Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
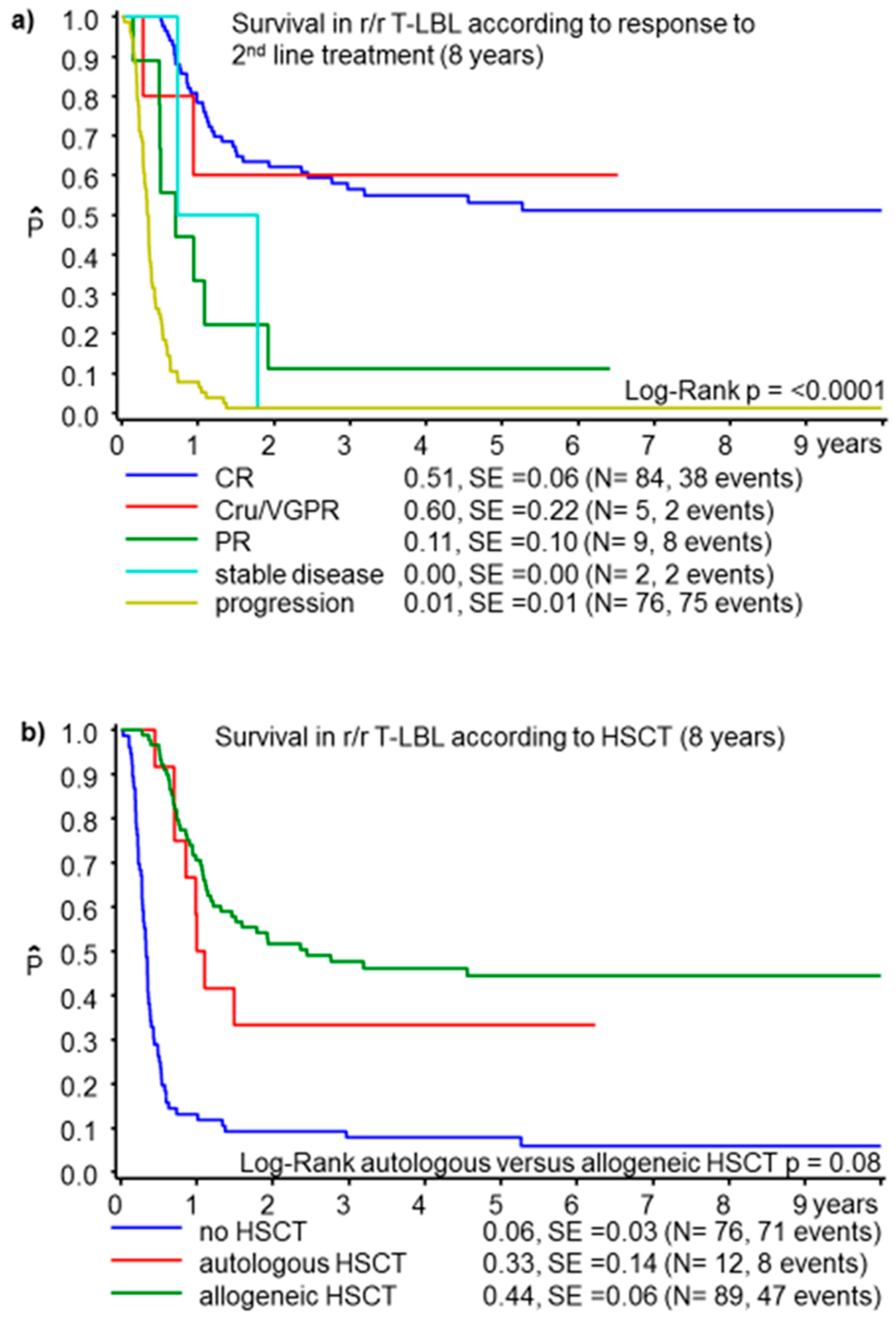
3.1. T-Cell Lymphoblastic Lymphoma
3.2. Precursor B-Cell Lymphoblastic Lymphoma
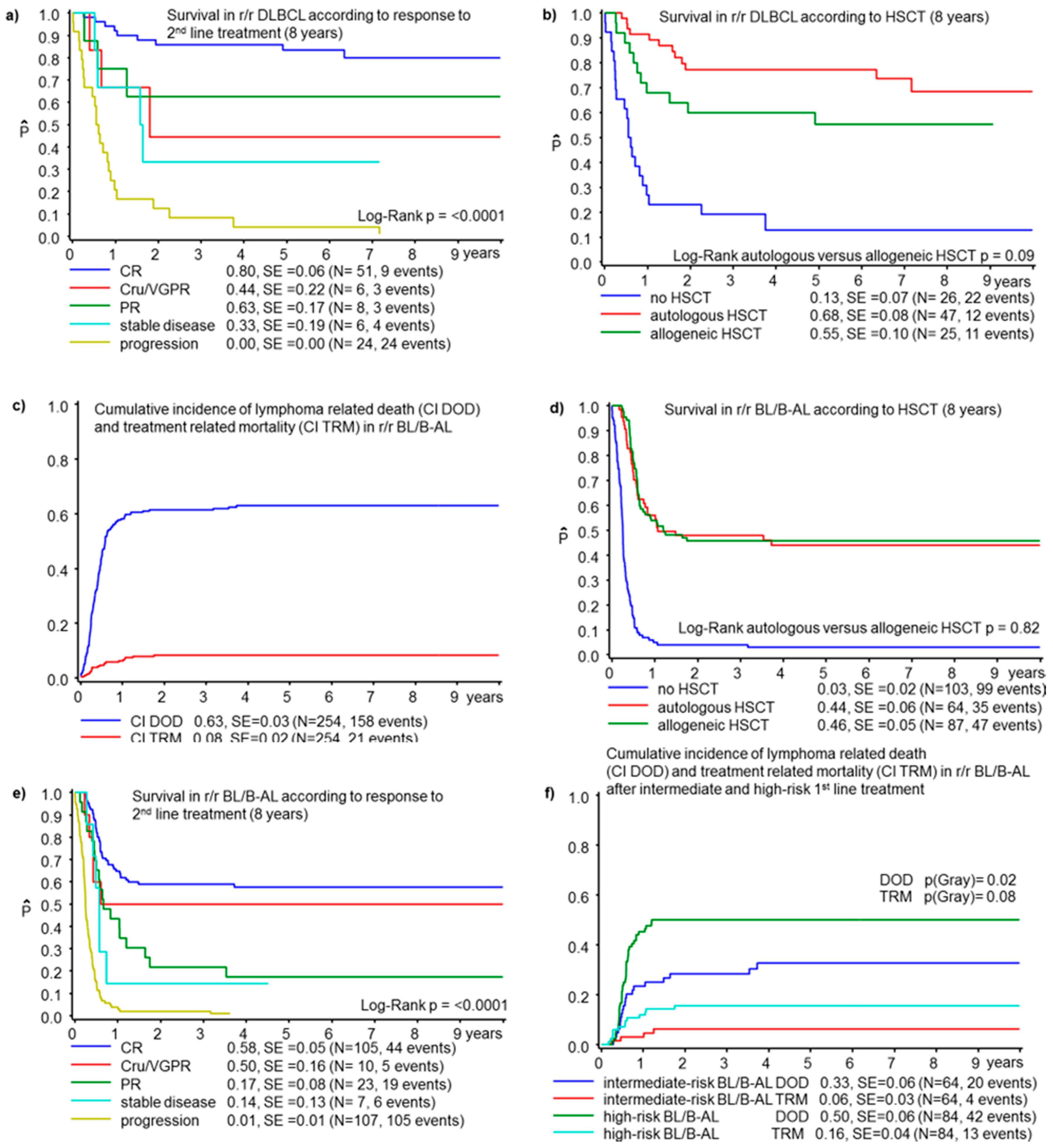
3.3. Diffuse Large B-Cell Lymphoma
3.4. Burkitt Lymphoma/Leukemia
3.4.1. Second-Line Treatment in r/r BL/B-AL
3.4.2. HSCT in BL/B-AL
3.4.3. Variables Associated with Survival in r/r BL/B-AL
3.5. Rituximab in First-Line Treatment of Mature B-NHL
4. Discussion
4.1. Lymphoblastic Lymphoma
4.2. Diffuse Large B-Cell Lymphoma
4.3. Burkitt Lymphoma/Leukemia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minard-Colin, V.; Brugieres, L.; Reiter, A.; Cairo, M.S.; Gross, T.G.; Woessmann, W.; Burkhardt, B.; Sandlund, J.T.; Williams, D.; Pillon, M.; et al. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J. Clin. Oncol. 2015, 33, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Schrappe, M.; Parwaresch, R.; Henze, G.; Muller-Weihrich, S.; Sauter, S.; Sykora, K.W.; Ludwig, W.D.; Gadner, H.; Riehm, H. Non-Hodgkin’s lymphomas of childhood and adolescence: Results of a treatment stratified for biologic subtypes and stage--a report of the Berlin-Frankfurt-Munster Group. J. Clin. Oncol. 1995, 13, 359–372. [Google Scholar] [CrossRef]
- Maramattom, L.V.; Hari, P.N.; Burns, L.J.; Carreras, J.; Arcese, W.; Cairo, M.S.; Costa, L.J.; Fenske, T.S.; Lill, M.; Freytes, C.O.; et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: A report from the center for international blood and marrow transplant research. Biol. Blood Marrow Transpl. 2013, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Le Deley, M.C.; Reiter, A.; Williams, D.; Delsol, G.; Oschlies, I.; McCarthy, K.; Zimmermann, M.; Brugieres, L. Prognostic factors in childhood anaplastic large cell lymphoma: Results of a large European intergroup study. Blood 2008, 111, 1560–1566. [Google Scholar] [CrossRef]
- Mitsui, T.; Mori, T.; Fujita, N.; Inada, H.; Horibe, K.; Tsurusawa, M.; Lymphoma Committee, J.P.L.L.S.G. Retrospective analysis of relapsed or primary refractory childhood lymphoblastic lymphoma in Japan. Pediatr. Blood Cancer 2009, 52, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Reiter, A.; Landmann, E.; Lang, P.; Lassay, L.; Dickerhoff, R.; Lakomek, M.; Henze, G.; von Stackelberg, A. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: A report from the berlin-frankfurt-muenster group. J. Clin. Oncol. 2009, 27, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Landmann, E.; Burkhardt, B.; Zimmermann, M.; Meyer, U.; Woessmann, W.; Klapper, W.; Wrobel, G.; Rosolen, A.; Pillon, M.; Escherich, G.; et al. Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica 2017, 102, 2086–2096. [Google Scholar] [CrossRef]
- Gross, T.G.; Hale, G.A.; He, W.; Camitta, B.M.; Sanders, J.E.; Cairo, M.S.; Hayashi, R.J.; Termuhlen, A.M.; Zhang, M.J.; Davies, S.M.; et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol. Blood Marrow Transpl. 2010, 16, 223–230. [Google Scholar] [CrossRef]
