Effectiveness and Safety of Robotic Radiosurgery for Optic Nerve Sheath Meningiomas: A Single Institution Series
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Variables
2.3. Cyberknife Treatment
2.4. Follow-Up
2.5. Analysis of the Dose Distribution
2.6. Statistics
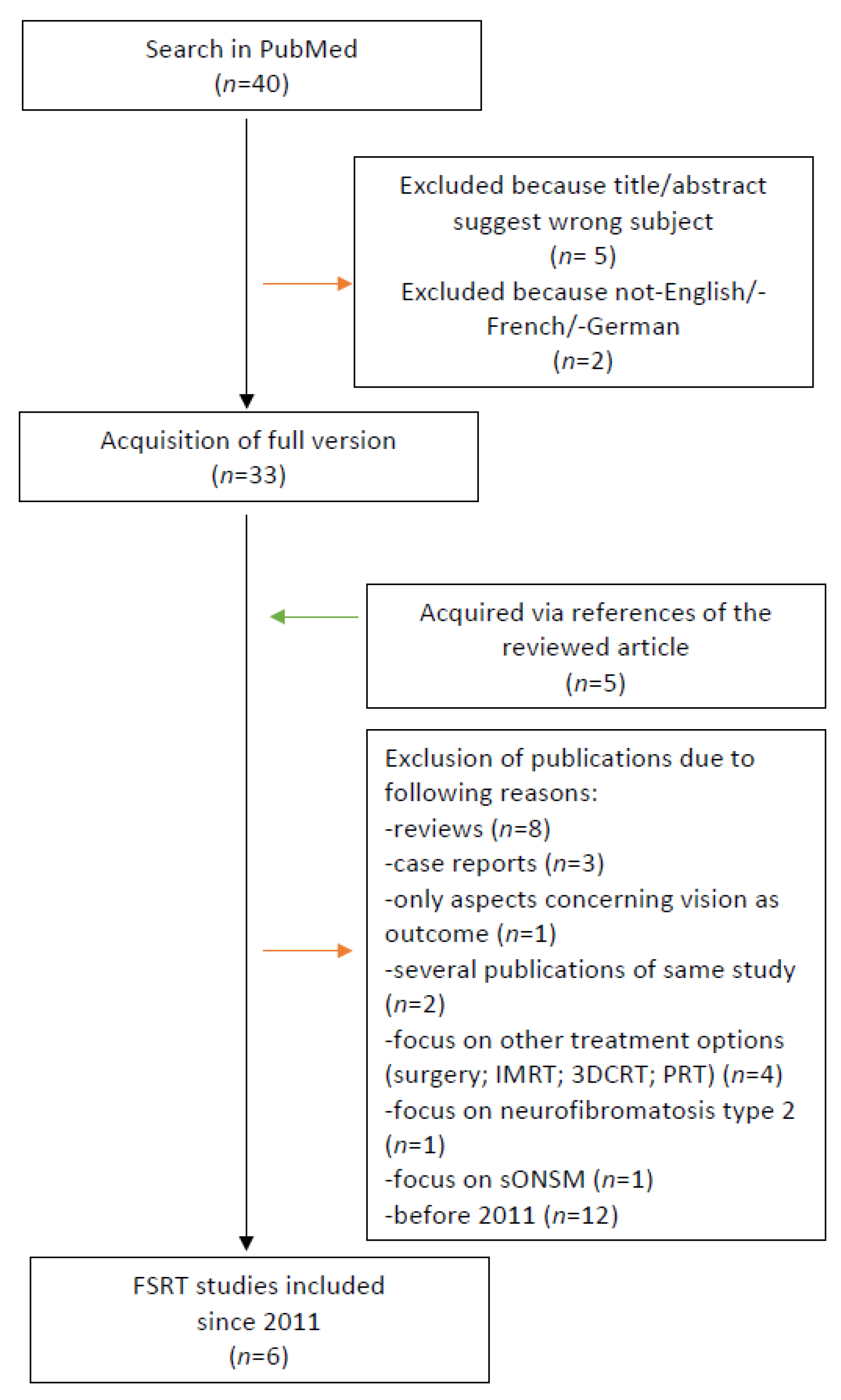
2.7. Research of the Literature
3. Results
3.1. Patient Characteristics
3.2. Treatment Characteristics
3.3. Local Tumor Control
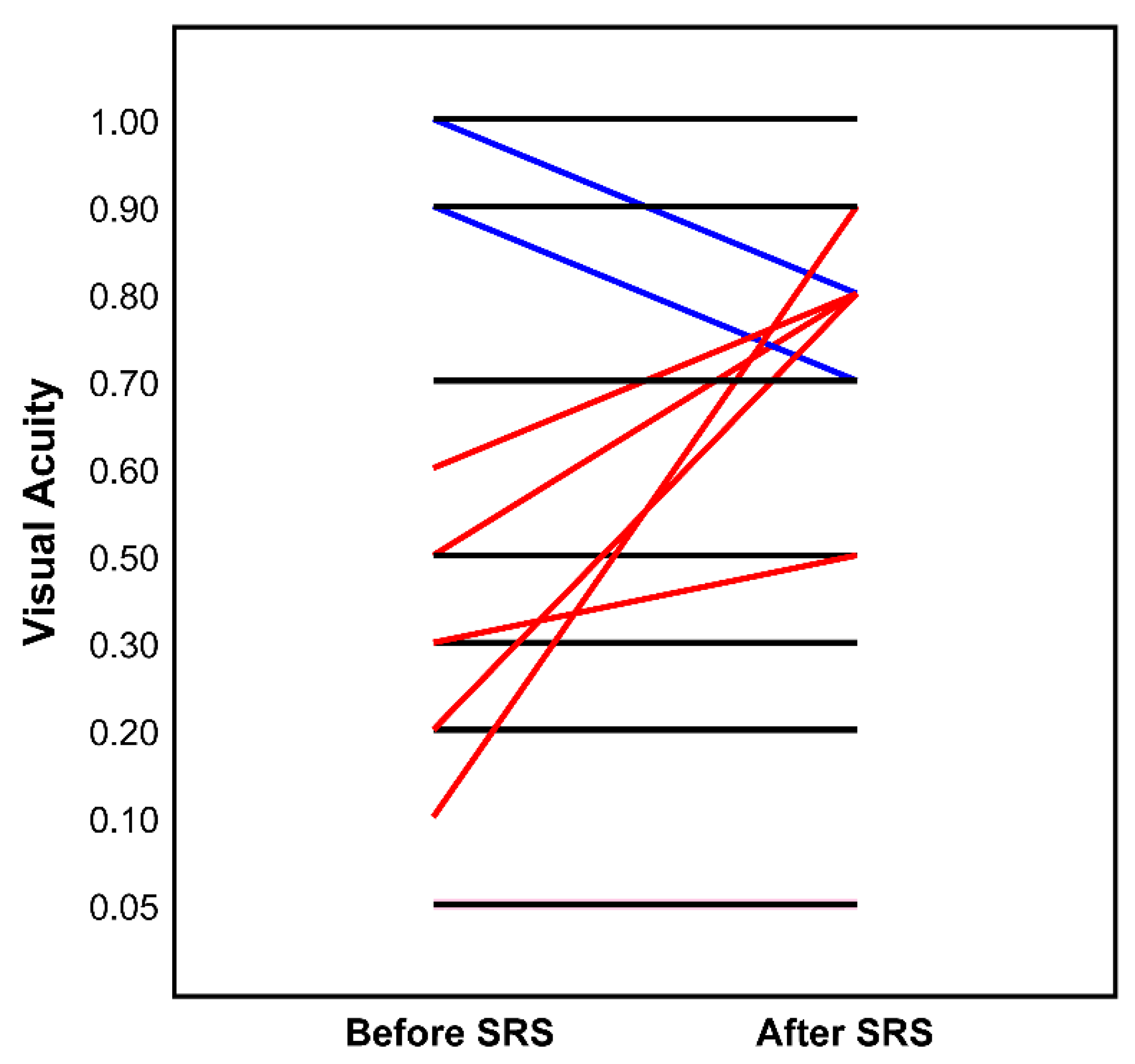
3.4. Morbidity and Outcome
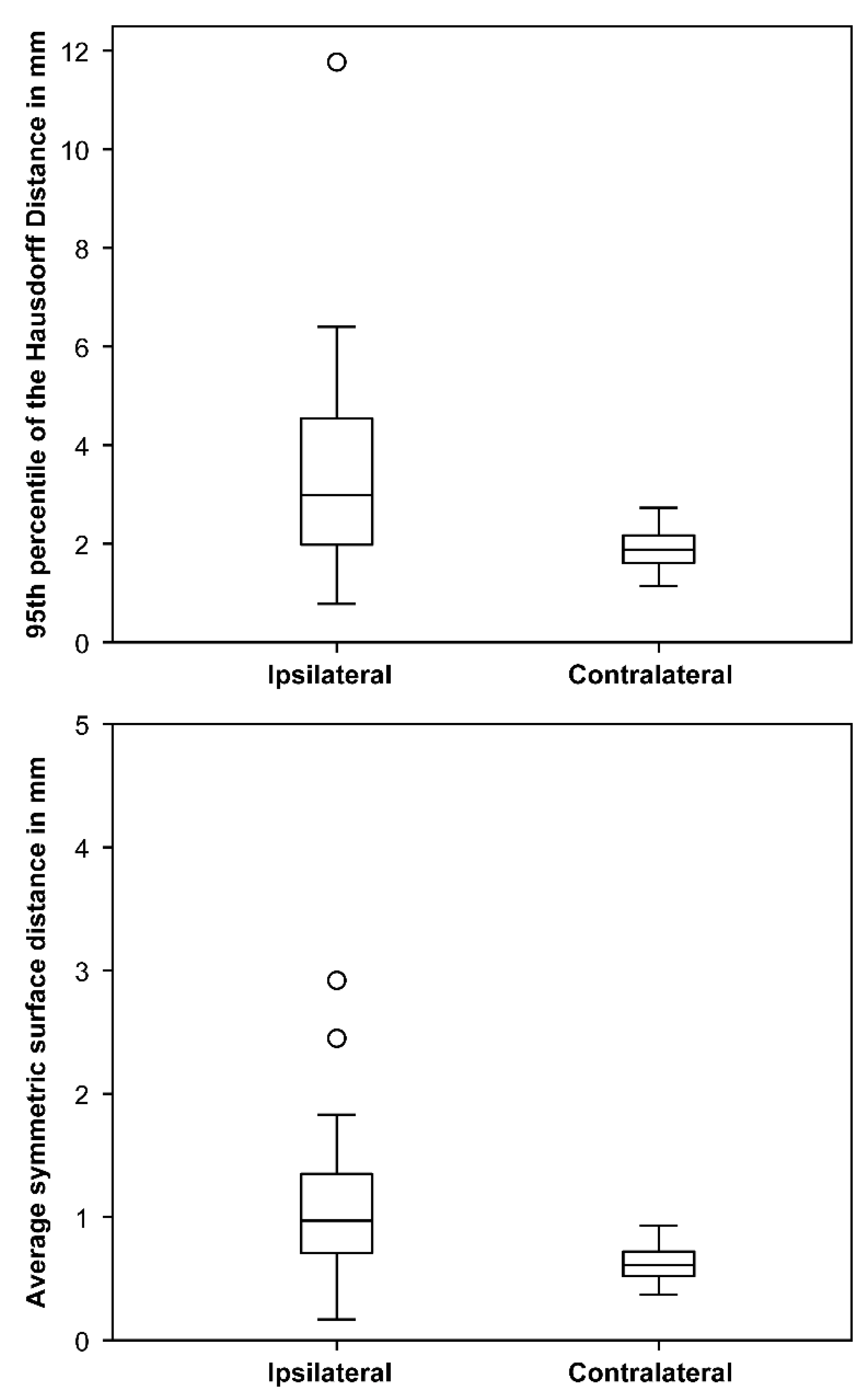
3.5. Optic Nerve Movement and Dose Uncertainties
3.6. Summary of the Literature
4. Discussion
4.1. Optic Nerve Movement and Dose Uncertainties
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulsen, F.; Doerr, S.; Wilhelm, H.; Becker, G.; Bamberg, M.; Claßen, J. Fractionated Stereotactic Radiotherapy in Patients with Optic Nerve Sheath Meningioma. Int. J. Radiat. Oncol. 2012, 82, 773–778. [Google Scholar] [CrossRef]
