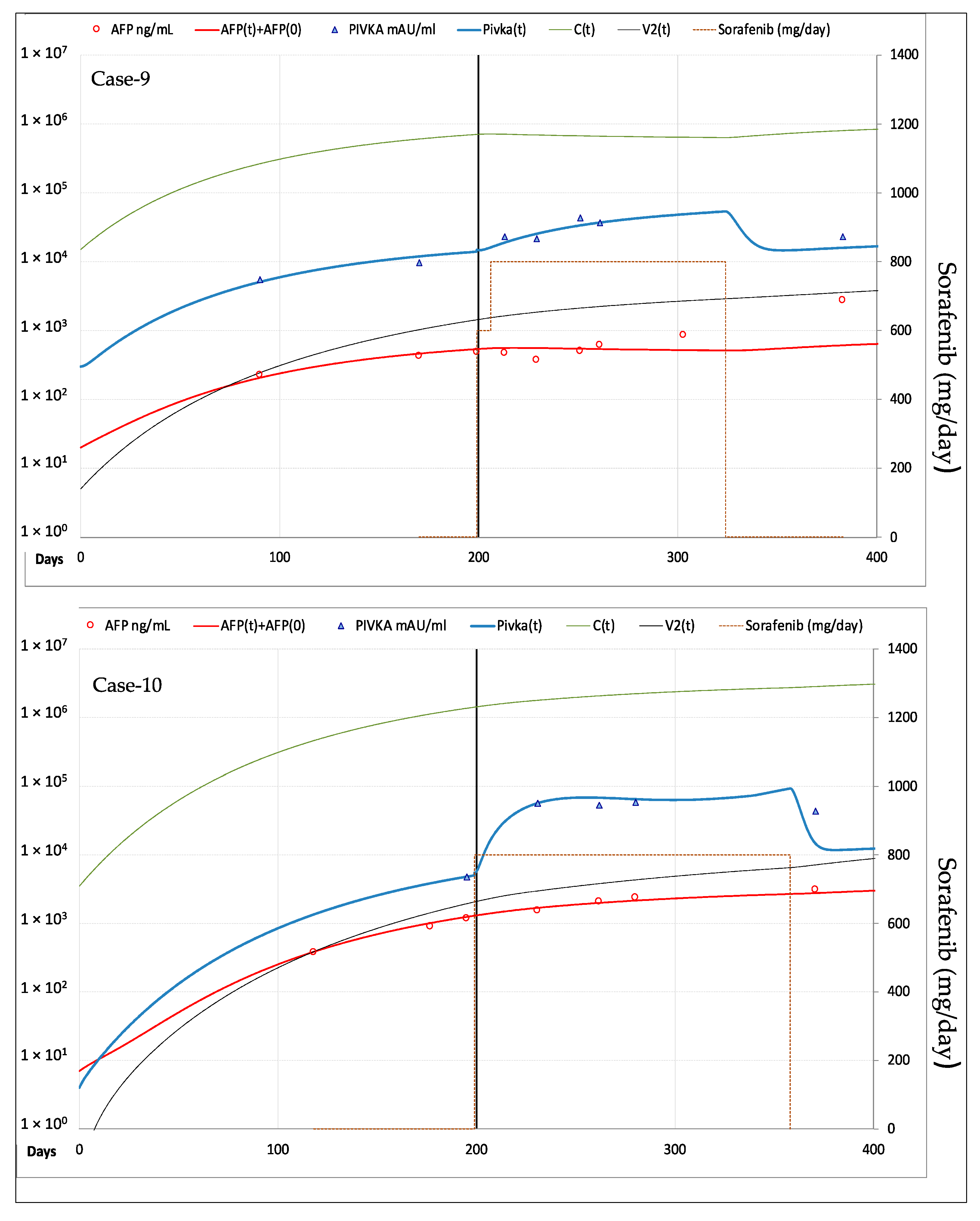
3.2.2. Fitting of AFP and PIVKA-II Serum Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GLOBOCAN 2018. The Global Cancer Observatory. International Agency for Research on Cancer 2020. Available online: http://gco.iarc.fr/today/online-analysis-table (accessed on 30 December 2020).
