Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation
Abstract
:Simple Summary
Abstract
1. Introduction
- Vascular normalization describes the correction of structural abnormalities by pruning immature branches, enhancing perivascular coverage, and reinstating the basal membrane [4]. This restores vascular functionality, in particular, the transportation of drugs to the tumor cells. Of all compounds used to achieve vascular normalization [8], VEGF inhibitors have been successful in clinical trials [9];
- Vascular permeabilization refers to the increase in capillary permeability due to the administration of inflammatory cytokines and vasomodulators, such as histamine, bradykinin, TNF-alpha, angiotensin II, botulinum neurotoxin and nitric oxide donors amongst others [5]. Some approaches use specific receptor-triggered endocytosis, i.e., employing the insulin-like growth factor 1 receptor to enable trafficking of compounds to the abluminal site [10];
- Overcoming the extracellular matrix (ECM) of tumors—extensive collagen networks are major obstacles for the penetration of therapeutic agents [11]. The use of collagen-degrading enzymes [12] or the downregulation of fibroblast activity [13] have shown great effectivity at improving the distribution of macromolecules;
- Hyperthermia is a simple, physical method that promotes drug delivery by increasing the local temperature of tissues to a range of 39–42 °C using tools such as microwaves, radiofrequency, and ultrasound. The induced capillary dilation increases perfusion and oxygenation, therefore enhancing the uptake and efficacy of chemotherapeutics [14];
- Sonodynamic therapy is a novel, rapidly developing treatment based on preferential uptake of sonosensitizing compounds in tumor tissues and subsequent activation of the drug by high-intensity focused ultrasound. This strategy is minimally invasive and may be administered to deeply situated tumors [18,19].
2. Materials and Methods
2.1. Animal Models
2.2. Synchrotron Microbeam Irradiation
2.3. Vascular Permeability Assay with FITC-Dextran
2.4. Semi-Thin Serial Sectioning and Transmission Electron Microscopy (TEM) of CAM
2.5. Immunostaining and Analysis of Glioblastoma Xenograft
2.6. Magnetic Resonance Imaging (MRI) of Glioma Xenografts
3. Results
3.1. Microbeams Induced a Transient Vascular “Permeability Window” in CAM without Impairing Tissue Perfusion
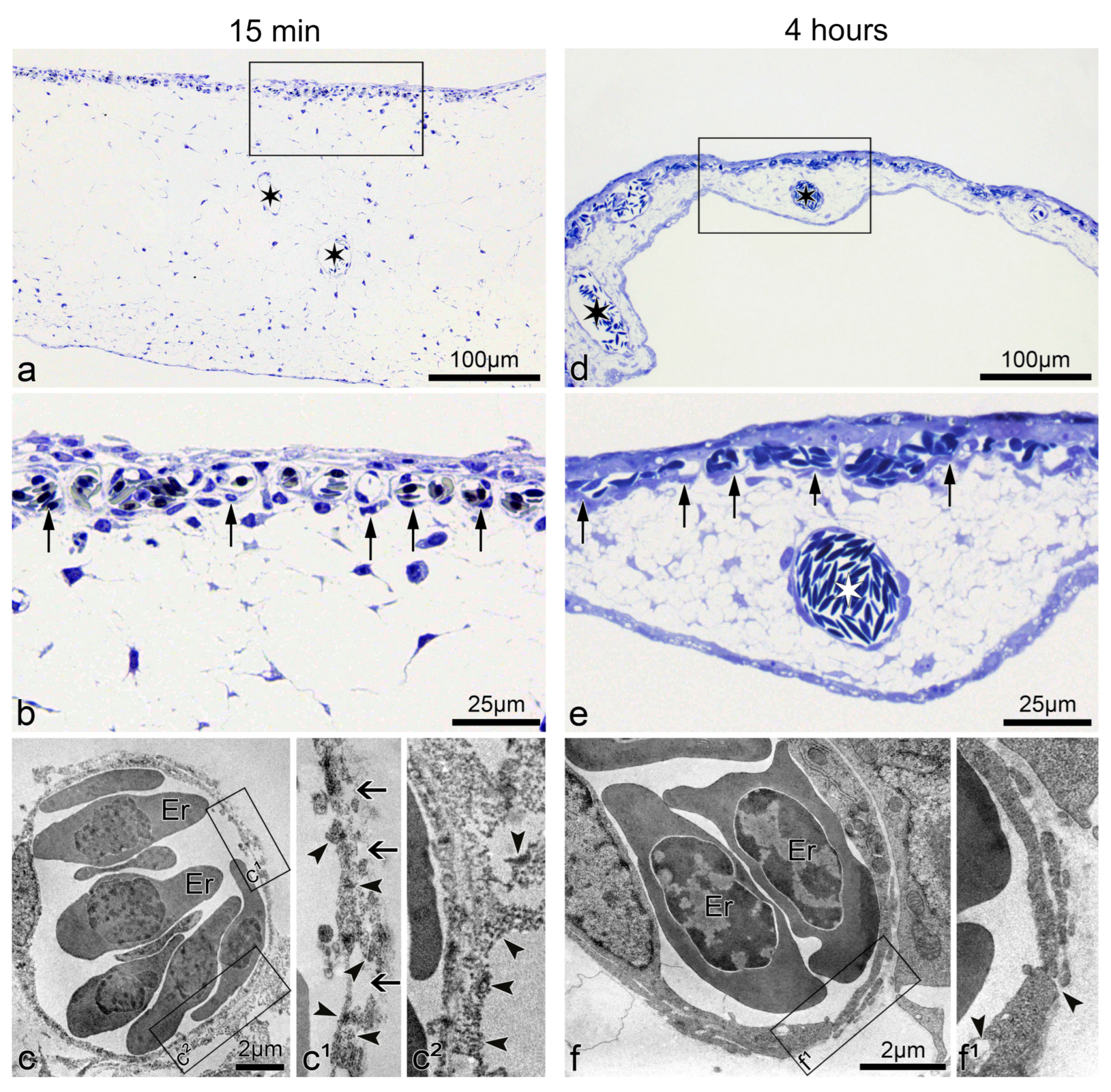
3.2. Time Course of the Structural Changes in the CAM during the Vascular “Permeability Window”
3.3. Microbeams also Induced Vascular Permeability in Human U-87 Glioblastoma Xenografts
3.4. Using the MRT-Induced Vascular Permeability to Enhance the Delivery of Cisplatin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2019, 21, 273. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y. Intratumoral drug delivery with nanoparticulate carriers. Pharm. Res. 2011, 28, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Tong, R.T.; Munn, L.L. Effect of Vascular Normalization by Antiangiogenic Therapy on Interstitial Hypertension, Peritumor Edema, and Lymphatic Metastasis: Insights from a Mathematical Model. Cancer Res. 2007, 67, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Corti, A. How to improve exposure of tumor cells to drugs—Promoter drugs increase tumor uptake and penetration of effector drugs. Adv. Drug Deliv. Rev. 2012, 64, 53–68. [Google Scholar] [CrossRef]
- Li, C.J.; Miyamoto, Y.; Kojima, Y.; Maeda, H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br. J. Cancer 1993, 67, 975–980. [Google Scholar] [CrossRef]