- Dutton, J.J. Optic nerve sheath meningiomas. Surv. Ophthalmol. 1992, 37, 167–183. [Google Scholar] [CrossRef]
- Spencer, W.H. Primary neoplasms of the optic nerve and its sheaths: Clinical features and current concepts of pathogenetic mechanisms. Trans. Am. Ophthalmol. Soc. 1972, 70, 490–528. [Google Scholar] [PubMed]
- Frisen, L.; Hoyt, W.F.; Tengroth, B.M. Optociliary veins, disc pallor and visual loss. Acta Ophthalmol. 2009, 51, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Pitz, S. Primary optic nerve sheath meningioma. Cancer 2007, 110, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R. Primary tumours of the optic nerve and its sheath. Eye 2004, 18, 1026–1037. [Google Scholar] [CrossRef]
- Conti, A.; Pontoriero, A.; Midili, F.; Iatì, G.; Siragusa, C.; Tomasello, C.; La Torre, D.; Cardali, S.M.; Pergolizzi, S.; De Renzis, C. CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: Intermediate-term results and radiobiological considerations. SpringerPlus 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Bosch, M.M.; Boltshauser, E.; Harpes, P.; Landau, K. Ophthalmologic Findings and Long-Term Course in Patients with Neurofibromatosis Type 2. Am. J. Ophthalmol. 2006, 141, 1068–1077. [Google Scholar] [CrossRef]
- Lekovic, G.P.; Schwartz, M.S.; Hanna, G.; Go, J. Intra-Orbital Meningioma Causing Loss of Vision in Neurofibromatosis Type 2: Case Series and Management Considerations. Front. Surg. 2018, 5, 60. [Google Scholar] [CrossRef]
- Network NCC. Central Nervous System Cancers 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdf (accessed on 30 March 2020).