- Ducassou, S.; Ferlay, C.; Bergeron, C.; Girard, S.; Laureys, G.; Pacquement, H.; Plantaz, D.; Lutz, P.; Vannier, J.P.; Uyttebroeck, A.; et al. Clinical presentation, evolution, and prognosis of precursor B-cell lymphoblastic lymphoma in trials LMT96, EORTC 58881, and EORTC 58951. Br. J. Haematol. 2011, 152, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Michaux, K.; Bergeron, C.; Gandemer, V.; Mechinaud, F.; Uyttebroeck, A.; Bertrand, Y.; SFCE; The EORTC Children Leukemia Group. Relapsed or Refractory Lymphoblastic Lymphoma in Children: Results and Analysis of 23 Patients in the EORTC 58951 and the LMT96 Protocols. Pediatr. Blood Cancer 2016, 63, 1214–1221. [Google Scholar] [CrossRef]
- Mitsui, T.; Fujita, N.; Koga, Y.; Fukano, R.; Osumi, T.; Hama, A.; Koh, K.; Kakuda, H.; Inoue, M.; Fukuda, T.; et al. The effect of graft-versus-host disease on outcomes after allogeneic stem cell transplantation for refractory lymphoblastic lymphoma in children and young adults. Pediatr. Blood Cancer 2020, 67, e28129. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Gerrard, M.; Sposto, R.; Auperin, A.; Pinkerton, C.R.; Michon, J.; Weston, C.; Perkins, S.L.; Raphael, M.; McCarthy, K.; et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007, 109, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Patte, C.; Auperin, A.; Gerrard, M.; Michon, J.; Pinkerton, R.; Sposto, R.; Weston, C.; Raphael, M.; Perkins, S.L.; McCarthy, K.; et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood 2007, 109, 2773–2780. [Google Scholar] [CrossRef]
- Gerrard, M.; Cairo, M.S.; Weston, C.; Auperin, A.; Pinkerton, R.; Lambilliote, A.; Sposto, R.; McCarthy, K.; Lacombe, M.J.; Perkins, S.L.; et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: Results of the FAB/LMB 96 international study. Br. J. Haematol. 2008, 141, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Seidemann, K.; Mann, G.; Zimmermann, M.; Burkhardt, B.; Oschlies, I.; Ludwig, W.D.; Klingebiel, T.; Graf, N.; Gruhn, B.; et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: A report of the BFM Group Study NHL-BFM95. Blood 2005, 105, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.; Di Tullio, M.T.; Garaventa, A.; Cesaro, S.; Putti, M.C.; Favre, C.; Lippi, A.; Surico, G.; Di Cataldo, A.; D’Amore, E.; et al. Long-term results of the first Italian Association of Pediatric Hematology and Oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92). Cancer 2004, 101, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.; Mussolin, L.; Carraro, E.; Conter, V.; Arico, M.; Vinti, L.; Garaventa, A.; Piglione, M.; Buffardi, S.; Sala, A.; et al. Detection of prognostic factors in children and adolescents with Burkitt and Diffuse Large B-Cell Lymphoma treated with the AIEOP LNH-97 protocol. Br. J. Haematol. 2016, 175, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Osumi, T.; Mori, T.; Fujita, N.; Saito, A.M.; Nakazawa, A.; Tsurusawa, M.; Kobayashi, R. Relapsed/refractory pediatric B-cell non-Hodgkin lymphoma treated with rituximab combination therapy: A report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr. Blood Cancer 2016, 63, 1794–1799. [Google Scholar] [CrossRef]
- Rigaud, C.; Auperin, A.; Jourdain, A.; Haouy, S.; Couec, M.L.; Aladjidi, N.; Gandemer, V.; Lambliotte, A.; Plat, G.; Landman-Parker, J.; et al. Outcome of relapse in children and adolescents with B-cell non-Hodgkin lymphoma and mature acute leukemia: A report from the French LMB study. Pediatr. Blood Cancer 2019, 66, e27873. [Google Scholar] [CrossRef]