- Nagamitsu, A.; Greish, K.; Maeda, H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: Cases of advanced solid tumors. Jpn. J. Clin. Oncol. 2009, 39, 756–766. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.; Moonen, C.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and Physical Vessel Modulation Strategies to Improve EPR-mediated Drug Targeting to Tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V.; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control Release 2015, 201, 78–89. [Google Scholar] [CrossRef]
- Magzoub, M.; Jin, S.; Verkman, A.S. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008, 22, 276–284. [Google Scholar] [CrossRef]
- Unemori, E.N.; Amento, E.P. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J. Biol. Chem. 1990, 265, 10681–10685. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Bolkestein, M.; Gasselhuber, A.; Yatvin, J.; van Rhoon, G.C.; Eggermont, A.M.M.; Haemmerich, D.; Koning, G.A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control Release 2013, 167, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.-M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Dimcevski, G.; Gilja, O.H.; Hoem, D.; Postema, M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: A clinical case study. Med. Phys. 2013, 40, 072902. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kulkarni, S.; Mutalik, S. Liquid metal based theranostic nanoplatforms: Application in cancer therapy, imaging and biosensing. Nanomedicine 2020, 26, 102175. [Google Scholar] [CrossRef]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Laissue, J.A.; Hlushchuk, R.; Graber, W.; Bravin, A.; Bräuer-Krisch, E.; Corde, S.; Blattmann, H.; Gruber, G.; Djonov, V. Microbeam radiation-induced tissue damage depends on the stage of vascular maturation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1522–1532. [Google Scholar] [CrossRef]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; Von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Button, T.M.; Le Duc, G.; Zhong, N.; Peña, L.A.; Smith, J.A.L.; Martinez, S.R.; Bacarian, T.; Tammam, J.; Ren, B. Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro-Oncology 2002, 4, 26–38. [Google Scholar] [CrossRef]
- Regnard, P.; Le Duc, G.; Bräuer-Krisch, E.; Troprès, I.; Siegbahn, E.A.; Kusak, A.; Clair, C.; Bernard, H.; Dallery, D.; Laissue, J.A.; et al. Irradiation of intracerebral 9L gliosarcoma by a single array of microplanar x-ray beams from a synchrotron: Balance between curing and sparing. Phys. Med. Biol. 2008, 53, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Boumendjel, A.; Khalil, E.; Serduc, R.; Brauer, E.; Siegbahn, E.A.; Laissue, J.A.; Boutonnat, J. Chalcone JAI-51 improves efficacy of synchrotron microbeam radiation therapy of brain tumors. J. Synchrotron Radiat. 2012, 19, 478–482. [Google Scholar] [CrossRef]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar] [CrossRef]
- Miura, M.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Hanson, A.L.; Nawrocky, M.M.; Micca, P.L.; Slatkin, D.N.; Laissue, J.A. Radiosurgical palliation of aggressive murine SCCVII squamous cell carcinomas using synchrotron-generated X-ray microbeams. Br. J. Radiol. 2006, 79, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Koonce, N.A.; Dings, R.P.M.; Siegel, E.; Moros, E.G.; Bräuer-Krisch, E.; Corry, P.M. Microbeam Radiation Therapy Alters Vascular Architecture and Tumor Oxygenation and is Enhanced by a Galectin-1 Targeted Anti-Angiogenic Peptide. Radiat. Res. 2012, 177, 804–812. [Google Scholar] [CrossRef]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Le Duc, G.; Maisin, C.; Bräuer-Krisch, E.; Siegbahn, E.A.; Renaud, L.; Khalil, E.; Rémy, C.; Poillot, C.; et al. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9l gliosarcoma vascular networks. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1503–1512. [Google Scholar] [CrossRef]