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Campigotto, M.; Giuffrè, M.; Colombo, A.; Visintin, A.; Aversano, A.; Budel, M.; Masutti, F.; Abazia, C.; Crocé, L.S. Comparison between hepatocellular carcinoma prognostic scores: A 10-year single-center experience and brief review of the current literature. WJH 2020, 12, 1239–1257. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Rimassa, L. Novel Therapies for Hepatocellular Carcinoma. Cancers 2020, 12, 3049. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kim, T.S.; Kim, J.H.; Kim, B.H.; Lee, Y.S.; Yoo, Y.J.; Kang, S.H.; Suh, S.J.; Jung, Y.K.; Seo, Y.S.; Yim, H.J.; et al. Complete response of advanced hepatocellular carcinoma to sorafenib: Another case and a comprehensive review. Clin. Mol. Hepatol. 2017, 23, 340–346. [Google Scholar] [CrossRef]
- Rimola, J.; Díaz-González, Á.; Darnell, A.; Varela, M.; Pons, F.; Hernandez-Guerra, M.; Delgado, M.; Castroagudin, J.; Matilla, A.; Sangro, B.; et al. Complete response under sorafenib in patients with hepatocellular carcinoma: Relationship with dermatologic adverse events. Hepatology 2018, 67, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Desmée, S.; Mentré, F.; Veyrat-Follet, C.; Guedj, J. Nonlinear mixed-effect models for prostate-specific antigen kinetics and link with survival in the context of metastatic prostate cancer: A comparison by simulation of two-stage and joint approaches. AAPS J. 2015, 17, 691–699. [Google Scholar] [CrossRef]
- Ait-Oudhia, S.; Mager, D.E.; Pokuri, V.; Tomaszewski, G.; Groman, A.; Zagst, P.; Fetterly, G.; Iyer, R. Bridging Sunitinib Exposure to Time-to-Tumor Progression in Hepatocellular Carcinoma Patients with Mathematical Modeling of an Angiogenic Biomarker. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Park, K. A Review of Modeling Approaches to Predict Drug Response in Clinical Oncology. Yonsei Med. J. 2017, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Jain, L.; Woo, S.; Gardner, E.R.; Dahut, W.L.; Kohn, E.C.; Kummar, S.; Mould, D.R.; Giaccone, G.; Yarchoan, R.; Venitz, J.; et al. Population pharmacokinetic analysis of sorafenib in patients with solid tumours. Br. J. Clin. Pharmacol. 2011, 72, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Asahina, Y.; Tsuchiya, K.; Tanaka, K.; Suzuki, Y.; Hoshioka, T.; Tamaki, S.; Kato, T.; Yasui, Y.; Hosokawa, T.; et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology 2011, 81, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Park, J.Y. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting responses in patients with hepatocellular carcinoma. BioMed Res. Int. 2013, 2013, 310427. [Google Scholar] [CrossRef]
- Kudo, M. Biomarkers and personalized Sorafenib therapy—Editorial. Liver Cancer 2014, 3, 399–404. [Google Scholar]
- Haruna, Y.; Yakushijin, T.; Kawamoto, S. Efficacy and safety of sorafenib plus vitamin K treatment for hepatocellular carcinoma: A phase II, randomized study. Cancer Med. 2021, 10, 914–922. [Google Scholar] [CrossRef]
- Akamine, T.; Ando, K.; Oki, E.; Saeki, H.; Nakashima, Y.; Imamura, Y.U.; Ohgaki, K.; Maehara, Y. Acute Liver Failure Due to Regorafenib May Be Caused by Impaired Liver Blood Flow: A Case Report. Anticancer Res. 2015, 35, 4037–4041. [Google Scholar]
- Zhao, B.; Zhao, H. Incidence and risk of regorafenib-induced hepatotoxicity. Oncotarget 2017, 8, 84102–84111. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Mehta, A.S.; Singal, A.G.; Block, T.; Marrero, J.A.; Lok, A.S. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2495–2503. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.D.; Jun, M.J.; Yun, S.C.; Shim, J.H.; Lee, H.C.; Lee, D.; An, J.; Lim, Y.S.; Chung, Y.H.; et al. Tumor Volume Doubling Time as a Dynamic Prognostic Marker for Patients with Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2923–2931. [Google Scholar] [CrossRef]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular carcinoma tumour volume doubling time: A systemic review and meta-analysis. Gut 2020. [Google Scholar] [CrossRef]