- Woessmann, W.; Zimmermann, M.; Meinhardt, A.; Müller, S.; Hauch, H.; Knörr, F.; Oschlies, I.; Klapper, W.; Niggli, F.; Kabickova, E.; et al. Progressive or relapsed Burkitt lymphoma or leukemia in children and adolescents after BFM-type first-line therapy. Blood 2020, 135, 1124–1132. [Google Scholar] [CrossRef]
- Fujita, N.; Kobayashi, R.; Atsuta, Y.; Iwasaki, F.; Suzumiya, J.; Sasahara, Y.; Inoue, M.; Koh, K.; Hori, T.; Goto, H.; et al. Hematopoietic stem cell transplantation in children and adolescents with relapsed or refractory B-cell non-Hodgkin lymphoma. Int. J. Hematol. 2019, 109, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, A.; Auperin, A.; Minard-Colin, V.; Aladjidi, N.; Zsiros, J.; Coze, C.; Gandemer, V.; Bertrand, Y.; Leverger, G.; Bergeron, C.; et al. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Societe Francaise des Cancers de l’Enfant study. Haematologica 2015, 100, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.; Auperin, A.; Perkins, S.L.; Pinkerton, R.; Harrison, L.; Goldman, S.; Patte, C. Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: A report from the FAB/LMB 96 study group. Br. J. Haematol. 2018, 182, 859–869. [Google Scholar] [CrossRef]
- Anoop, P.; Sankpal, S.; Stiller, C.; Tewari, S.; Lancaster, D.L.; Khabra, K.; Taj, M.M. Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk. Lymphoma 2012, 53, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, E.S.; Lee, S.H.; Koo, H.H.; Kim, H.S.; Lyu, C.J.; Jun, S.E.; Lim, Y.T.; Baek, H.J.; Kook, H.; et al. Clinical outcome of relapsed or refractory burkitt lymphoma and mature B-cell lymphoblastic leukemia in children and adolescents. Cancer Res. Treat. 2014, 46, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Minard-Colin, V.; Auperin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Bollard, C.M.; et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, K.; Attarbaschi, A.; Abla, O.; Alexander, S.; Bomken, S.; Bubanska, E.; Chiang, A.; Csoka, M.; Fedorova, A.; Kabickova, E.; et al. Non-anaplastic peripheral T cell lymphoma in children and adolescents-an international review of 143 cases. Ann. Hematol. 2016, 95, 1295–1305. [Google Scholar] [CrossRef]
- Kontny, U.; Oschlies, I.; Woessmann, W.; Burkhardt, B.; Lisfeld, J.; Salzburg, J.; Janda, A.; Attarbaschi, A.; Niggli, F.; Zimmermann, M.; et al. Non-anaplastic peripheral T-cell lymphoma in children and adolescents--a retrospective analysis of the NHL-BFM study group. Br. J. Haematol. 2015, 168, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, M.; Waxman, I.M.; Sposto, R.; Auperin, A.; Perkins, S.L.; Goldman, S.; Harrison, L.; Pinkerton, R.; McCarthy, K.; Raphael, M.; et al. Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood 2013, 121, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Lisfeld, J.; Burkhardt, B.; Group, N.-B.S. Therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med. 2013, 369, 282. [Google Scholar] [CrossRef]