- Serduc, R.; Vérant, P.; Vial, J.C.; Farion, R.; Rocas, L.; Rémy, C.; Fadlallah, T.; Brauer, E.; Bravin, A.; Laissue, J.; et al. In vivo two-photon microscopy study of short-term effects of microbeam irradiation on normal mouse brain microvasculature. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.R.; Christen, T.; Laissue, J.A.; Farion, R.R.; Bouchet, A.; van der Sanden, B.; Segebarth, C.; Brauer-Krisch, E.; Le Duc, G.G.; Bravin, A.; et al. Brain tumor vessel response to synchrotron microbeam radiation therapy: A short-term in vivo study. Phys. Med. Biol. 2008, 53, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Gebbers, J.O.; Laissue, J.A. Subacute neuropathological effects of microplanar beams of x-rays from a synchrotron wiggler. Proc. Natl. Acad. Sci. USA 1995, 92, 8783–8787. [Google Scholar] [CrossRef] [PubMed]
- Laissue, J.A.; Blattmann, H.; Di Michiel, M.; Slatkin, D.N.; Lyubimova, N.; Guzman, R.; Michiel, D.; Zimmermann, A.; Birrer, S.; Bey, T.; et al. The weaning piglet cerebellum: A surrogate for tolerance to MRT (microbeam radiation therapy) in paediatric neuro-oncology. Proc. SPIE 2001, 65–73. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Morris, G.M.; Le Duc, G.; Huang, X.; Ren, B.; Bacarian, T.; Allen, J.C.; Kalef-Ezra, J.; Orion, I.; Rosen, E.M.; et al. Response of avian embryonic brain to spatially segmented x-ray microbeams. Cell. Mol. Biol. 2001, 47, 485–493. [Google Scholar]
- Laissue, J.A.; Blattmann, H.; Wagner, H.P.; Grotzer, M.A.; Slatkin, D.N. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev. Med. Child Neurol. 2007, 49, 577–581. [Google Scholar] [CrossRef]
- Laissue, J.A.; Lyubimova, N.; Wagner, H.-P.; Archer, D.W.; Slatkin, D.N.; Di Michiel, M.; Nemoz, C.; Renier, M.; Brauer, E.; Spanne, P.O.; et al. Microbeam Radiation Therapy. 6 October 1999, Volume 3770. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/3770/1/Microbeam-radiation-therapy/10.1117/12.368185.short?SSO=1 (accessed on 30 April 2020).
- Potez, M.; Bouchet, A.; Wagner, J.; Donzelli, M.; Bräuer-Krisch, E.; Hopewell, J.W.; Laissue, J.; Djonov, V. Effects of Synchrotron X-Ray Micro-beam Irradiation on Normal Mouse Ear Pinnae. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 680–689. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef]
- Djonov, V.G.; Galli, A.B.; Burri, P.H. Intussusceptive arborization contributes to vascular tree formation in the chick chorio-allantoic membrane. Anat. Embryol. 2000, 202, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Perra, M.T.; Longo, V.; Maxia, C.; Annese, T.; Piras, F.; Murtas, D.; Sirigu, P. Erythropoietin is involved in angiogenesis in human primary melanoma. Int. J. Exp. Pathol. 2010, 91, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on anti-angiogenesis. Curr. Pharm. Biotechnol. 2000, 1, 73–82. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM): A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.-C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Chu, P.-Y.; Koh, A.P.-F.; Antony, J.; Huang, R.Y.-J. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Che, P.P.; Sarogni, P.; Sminia, P.; Giovannetti, E.; Voliani, V. Tumor grafted—Chick chorioallantoic membrane as an alternative model for biological cancer research and conventional/nanomaterial-based theranostics evaluation. Expert Opin. Drug Metab. Toxicol. 2021, 1–22. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Bravin, A.; Lerch, M.; Rosenfeld, A.; Stepanek, J.; Di Michiel, M.; Laissue, J.A. MOSFET dosimetry for microbeam radiation therapy at the European Synchrotron Radiation Facility. Med. Phys. 2003, 30, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.W. Collimator for Producing an Array of Microbeams. 1997. Available online: https://www.surechembl.org/document/US-5771270-A (accessed on 22 April 2021).