- Weitz, I.C.; Liebman, H.A. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: A critical review. Hepatology 1993, 18, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Shiraha, H.; Ueda, N.; Takaoka, N.; Nakanishi, Y.; Matsuo, N.; Tanaka, S.; Nishina, S.; Suzuki, M.; Takaki, A.; et al. Des-gamma-carboxyl prothrombin-promoted vascular endothelial cell proliferation and migration. J. Biol. Chem. 2007, 282, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shiraha, H.; Fujikawa, T.; Takaoka, N.; Ueda, N.; Nakanishi, Y.; Koike, K.; Takaki, A.; Shiratori, Y. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J. Biol. Chem. 2005, 280, 6409–6415. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Ciruolo, M.; Abate, M.L.; Carucci, P.; Rolle, E.; Rosso, C.; Olivero, A.; Troshina, G.; Risso, A.; Nicolosi, A.; et al. Alpha-Fetoprotein, Protein Induced by Vitamin K Absence or Antagonist II and Glypican-3 for the Detection and Prediction of Hepatocellular Carcinoma in Patients with Cirrhosis of Viral Etiology. Cancers 2020, 12, 3218. [Google Scholar] [CrossRef] [PubMed]
- Ricco, G.; Cosma, C.; Bedogni, G.; Biasiolo, A.; Guarino, M.; Pontisso, P.; Morisco, F.; Oliveri, F.; Cavallone, D.; Bonino, F.; et al. Modeling the time-related fluctuations of AFP and PIVKA-II serum levels in patients with cirrhosis undergoing surveillance for hepatocellular carcinoma. Cancer Biomark. 2020, 29, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Suzuki, H.; Okano, H.; Oyamada, T.; Yasuda, Y.; Sakamoto, A. Hypoxia-induced des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Int. J. Oncol. 2010, 36, 161–170. [Google Scholar] [PubMed]
- Suzuki, T.; Shinjo, S.; Arai, T.; Kanai, M.; Goda, N. Hypoxia and fatty liver. World J. Gastroenterol. 2014, 20, 15087–15097. [Google Scholar] [CrossRef]
- Ribba, B.; Watkin, E.; Tod, M.; Girard, P.; Grenier, E.; You, B.; Giraudo, E.; Freyer, G. A model of vascular tumour growth in mice combining longitudinal tumour size data with histological biomarkers. Eur. J. Cancer 2011, 47, 479–490. [Google Scholar] [CrossRef]
- Kareva, I. (Ed.) Chapter 5—Angiogenesis Regulators as a Possible Key to Accelerated Growth of Secondary Tumors Following Primary Tumor Resection. In Understanding Cancer from a Systems Biology Point of View; Academic Press: Cambridge, MA, USA, 2018; pp. 61–77. ISBN 9780128136737. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Edeline, J.; Icard, N.; Lenoir, L.; Laffont, S.; Mesbah, H.; Breton, M.; Sulpice, L.; Boudjema, K.; et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J. Nucl. Med. 2015, 56, 339–346. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bargellini, I.; Cela, M.; Cincione, I.; Sacco, R. Comparison between Y90 Radioembolization Plus Sorafenib and Y90 Radioembolization alone in the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers 2020, 12, 897. [Google Scholar] [CrossRef] [PubMed]




| Clinical Features | Model Set-Up | Model Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
| Gender | F | M | M | M | F | M | M | M | M | M |
| Age at treatment start | 79 | 72 | 70 | 65 | 64 | 56 | 76 | 61 | 67 | 69 |
| Liver disease etiology | HBV | HCV | GH | HBV | HCV | HBV | HCV | HBV | HCV | HCV |
| HCC Staging (BCLC) | C | C | C | B | C | C | B | C | C | C |
