Full-Thickness Tumor Resection of Oral Cancer Involving the Facial Skin—Microsurgical Reconstruction of Extensive Defects after Radical Treatment of Advanced Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Therapeutic Procedures
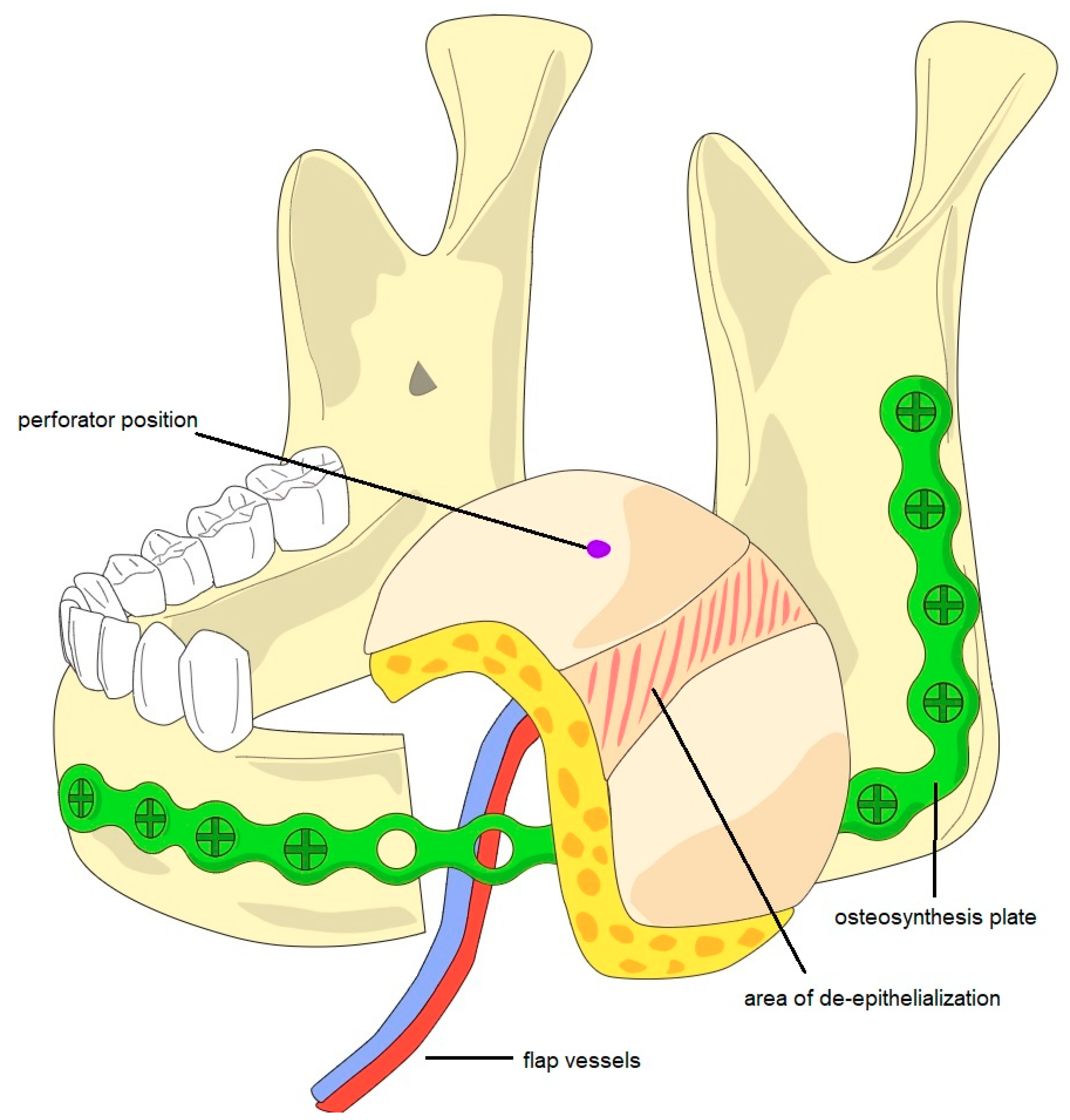
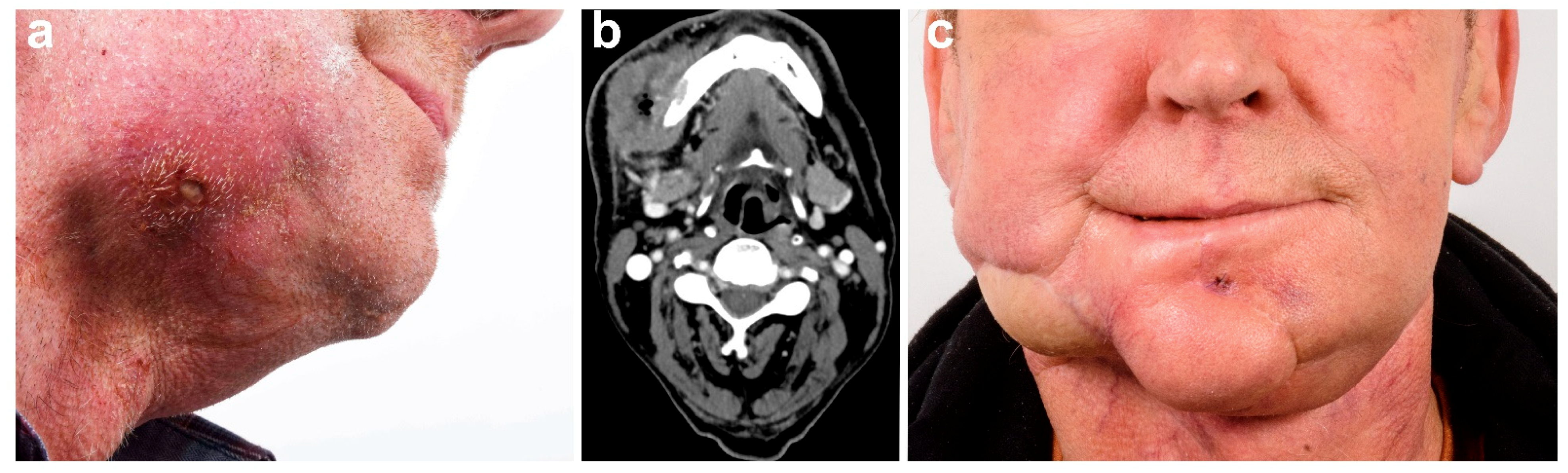
2.3. Surgical Technique
2.4. Perioperative Management
2.5. Outcome
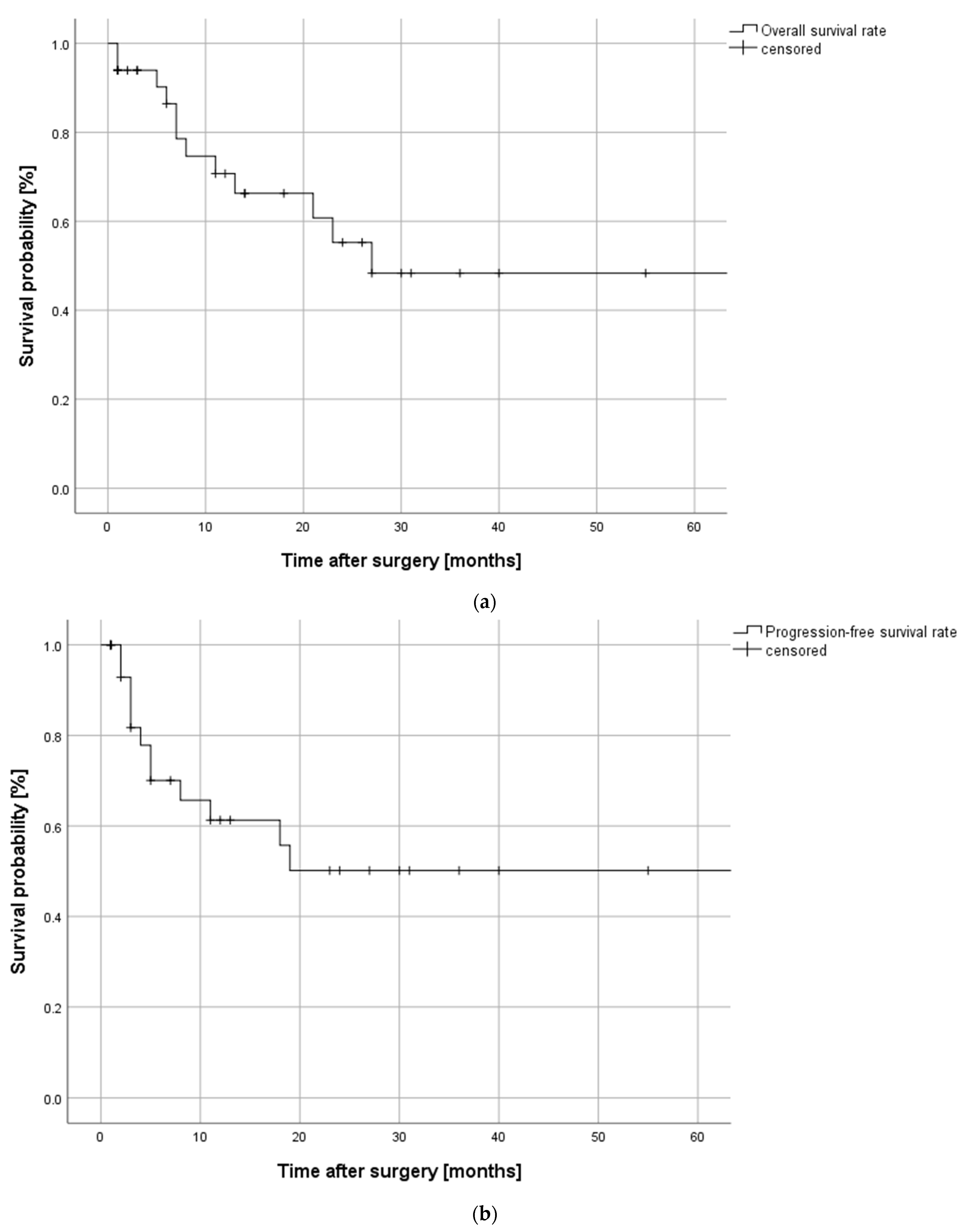
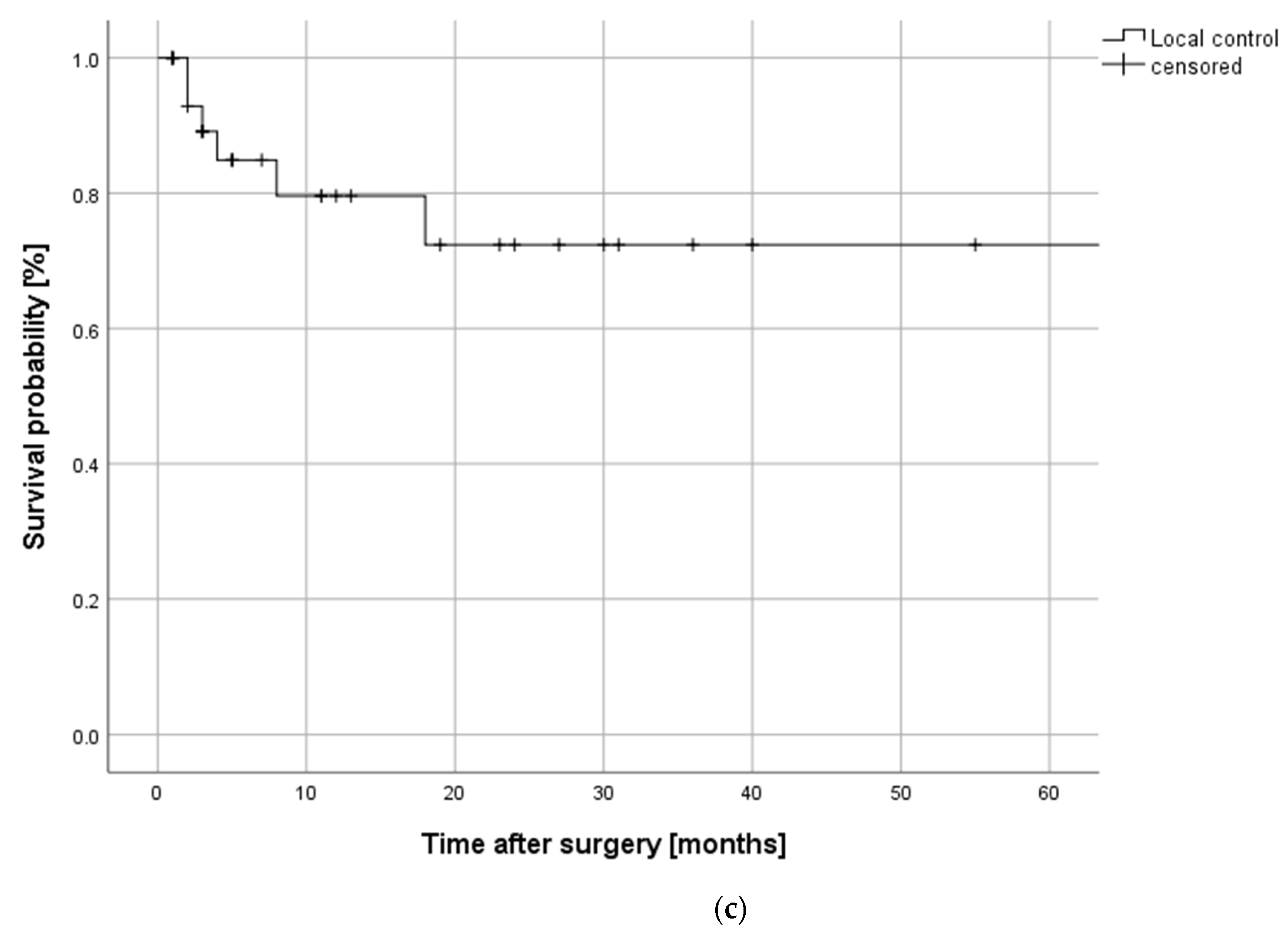
2.5.1. Oncological Outcome
2.5.2. Functional Outcome
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G., Jr.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Urken, M.L.; Weinberg, H.; Buchbinder, D.; Moscoso, J.F.; Lawson, W.; Catalano, P.J.; Biller, H.F. Microvascular free flaps in head and neck reconstruction. Report of 200 cases and review of complications. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 633–640. [Google Scholar] [CrossRef]
- McConnel, F.M.; Pauloski, B.R.; Logemann, J.A.; Rademaker, A.W.; Colangelo, L.; Shedd, D.; Carroll, W.; Lewin, J.; Johnson, J. Functional results of primary closure vs flaps in oropharyngeal reconstruction: A prospective study of speech and swallowing. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, G.; Chen, S.H.; Elia, R.; Sitpahul, N.; Chan, J.C.Y.; Losco, L.; Cigna, E.; Ribuffo, D.; Chen, H.C. Outcomes following head neck free flap reconstruction requiring interposition vein graft or vascular bridge flap. Head Neck 2019, 41, 2914–2920. [Google Scholar] [CrossRef]