- Pillon, M.; Carraro, E.; Mussolin, L.; Conter, V.; Tondo, A.; Arico, M.; Mura, R.; Sala, A.; Vinti, L.; Buffardi, S.; et al. Primary mediastinal large B-cell lymphoma: Outcome of a series of pediatric patients treated with high-dose methotrexate and cytarabine plus anti-CD20. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.C.; Weitzman, S.; Weinstein, H.; Chang, M.; Cairo, M.; Hutchison, R.; Shiramizu, B.; Wiley, J.; Woods, D.; Barnich, M.; et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2009, 52, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.A.A.; Beishuizen, A.; Bhojwani, D.; Burkhardt, B.; Minard-Colin, V.; Norris, R.E.; Kabickova, E.; Pinarli, F.G.; Tacyildiz, N.; Howes, A.; et al. Ibrutinib plus CIT for R/R mature B-NHL in children (SPARKLE trial): Initial safety, pharmacokinetics, and efficacy. Leukemia 2020, 34, 2271–2275. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Klapper, W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br. J. Haematol. 2008, 142, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.D.J.; Scobie, N.; Norga, K.; Ligas, F.; Chiodin, D.; Burke, A.; Minard-Colin, V.; Adamson, P.; Marshall, L.V.; Balakumaran, A.; et al. ACCELERATE and European Medicine Agency Paediatric Strategy Forum for medicinal product development for mature B-cell malignancies in children. Eur. J. Cancer 2019, 110, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Takimoto, T.; Nakazawa, A.; Fujita, N.; Akazai, A.; Yamato, K.; Yazaki, M.; Deguchi, T.; Hashii, Y.; Kato, K.; et al. Inferior outcomes of stage III T lymphoblastic lymphoma relative to stage IV lymphoma and T-acute lymphoblastic leukemia: Long-term comparison of outcomes in the JACLS NHL T-98 and ALL T-97 protocols. Int. J. Hematol 2014, 99, 743–749. [Google Scholar] [CrossRef]
- Willasch, A.M.; Peters, C.; Sedlacek, P.; Dalle, J.H.; Kitra-Roussou, V.; Yesilipek, A.; Wachowiak, J.; Lankester, A.; Prete, A.; Hamidieh, A.A.; et al. Myeloablative conditioning for allo-HSCT in pediatric ALL: FTBI or chemotherapy?-A multicenter EBMT-PDWP study. Bone Marrow Transpl. 2020, 55, 1540–1551. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Levine, J.E.; Harris, R.E.; Loberiza, F.R., Jr.; Armitage, J.O.; Vose, J.M.; Van Besien, K.; Lazarus, H.M.; Horowitz, M.M.; Bashey, A.; Bolwell, B.J.; et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 2003, 101, 2476–2482. [Google Scholar] [CrossRef]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Ludwig, W.D.; Yakisan, E.; Zimmermann, M.; Mann, G.; Chott, A.; Ebell, W.; Klingebiel, T.; et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 1999, 94, 3294–3306. [Google Scholar] [PubMed]
- Grigg, A.; Ritchie, D. Graft-versus-lymphoma effects: Clinical review, policy proposals, and immunobiology. Biol. Blood Marrow Transpl. 2004, 10, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.L. Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Dan Med. Bull 2007, 54, 112–139. [Google Scholar]
- Schmitz, N.; Dreger, P.; Glass, B.; Sureda, A. Allogeneic transplantation in lymphoma: Current status. Haematologica 2007, 92, 1533–1548. [Google Scholar] [CrossRef][Green Version]
- Giulino-Roth, L.; Ricafort, R.; Kernan, N.A.; Small, T.N.; Trippett, T.M.; Steinherz, P.G.; Prockop, S.E.; Scaradavou, A.; Chiu, M.; O’Reilly, R.J.; et al. Ten-year follow-up of pediatric patients with non-Hodgkin lymphoma treated with allogeneic or autologous stem cell transplantation. Pediatr. Blood Cancer 2013, 60, 2018–2024. [Google Scholar] [CrossRef]