| Prior HCC treatments | PEI, TARE | - | - | TACE | TACE | RFTA | TACE | - | TACE, PEI | - |
| HCC volume (cm3) | 139 | 32 | 52.2 | 6.4 | 24.5 | 6.1 | 200 | 89 | 29.1 | 116 |
| Vascular invasion | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes |
| Lymph-node mts | No | No | No | No | No | Yes | No | No | No | No |
| AFP at BL (ng/mL) | 21,531 | 5905 | 29,953 | 635 | 513 | 1741 | 55 | 71 | 417 | 1167 |
| PIVKA at BL (AI/mL) | 30,362 | 108,460 | 592 | 151 | 388 | 147 | 6000 | 589 | 9799 | 4712 |
| Treatment | SOR | SOR | RGR | SOR | SOR | SOR | SOR | SOR | SOR | SOR |
| Duration (months) | 60 | 27 | 12 | 11.5 | 25.7 | 18.4 | 9.3 | 18.9 | 5.1 | 5.7 |
| Target Response * | CR | PR | PR | CR | CR | CR | SD | SD | PD | PD |
| Overall Response * | CR | PD | PR | CR ** | PD | PD | PD | PD | PD | PD |
| Response * | Model Set-Up | Model Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | PR | CR | CR | CR | SD | SD | PD | PD | |
| Model Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
| ξ1 | 0.360 | 0.250 | 0.320 | 0.317 | 0.315 | 0.315 | 0.360 | 0.330 | 0.440 | 0.355 |
| ξ2 | 0.931 | 0.959 | 0.932 | 0.917 | 0.910 | 0.911 | 0.924 | 0.926 | 0.90 | 0.923 |
| ξ4 | 0.11 | 0.11 | 0.11 | 0.12 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| ω1 | 2.20 × 10−3 | 1.50 × 10−3 | 6.50 × 10−3 | 1.00 × 10−3 | 2.00 × 10−4 | 3.00 × 10−3 | 7.00 × 10−6 | 6.00 × 10−6 | 3.00 × 10−4 | 1.00 × 10−4 |
| ω2 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.12 | 0.30 | 0.10 | 0.10 | 0.10 |
| π1 | 2.50 × 10−4 | 1.00 × 10−2 | 7.00 × 10−5 | 2.00 × 10−4 | 2.00 × 10−4 | 9.80 × 10−2 | 3.00 × 10−3 | 5.00 × 10−5 | 8.00 × 10−3 | 2.00 × 10−4 |
| π2 | 1.22 | 1.15 | 1.14 | 1.14 | 1.20 | 0.62 | 0.88 | 1.13 | 1.00 | 1.14 |
| π3 | 0.2 | 0.3 | 0.4 | 0.4 | 0.4 | 0.5 | 0.20 | 0.4 | 0.4 | 0.4 |
| μ1 | 1.30 × 10−5 | 1.30 × 10−5 | 7.00 × 10−6 | 3.00 × 10−5 | 5.00 × 10−6 | 9.00 × 10−5 | 8.00× 10−6 | 7.00 × 10−6 | 7.00 × 10−6 | 7.00 × 10−6 |
| μ2 | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| ϑ1 | 220 | 50 | 30 | 500 | 150 | 100 | 30 | 13 | 3 | 1 |
| α2 | 4.20 × 10−3 | 4.20 × 10−3 | 2.00 × 10−3 | 4.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 |
| α3 | 0.350 | 0.270 | 0.002 | 0.200 | 0.400 | 0.200 | 0 | 0 | 0 | 0 |
| ψ1 | 10 | 5 | 5 | 10 | 70 | 50 | 10 | 3 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombatto, P.; Demirtas, C.O.; Ricco, G.; Civitano, L.; Boraschi, P.; Scalise, P.; Cavallone, D.; Oliveri, F.; Romagnoli, V.; Bleve, P.; et al. Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib. Cancers 2021, 13, 2064. https://doi.org/10.3390/cancers13092064
Colombatto P, Demirtas CO, Ricco G, Civitano L, Boraschi P, Scalise P, Cavallone D, Oliveri F, Romagnoli V, Bleve P, et al. Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib. Cancers. 2021; 13(9):2064. https://doi.org/10.3390/cancers13092064
Chicago/Turabian StyleColombatto, Piero, Coskun Ozer Demirtas, Gabriele Ricco, Luigi Civitano, Piero Boraschi, Paola Scalise, Daniela Cavallone, Filippo Oliveri, Veronica Romagnoli, Patrizia Bleve, and et al. 2021. "Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib" Cancers 13, no. 9: 2064. https://doi.org/10.3390/cancers13092064
APA StyleColombatto, P., Demirtas, C. O., Ricco, G., Civitano, L., Boraschi, P., Scalise, P., Cavallone, D., Oliveri, F., Romagnoli, V., Bleve, P., Coco, B., Salvati, A., Urbani, L., Bonino, F., & Brunetto, M. R. (2021). Modeling Hepatocellular Carcinoma Cells Dynamics by Serological and Imaging Biomarkers to Explain the Different Responses to Sorafenib and Regorafenib. Cancers, 13(9), 2064. https://doi.org/10.3390/cancers13092064