- Podrecca, S.; Salvatori, P.; Squadrelli Saraceno, M.; Fallahdar, D.; Calabrese, L.; Cantu, G.; Molinari, R. Review of 346 patients with free-flap reconstruction following head and neck surgery for neoplasm. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, K.A.; Kobayashi, M.; Evans, G.R.D. Current options in head and neck reconstruction. Plast. Reconstr. Surg. 2006, 118, 122e–133e. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sanchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Group, E.W. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Madhura, M.G.; Rao, R.S.; Patil, S.; Fageeh, H.N.; Alhazmi, A.; Awan, K.H. Advanced diagnostic aids for oral cancer. Dis. Mon. 2020, 66, 101034. [Google Scholar] [CrossRef]
- Hinni, M.L.; Ferlito, A.; Brandwein-Gensler, M.S.; Takes, R.P.; Silver, C.E.; Westra, W.H.; Seethala, R.R.; Rodrigo, J.P.; Corry, J.; Bradford, C.R.; et al. Surgical margins in head and neck cancer: A contemporary review. Head Neck 2013, 35, 1362–1370. [Google Scholar] [CrossRef]
- Hosni, A.; Chiu, K.; Huang, S.H.; Xu, W.; Huang, J.; Bayley, A.; Bratman, S.V.; Cho, J.; Giuliani, M.; Kim, J.; et al. Non-operative management for oral cavity carcinoma: Definitive radiation therapy as a potential alternative treatment approach. Radiother. Oncol. 2020, 154, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Noronha, V.; Thiagarajan, S.; Joshi, A.; Chandrasekharan, A.; Talreja, V.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Juvekar, S.; et al. Salvage surgery in head and neck cancer: Does it improve outcomes? Eur. J. Surg. Oncol. 