- Okur, F.V.; Krance, R. Stem cell transplantation in childhood non-Hodgkin’s lymphomas. Curr. Hematol. Malig. Rep. 2010, 5, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Aukema, S.M.; Theil, L.; Rohde, M.; Bauer, B.; Bradtke, J.; Burkhardt, B.; Bonn, B.R.; Claviez, A.; Gattenlohner, S.; Makarova, O.; et al. Sequential karyotyping in Burkitt lymphoma reveals a linear clonal evolution with increase in karyotype complexity and a high frequency of recurrent secondary aberrations. Br. J. Haematol. 2015, 170, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Pillon, M.; d’Amore, E.S.; Conter, V.; Piglione, M.; Lo Nigro, L.; Garaventa, A.; Buffardi, S.; Arico, M.; Rosolen, A. Minimal disseminated disease in high-risk Burkitt’s lymphoma identifies patients with different prognosis. J. Clin. Oncol. 2011, 29, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Schlesner, M.; Hoffmann, S.; Kreuz, M.; Leich, E.; Burkhardt, B.; Rosolowski, M.; Ammerpohl, O.; Wagener, R.; Bernhart, S.H.; et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat. Genet. 2012, 44, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Baleydier, F.; Lengline, E.; Ben Abdelali, R.; Petit, A.; Villarese, P.; Cieslak, A.; Minard-Colin, V.; Rullier, A.; Moreau, A.; et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J. Clin. Oncol. 2012, 30, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Bonn, B.R.; Rohde, M.; Zimmermann, M.; Krieger, D.; Oschlies, I.; Niggli, F.; Wrobel, G.; Attarbaschi, A.; Escherich, G.; Klapper, W.; et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood 2013, 121, 3153–3160. [Google Scholar] [CrossRef] [PubMed]
- Balbach, S.T.; Makarova, O.; Bonn, B.R.; Zimmermann, M.; Rohde, M.; Oschlies, I.; Klapper, W.; Rossig, C.; Burkhardt, B. Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia 2016, 30, 970–973. [Google Scholar] [CrossRef]
- Lopez, C.; Kleinheinz, K.; Aukema, S.M.; Rohde, M.; Bernhart, S.H.; Hubschmann, D.; Wagener, R.; Toprak, U.H.; Raimondi, F.; Kreuz, M.; et al. Genomic and transcriptomic changes complement each other in the pathogenesis of sporadic Burkitt lymphoma. Nat. Commun. 2019, 10, 1459. [Google Scholar] [CrossRef] [PubMed]
- Reutter, K.; Sandmann, S.; Rohde, J.; Müller, S.; Wöste, M.; Khanam, T.; Michgehl, U.; Klapper, W.; Wößmann, W.; Seggewiß, J.; et al. Reconstructing clonal evolution in relapsed and non-relapsed Burkitt lymphoma. Leukemia 2020. [Google Scholar] [CrossRef] [PubMed]
- Khanam, T.; Sandmann, S.; Seggewiss, J.; Ruether, C.M.; Zimmermann, M.; Norvil, A.B.; Bartenhagen, C.; Randau, G.; Mueller, S.; Herbrüggen, H.; et al. Integrative genomic analysis of pediatric T- cell lymphoblastic lymphoma reveals candidates of clinical significance. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Lovisa, F.; Carraro, E.; Primerano, S.; D’Amore, E.S.G.; Bonvini, P.; Nigro, L.L.; Vito, R.; Vinti, L.; Farruggia, P.; et al. Clinical impact of miR-223 expression in pediatric T-Cell lymphoblastic lymphoma. Oncotarget 2017, 8, 107886–107898. [Google Scholar] [CrossRef] [PubMed]

| Patients’ Characteristics and Association with Outcome | n | OS 8 y (±SE) (%) | p Value (Log-Rank) |
|---|---|---|---|