- Bräuer-Krisch, E.; Requardt, H.; Brochard, T.; Berruyer, G.; Renier, M.; Laissue, J.A.; Bravin, A. New technology enables high precision multislit collimators for microbeam radiation therapy. Rev. Sci. Instrum. 2009, 80, 074301. [Google Scholar] [CrossRef] [PubMed]
- FITC-Labelled Polysaccharides. Sigma-Aldrich. 2020. Available online: https://www.sigmaaldrich.com/technical-documents/articles/chemistry/fluorescently-labeled-dextrane.html (accessed on 13 April 2021).
- FluoSpheres Fluorescent Microspheres—Product Information 2005 by Molecular Probes. Available online: https://tools.thermofisher.com/content/sfs/manuals/mp05000.pdf (accessed on 30 April 2020).
- Working with FluoSpheres Fluorescent Microspheres—Product Information 2004 by Molecular Probes. Available online: http://tools.thermofisher.com/content/sfs/manuals/mp05001.pdf (accessed on 30 April 2020).
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive angiogenesis: Its role in embryonic vascular network formation. Circ. Res. 2000, 86, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.E.; Fritzell, S.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. The effect of locally delivered cisplatin is dependent on an intact immune function in an experimental glioma model. Sci. Rep. 2019, 9, 5632. [Google Scholar] [CrossRef]
- Jacobs, S.; McCully, C.L.; Murphy, R.F.; Bacher, J.; Balis, F.M.; Fox, E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother. Pharmacol. 2010, 65, 817–824. [Google Scholar] [CrossRef]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum Neurotoxicity Pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Luo, Z.; Dai, L.; Guo, X.; Cai, K. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today 2017, 15, 56–90. [Google Scholar] [CrossRef]
- Huang, P.; Wang, D.; Su, Y.; Huang, W.; Zhou, Y.; Cui, D.; Zhu, X.; Yan, D. Combination of Small Molecule Prodrug and Nanodrug Delivery: Amphiphilic Drug–Drug Conjugate for Cancer Therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Schraffordt Koops, H.; Liénard, D.; Kroon, B.B.; van Geel, A.N.; Hoekstra, H.J.; Lejeune, F.J. Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: A multicenter trial. J. Clin. Oncol. 1996, 14, 2653–2665. [Google Scholar] [CrossRef]
- Nakata, H.; Yoshimine, T.; Murasawa, A.; Kumura, E.; Harada, K.; Ushio, Y.; Hayakawa, T. Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir. 1995, 136, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Ferrara, N. Vascular Endothelial Growth Factor Signaling Pathways: Therapeutic Perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Storkebaum, E.; de Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatasso, S.; Fernandez-Palomo, C.; Hlushchuk, R.; Fazzari, J.; Tschanz, S.; Pellicioli, P.; Krisch, M.; Laissue, J.A.; Djonov, V. Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation. Cancers 2021, 13, 2103. https://doi.org/10.3390/cancers13092103
Sabatasso S, Fernandez-Palomo C, Hlushchuk R, Fazzari J, Tschanz S, Pellicioli P, Krisch M, Laissue JA, Djonov V. Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation. Cancers. 2021; 13(9):2103. https://doi.org/10.3390/cancers13092103
Chicago/Turabian StyleSabatasso, Sara, Cristian Fernandez-Palomo, Ruslan Hlushchuk, Jennifer Fazzari, Stefan Tschanz, Paolo Pellicioli, Michael Krisch, Jean A. Laissue, and Valentin Djonov. 2021. "Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation" Cancers 13, no. 9: 2103. https://doi.org/10.3390/cancers13092103
APA StyleSabatasso, S., Fernandez-Palomo, C., Hlushchuk, R., Fazzari, J., Tschanz, S., Pellicioli, P., Krisch, M., Laissue, J. A., & Djonov, V. (2021). Transient and Efficient Vascular Permeability Window for Adjuvant Drug Delivery Triggered by Microbeam Radiation. Cancers, 13(9), 2103. https://doi.org/10.3390/cancers13092103