2020, 46, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Bodem, J.; Freudlsperger, C.; Zittel, S.; Weichert, W.; Hoffmann, J.; Freier, K. Outcome of heavily pretreated recurrent oral squamous cell carcinoma after salvage resection: A monocentric retrospective analysis. J. Cranio-Maxillofac. Surg. 2016, 44, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Zittel, S.; Moratin, J.; Metzger, K.; Ristow, O.; Krisam, J.; Bodem, J.; Engel, M.; Freudlsperger, C.; Hoffmann, J.; et al. Prospective feasibility analysis of salvage surgery in recurrent oral cancer in terms of quality of life. Oral Oncol. 2020, 102, 104580. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Leemans, C.R. Salvage surgery in head and neck cancer. Oral Dis. 2021, 27, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Baek, C.H.; Park, W.; Choi, N.; Gu, S.; Sohn, I.; Chung, M.K. Free flap outcome of salvage surgery compared to primary surgery for head and neck defects: A propensity score analysis. Oral Oncol. 2016, 62, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef]
- Elbers, J.B.W.; Veldhuis, L.I.; Bhairosing, P.A.; Smeele, L.E.; Jozwiak, K.; van den Brekel, M.W.M.; Verheij, M.; Al-Mamgani, A.; Zuur, C.L. Salvage surgery for advanced stage head and neck squamous cell carcinoma following radiotherapy or chemoradiation. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 647–655. [Google Scholar] [CrossRef]
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377. [Google Scholar] [CrossRef]
- Mijiti, A.; Kuerbantayi, N.; Zhang, Z.Q.; Su, M.Y.; Zhang, X.H.; Huojia, M. Influence of preoperative radiotherapy on head and neck free-flap reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2165–2180. [Google Scholar] [CrossRef]