| Sex | |||
| Male | 464 | 34 ± 2 | |
| Female | 175 | 34 ± 4 | 0.70 |
| Age | |||
| <10 years | 287 | 38 ± 3 | |
| >10 years | 352 | 31 ± 3 | 0.084 |
| Period of diagnosis | |||
| 2000–07 | 324 | 34 ± 3 | |
| 2008–16 | 315 | 34 ± 3 | 0.65 |
| Initial stage of disease | |||
| I | 12 | 53 ± 16 | |
| II | 34 | 68 ± 8 | |
| III | 361 | 34 ± 3 | |
| IV | 218 | 28 ± 3 | 0.0003 |
| BM involvement at initial diagnosis | |||
| Yes | 191 | 25 ± 3 | |
| No | 435 | 38 ± 3 | 0.0003 |
| BM involvement at relapse | |||
| Yes | 198 | 28 ± 3 | |
| No | 435 | 36 ± 3 | 0.0002 |
| CNS involvement at initial diagnosis | |||
| Yes | 76 | 27 ± 5 | |
| No | 550 | 36 ± 2 | 0.011 |
| CNS involvement at relapse | |||
| Yes | 128 | 32 ± 4 | |
| No | 505 | 34 ± 2 | 0.76 |
| Local relapse | |||
| Yes | 527 | 33 ± 2 | |
| No | 103 | 37 ± 5 | 0.34 |
| Response to first-line treatment | |||
| Refractory | 43 | 30 ± 7 | |
| Progression | 118 | 28 ± 4 | |
| Relapse | 478 | 36 ± 2 | 0.001 |
| Time to r/r disease | |||
| <3 months from the initial diagnosis | 76 | 25 ± 5 | |
| 3–6 months | 232 | 27 ± 3 | |
| 6–9 months | 104 | 34 ± 5 | |
| >9 months | 217 | 45 ± 4 | <0.0001 |
| Response to second-line treatment | |||
| 2nd CR | 294 | 61 ± 3 | |
| CRu or VGPR | 29 | 44 ± 12 | |
| PR | 54 | 34 ± 7 | |
| SD | 18 | 27 ± 11 | |
| Progression | 238 | 1 ± 1 | <0.0001 |
| HSCT | |||
| No HSCT | 238 | 8 ± 2 | |
| Autologous HSCT | 150 | 55 ± 5 | |
| Allogeneic HSCT | 251 | 47 ± 3 | <0.0001 |
| Patients’ Characteristics | No | pOS 8 y (±SE) | p Value (Log-Rank) | No HSCT n = 76 | Autologous HSCT n = 12 | Allogeneic HSCT n = 89 | p Value No HSCT vs. HSCT (Auto or Allo) (chi2) | p Value Auto vs. Allo (chi2) | |
|---|---|---|---|---|---|---|---|---|---|
| n = 177 | (%) | (%) | (%) | (%) | |||||
| Diagnosis | 2000–07 | 84 | 27 ± 5 | 46 | 58 | 47 | |||
| 2008–16 | 93 | 27 ± 5 | 0.82 | 54 | 42 | 53 | 0.7454 | 0.4685 | |
| Sex | Male | 137 | 28 ± 4 | 80 | 63 | 74 | |||
| Female | 40 | 24 ± 7 | 0.64 | 20 | 17 | 26 | 0.4297 | 0.4893 | |
| Age | <10 years | 83 | 35 ± 5 | 38 | 42 | 55 | |||
| ≥10 years | 94 | 20 ± 4 | 0.0032 | 62 | 58 | 45 | 0.0434 | 0.3827 | |
| Stage | I | 0 | 0 | 0 | 0 | ||||
| II | 4 | 50 ± 25 | 1 | 0 | 4 | ||||
| III | 119 | 21 ± 4 | 82 | 92 | 61 | ||||
| IV | 42 | 38 ± 8 | 0.019 | 17 | 8 | 35 | 0.0580 | 0.1133 | |
| Initial CNS disease | Yes | 11 | 51 ± 16 | 4 | 8 | 8 | |||
| No | 155 | 26 ± 4 | 0.19 | 96 | 92 | 92 | 0.2823 | 0.9907 | |
| Initial BM disease | Yes | 35 | 33 ± 8 | 16 | 8 | 28 | |||
| No | 131 | 24 ± 4 | 0.11 | 84 | 92 | 72 | 0.1268 | 0.1488 | |
| CNS disease at relapse | Yes | 33 | 45 ± 9 | 9 | 50 | 23 | |||
| No | 144 | 24 ± 4 | 0.14 | 91 | 50 | 77 | 0.0052 | 0.0406 | |
| BM disease at relapse | Yes | 62 | 27 ± 6 | 38 | 8 | 36 | |||
| No | 115 | 27 ± 4 | 0.41 | 62 | 92 | 64 | 0.4490 | 0.0555 | |
| Local relapse | Yes | 144 | 22 ± 4 | 90 | 67 | 76 | |||
| No | 33 | 49 ± 9 | 0.003 | 10 | 33 | 24 | 0.0162 | 0.4631 | |
| Response to first-line treatment | Refractory | 9 | 22 ± 14 | 9 | 0 | 2 | |||
| Progression | 19 | 37 ± 11 | 15 | 17 | 7 | ||||
| Relapse | 149 | 26 ± 4 | 0.46 | 76 | 84 | 91 | 0.0271 | 0.4374 | |
| Time to relapse | <3 months | 17 | 35 ± 12 | 12 | 9 | 8 | |||