- Halle, M.; Eriksson, B.O.; Docherty Skogh, A.C.; Sommar, P.; Hammarstedt, L.; Gahm, C. Improved Head and Neck Free Flap Outcome-Effects of a Treatment Protocol Adjustment from Pre- to Postoperative Radiotherapy. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1253. [Google Scholar] [CrossRef]
- Benatar, M.J.; Dassonville, O.; Chamorey, E.; Poissonnet, G.; Ettaiche, M.; Pierre, C.S.; Benezery, K.; Hechema, R.; Demard, F.; Santini, J.; et al. Impact of preoperative radiotherapy on head and neck free flap reconstruction: A report on 429 cases. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Kesting, M.R.; Holzle, F.; Wales, C.; Steinstraesser, L.; Wagenpfeil, S.; Mucke, T.; Rohleder, N.H.; Wolff, K.D.; Hasler, R.J. Microsurgical reconstruction of the oral cavity with free flaps from the anterolateral thigh and the radial forearm: A comparison of perioperative data from 161 cases. Ann. Surg. Oncol. 2011, 18, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Lazzeri, D.; Agostini, V.; Shokrollahi, K. Anterolateral thigh flap as the ideal flap to full-thickness cheek reconstruction. J. Craniofac. Surg. 2010, 21, 1897–1898. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Lazzeri, D.; Spinelli, G. Anterolateral thigh flap: Systematic literature review of specific donor-site complications and their management. J. Cranio-Maxillofac. Surg. 2013, 41, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Jonas, R.; Engel, M.; Freier, K.; Hoffmann, J.; Freudlsperger, C. A comparison of free anterolateral thigh and latissimus dorsi flaps in soft tissue reconstruction of extensive defects in the head and neck region. J. Cranio-Maxillofac. Surg. 2014, 42, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, S.; Boyd, J.B.; McCabe, S.; Gullane, P.; Rotstein, L.; Brown, D.; Yoo, J. Recipient vessels in head and neck microsurgery: Radiation effect and vessel access. Plast. Reconstr. Surg. 1993, 92, 628–632. [Google Scholar] [CrossRef]
- Demirkan, F.; Wei, F.C.; Chen, H.C.; Chen, I.H.; Hau, S.P.; Liau, C.T. Microsurgical reconstruction in recurrent oral cancer: Use of a second free flap in the same patient. Plast. Reconstr. Surg. 1999, 103, 829–838. [Google Scholar] [CrossRef]
- Jeng, S.F.; Kuo, Y.R.; Wei, F.C.; Su, C.Y.; Chien, C.Y. Reconstruction of concomitant lip and cheek through-and-through defects with combined free flap and an advancement flap from the remaining lip. Plast. Reconstr. Surg. 2004, 113, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, M.; Oshima, J.; Aihara, Y.; Nishijima, A.; Sekido, M. Free-flap reconstruction for full-thickness oral defects involving the oral commissure combined with oral modiolus reconstruction using a fascial sling. Microsurgery 2020, 40, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Savant, D.N.; Patel, S.G.; Deshmukh, S.P.; Gujarati, R.; Bhathena, H.M.; Kavarana, N.M. Folded free radial forearm flap for reconstruction of full-thickness defects of the cheek. Head Neck 1995, 17, 293–296. [Google Scholar] [CrossRef]
- Katou, F.; Shirai, N.; Kamakura, S.; Ohki, H.; Motegi, K. Full-thickness reconstruction of cheek defect involving oral commissure with forearm tendinocutaneous flap. Br. J. Oral Maxillofac. Surg. 1996, 34, 26–27. [Google Scholar] [CrossRef]

| Parameter | Number of Cases (%) |
|---|---|
| Gender | |
| Female | 12 (36.4) |
| Male | 21 (63.6) |
| Age | |
| Mean: 67 ± 13.7 years | |
| Range: 25–95 years | |
| Risk Factors | |
| Tobacco | 17 (51.5) |
| Alcohol | 10 (30.3) |
| Primary or Recurrent Tumor | |
| Primary tumor | 21 (63.6) |
| Recurrent tumor (local recurrence) | 12 (36.4) |
| Tumor Localization | |
| Mandible + Floor of the mouth | 20 (60.6) |
| Buccal mucosa | 8 (24.2) |
| Maxilla | 5 (15.2) |
| T Stage | |
| T1 | - |
| T2 | 1 (3.0) |
| T3 | 6 (18.2) |
| T4 | 26 (78.8) |
| N Stage | |
| 0 | 21 (63.6) |
| 1 | 1 (3.0) |
| 2a | - |
| 2b | 1 (3.0) |
| 2c | 2 (6.1) |
| 3 | 8 (24.2) |
| M Stage | |
| 0 | 33 (100) |
| 1 | - |
| UICC Stage | |
| 1 | - |
| 2 | - |
| 3 | 4 (12.1) |
| 4 | 29 (87.9) |
| Histopathological Tumor Features | |
| Lymphatic invasion | 15 (45.5) |
| Vascular invasion | 4 (12.1) |
| Perineural invasion | 12 (36.4) |
| Affected Subsite of the Oral Cavity | Free Flap (no.) | Success Rate |
|---|---|---|
| Mandible + Floor of the Mouth | ||
| ALT (n = 15) | 12/15 (80%) | |
| RFF (n = 1) | 1/1 (100%) | |
| Scapula (n = 1) | 1/1 (100%) | |
| Fibula (n = 2) | 2/2 (100%) | |
| Buccal Mucosa | ||
| ALT (n = 7) | 7/7 (100%) | |
| RFF (n = 1) | 1/1 (100%) | |
| Maxilla | ||
| ALT (n = 2) | 2/2 (100%) | |
| RFF (n = 1) | 1/1 (100%) | |
| Scapula (n = 2) | 0/2 (0%) |
| Type of Free Flap | Revision Rate | Success Rate |
|---|---|---|
| ALT | 4/24 (16.7%) | 21/24 (87.5%) |
| RFF | 0/3 | 3/3 (100%) |
| Scapula | 3/3 (100%) | 1/3 (33.3%) |
| Fibula | 0/2 | 2/2 (100%) |
| Latissimus dorsi | 0/1 | 1/1 (100%) |
| Advantages of ALT Flaps |
|
|
|
|
|