| 3–6 months | 30 | 7 ± 5 | 26 | 18 | 10 | ||||
| 6–9 months | 20 | 12 ± 8 | 15 | 9 | 9 | ||||
| ≥9 months | 107 | 35 ± 5 | <0.0001 | 47 | 64 | 73 | 0.0091 | 0.8689 | |
| Response to second-line treatment | CR | 84 | 51 ± 6 | 9 | 91 | 75 | |||
| Cru/VGPR | 5 | 60 ± 22 | 0 | 0 | 6 | ||||
| PR | 9 | 11 ± 10 | 3 | 0 | 8 | ||||
| SD | 2 | 0 | 0 | 0 | 2 | ||||
| PD | 76 | 1 ± 1 | <0.0001 | 88 | 9 | 9 | <0.0001 | 0.7282 | |
| HSCT | No | 76 | 6 ± 3 | ||||||
| Autologous | 12 | 33 ± 13 | |||||||
| Allogeneic | 89 | 44 ± 6 | <0.0001 | ||||||
| Patients’ Characteristics | No | pOS 8 y (±SE) | p Value (Log-Rank) | No HSCT n = 26 | Autologous HSCT n =47 | Allogeneic HSCT n = 25 | p Value No HSCT vs. HSCT (Auto or Allo) (chi2) | p Value Auto vs. Allo (chi2) | |
|---|---|---|---|---|---|---|---|---|---|
| n = 98 | (%) | (%) | (%) | (%) | |||||
| Diagnosis | 2000–07 | 57 | 51 ± 7 | 77 | 55 | 44 | |||
| 2008–16 | 41 | 52 ± 8 | 0.93 | 23 | 44 | 56 | 0.0237 | 0.3603 | |
| Sex | Male | 60 | 58 ± 7 | 62 | 70 | 44 | |||
| Female | 38 | 40 ± 8 | 0.12 | 39 | 30 | 56 | 0.9694 | 0.0298 | |
| Age | <10 years | 39 | 55 ± 9 | 42 | 40 | 36 | |||
| >10 years | 59 | 47 ± 7 | 0.45 | 58 | 69 | 64 | 0.7602 | 0.7138 | |
| Stage | I | 4 | 75 ± 22 | 4 | 6 | 0 | |||
| II | 10 | 80 ± 13 | 4 | 17 | 4 | ||||
| III | 64 | 44 ± 7 | 72 | 60 | 72 | ||||
| IV | 19 | 52 ± 12 | 0.32 | 20 | 17 | 24 | 0.6847 | 0.2025 | |
| Initial CNS disease | Yes | 10 | 48 ± 16 | 8 | 6 | 20 | |||
| No | 87 | 51 ± 6 | 0.50 | 92 | 94 | 80 | 0.6594 | 0.0801 | |
| Initial BM disease | Yes | 11 | 64 ± 15 | 12 | 13 | 8 | |||
| No | 86 | 49 ± 6 | 0.68 | 88 | 87 | 92 | 0.9703 | 0.5763 | |
| CNS disease at relapse | Yes | 13 | 42 ± 15 | 12 | 11 | 20 | |||
| No | 83 | 50 ± 6 | 0.47 | 88 | 89 | 80 | 0.7267 | 0.3085 | |
| BM disease at relapse | Yes | 12 | 67 ± 14 | 12 | 11 | 16 | |||
| No | 84 | 47 ± 6 | 0.41 | 88 | 89 | 84 | 0.8622 | 0.5582 | |
| Local relapse | Yes | 89 | 51 ± 6 | 96 | 98 | 88 | |||
| No | 5 | 40 ± 22 | 0.44 | 4 | 2 | 12 | 0.7316 | 0.0819 | |
| Response to first-line treatment | Refractory | 9 | 33 ± 16 | 8 | 9 | 12 | |||
| Progression | 22 | 35 ± 10 | 31 | 17 | 24 | ||||
| Relapse | 67 | 58 ± 7 | 0.016 | 62 | 75 | 64 | 0.4920 | 0.6487 | |
| Time to relapse | <3 months | 12 | 25 ± 13 | 23 | 6 | 13 | |||
| 3–6 months | 29 | 44 ± 11 | 27 | 28 | 38 | ||||
| 6–9 months | 20 | 43 ± 13 | 15 | 28 | 13 | ||||
| >9 months | 36 | 70 ± 8 | 0.011 | 35 | 38 | 38 | 0.2694 | 0.4198 | |
| Response to second-line treatment | CR | 51 | 80 ± 6 | 16 | 59 | 83 | |||
| Cru/VGPR | 6 | 44 ± 22 | 0 | 11 | 4 | ||||
| PR | 8 | 63 ± 17 | 0 | 13 | 8 | ||||
| SD | 6 | 33 ± 19 | 0 | 13 | 0 | ||||
| PD | 24 | 0 | <0.0001 | 84 | 4 | 4 | <0.0001 | 0.2235 | |
| HSCT | No | 26 | 13 ± 7 | ||||||
| Autologous | 47 | 68 ± 8 | |||||||
| Allogeneic | 25 | 55 ± 10 | <0.0001 | ||||||
| Patients’ Charateristics | No | pOS 8 y (±SE) | p Value (Log-Rank) | No HSCT n = 103 | Autologous HSCT n = 64 | Allogeneic HSCT n = 87 | p Value No HSCT vs. HSCT (Auto or Allo) (chi2) | p Value Auto vs. Allo (chi2) | |
|---|---|---|---|---|---|---|---|---|---|
| n = 254 | (%) | (%) | (%) | (%) | |||||
| Diagnosis | 2000–07 | 135 | 26 ± 4 | 54 | 64 | 44 | |||
| 2008–16 | 119 | 30 ± 4 | 0.32 | 46 | 36 | 56 | 0.7477 | 0.0132 | |
| Sex | Male | 207 | 29 ± 3 | 77 | 92 | 79 | |||
| Female | 47 | 26 ± 6 | 0.30 | 23 | 8 | 21 | 0.1040 | 0.0295 | |
| Age | <10 years | 134 | 32 ± 4 | 49 | 47 | 62 | |||
| ≥10 years | 120 | 24 ± 4 | 0.18 | 52 | 53 | 38 | 0.2668 | 0.0633 | |
| Stage | I | 3 | 67 ± 27 | 1 | 3 | 0 | |||
| II | 14 | 57 ± 13 | 6 | 6 | 5 | ||||
| III | 107 | 31 ± 5 | 41 | 59 | 31 | ||||
| IV | 129 | 22 ± 4 | 0.15 | 52 | 31 | 64 | 0.9807 | 0.0005 | |
| Initial CNS disease | Yes | 46 | 13 ± 5 | 19 | 11 | 23 | |||
| No | 207 | 31 ± 3 | 0.039 | 81 | 89 | 77 | 0.8800 | 0.0562 | |
| Initial BM disease | Yes | 121 | 20 ± 4 | 49 | 30 | 60 | |||
| No | 132 | 36 ± 4 | 0.069 | 51 | 70 | 40 | 0.7548 | 0.0003 | |
| CNS disease at relapse | Yes | 67 | 25 ± 5 | 20 | 27 | 35 | |||
| No | 185 | 29 ± 3 | 0.74 | 80 | 73 | 65 | 0.0386 | 0.3285 | |
| BM disease at relapse | Yes | 97 | 25 ± 4 | 46 | 21 | 43 | |||
| No | 155 | 30 ± 4 | 0.091 | 54 | 79 | 57 | 0.0413 | 0.0050 | |
| Local relapse | Yes | 203 | 28 ± 3 | 87 | 79 | 75 | |||
| No | 48 | 29 ± 7 | 0.44 | 13 | 21 | 25 | 0.0388 | 0.5061 | |
| Response to first-line treatment | Refractory | 12 | 8 ± 8 | 6 | 6 | 2 | |||
| Progression | 56 | 12 ± 4 | 32 | 16 | 15 | ||||
| Relapse | 186 | 43 ± 4 | <0.0001 | 62 | 78 | 83 | 0.0037 | 0.4589 | |
| Time to relapse | <3 months | 36 | 17 ± 6 | 19 | 11 | 12 | |||
| 3–6 months | 142 | 22 ± 4 | 62 | 63 | 46 | ||||
| 6–9 months | 46 | 38 ± 7 | 14 | 19 | 24 | ||||
| ≥9 months | 26 | 57 ± 10 | <0.0001 | 6 | 8 | 18 | 0.0400 | 0.1770 | |
| Response to second-line treatment | CR | 105 | 58 ± 5 | 5 | 62 | 70 | |||
| Cru/VGPR | 10 | 50 ± 16 | 0 | 6 | 7 | ||||
| PR | 23 | 17 ± 8 | 1 | 16 | 14 | ||||
| SD | 7 | 14 ± 13 | 0 | 6 | 3 | ||||
| PD | 107 | 1 ± 1 | <0.0001 | 94 | 10 | 6 | <0.0001 | 0.7609 | |
| HSCT | No | 103 | 3 ± 2 | ||||||
| Autologous | 64 | 44 ± 6 | |||||||
| Allogeneic | 87 | 46 ± 5 | <0.0001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhardt, B.; Taj, M.; Garnier, N.; Minard-Colin, V.; Hazar, V.; Mellgren, K.; Osumi, T.; Fedorova, A.; Myakova, N.; Verdu-Amoros, J.; et al. Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations. Cancers 2021, 13, 2075. https://doi.org/10.3390/cancers13092075
Burkhardt B, Taj M, Garnier N, Minard-Colin V, Hazar V, Mellgren K, Osumi T, Fedorova A, Myakova N, Verdu-Amoros J, et al. Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations. Cancers. 2021; 13(9):2075. https://doi.org/10.3390/cancers13092075
Chicago/Turabian StyleBurkhardt, Birgit, Mary Taj, Nathalie Garnier, Veronique Minard-Colin, Volkan Hazar, Karin Mellgren, Tomoo Osumi, Alina Fedorova, Natalia Myakova, Jaime Verdu-Amoros, and et al. 2021. "Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations" Cancers 13, no. 9: 2075. https://doi.org/10.3390/cancers13092075
APA StyleBurkhardt, B., Taj, M., Garnier, N., Minard-Colin, V., Hazar, V., Mellgren, K., Osumi, T., Fedorova, A., Myakova, N., Verdu-Amoros, J., Andres, M., Kabickova, E., Attarbaschi, A., Chiang, A. K. S., Bubanska, E., Donska, S., Hjalgrim, L. L., Wachowiak, J., Pieczonka, A., ... Pillon, M. (2021). Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations. Cancers, 13(9), 2075. https://doi.org/10.3390/cancers13092075