| Disadvantages of ALT Flaps |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moratin, J.; Mrosek, J.; Horn, D.; Metzger, K.; Ristow, O.; Zittel, S.; Engel, M.; Freier, K.; Hoffmann, J.; Freudlsperger, C. Full-Thickness Tumor Resection of Oral Cancer Involving the Facial Skin—Microsurgical Reconstruction of Extensive Defects after Radical Treatment of Advanced Squamous Cell Carcinoma. Cancers 2021, 13, 2122. https://doi.org/10.3390/cancers13092122
Moratin J, Mrosek J, Horn D, Metzger K, Ristow O, Zittel S, Engel M, Freier K, Hoffmann J, Freudlsperger C. Full-Thickness Tumor Resection of Oral Cancer Involving the Facial Skin—Microsurgical Reconstruction of Extensive Defects after Radical Treatment of Advanced Squamous Cell Carcinoma. Cancers. 2021; 13(9):2122. https://doi.org/10.3390/cancers13092122
Chicago/Turabian StyleMoratin, Julius, Jan Mrosek, Dominik Horn, Karl Metzger, Oliver Ristow, Sven Zittel, Michael Engel, Kolja Freier, Juergen Hoffmann, and Christian Freudlsperger. 2021. "Full-Thickness Tumor Resection of Oral Cancer Involving the Facial Skin—Microsurgical Reconstruction of Extensive Defects after Radical Treatment of Advanced Squamous Cell Carcinoma" Cancers 13, no. 9: 2122. https://doi.org/10.3390/cancers13092122
APA StyleMoratin, J., Mrosek, J., Horn, D., Metzger, K., Ristow, O., Zittel, S., Engel, M., Freier, K., Hoffmann, J., & Freudlsperger, C. (2021). Full-Thickness Tumor Resection of Oral Cancer Involving the Facial Skin—Microsurgical Reconstruction of Extensive Defects after Radical Treatment of Advanced Squamous Cell Carcinoma. Cancers, 13(9), 2122. https://doi.org/10.3390/cancers13092122