- Hénaux, P.-L.; Bretonnier, M.; Le Reste, P.-J.; Morandi, X. Modern Management of Meningiomas Compressing the Optic Nerve: A Systematic Review. World Neurosurg. 2018, 118, e677–e686. [Google Scholar] [CrossRef]
- Bloch, O.; Sun, M.; Kaur, G.; Barani, I.J.; Parsa, A.T. Fractionated radiotherapy for optic nerve sheath meningiomas. J. Clin. Neurosci. 2012, 19, 1210–1215. [Google Scholar] [CrossRef]
- Eckert, F.; Clasen, K.; Kelbsch, C.; Tonagel, F.; Bender, B.; Tabatabai, G.; Zips, D.; Thorwarth, D.; Frey, B.; Becker, G.; et al. Retrospective analysis of fractionated intensity-modulated radiotherapy (IMRT) in the interdisciplinary management of primary optic nerve sheath meningiomas. Radiat. Oncol. 2019, 14, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Arvold, N.D.; Lessell, S.; Bussiere, M.; Beaudette, K.; Rizzo, J.F.; Loeffler, J.S.; Shih, H.A. Visual Outcome and Tumor Control After Conformal Radiotherapy for Patients With Optic Nerve Sheath Meningioma. Int. J. Radiat. Oncol. 2009, 75, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Welzel, T.; Rieken, S.; Debus, J.; Combs, S.E. Prior surgical intervention and tumor size impact clinical outcome after precision radiotherapy for the treatment of optic nerve sheath meningiomas (ONSM). Radiat. Oncol. 2011, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Metellus, P.; Kapoor, S.; Kharkar, S.; Batra, S.; Jackson, J.F.; Kleinberg, L.; Miller, N.R.; Rigamonti, D. Fractionated Conformal Radiotherapy for Management of Optic Nerve Sheath Meningiomas: Long-Term Outcomes of Tumor Control and Visual Function at a Single Institution. Int. J. Radiat. Oncol. 2011, 80, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Mimura, O.; Masai, N.; Ohashi, A.; Ikenaga, K.; Okuno, Y.; Nishiguchi, I.; Oh, R. Early intervention using high-precision radiotherapy preserved visual function for five consecutive patients with optic nerve sheath meningioma. Int. J. Clin. Oncol. 2018, 23, 826–834. [Google Scholar] [CrossRef]
- Romanelli, P.; Bianchi, L.; Muacevic, A.; Beltramo, G. Staged image guided robotic radiosurgery for optic nerve sheath meningiomas. Comput. Aided Surg. 2011, 16, 257–266. [Google Scholar] [CrossRef]
- Inoue, T.; Okuno, Y.; Nishiguchi, I.; Ikenaga, K.; Mimura, O. Rapid recovery of vision following early intervention with fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Int. Med. Case Rep. J. 2018, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, R.; Cella, L.; Conson, M.; Tranfa, F.; Strianese, D.; Liuzzi, R.; Solla, R.; Farella, A.; Salvatore, M.; Bonavolontà, G. Fractionated Stereotactic Radiation Therapy for Orbital Optic Nerve Sheath Meningioma—A Single Institution Experience and a Short Review of the Literature. J. Radiat. Res. 2011, 52, 82–87. [Google Scholar] [CrossRef]
- Saeed, P.; Rootman, J.; Nugent, R.A.; White, V.A.; Mackenzie, I.R.; Koornneef, L. Optic nerve sheath meningiomas. Ophthalmology 2003, 110, 2019–2030. [Google Scholar] [CrossRef]
- Colenbrander, A. Aspects of vision loss—Visual functions and functional vision. Vis. Impair. Res. 2003, 5, 115–136. [Google Scholar] [CrossRef]
- WHO. Consultation on Development of Standards for Characterization of Vision Loss and Visual Functioning: Genveva, 4–5 September 2003; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Romanelli, P.; Wowra, B.; Muacevic, A. Multisession CyberKnife radiosurgery for optic nerve sheath meningiomas. Neurosurg. Focus 2007, 23, E10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, D.; Zhang, Z.; Zhang, Y.; Li, Y.; Liu, X.; Jia, Q.; Zheng, L.; Song, G. Long-term results of Gamma Knife surgery for optic nerve sheath meningioma. J. Neurosurg. 2010, 113, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Bianchi, S.; Milanesi, I.; Bergantin, A.; Bianchi, L.; Broggi, G.; Fariselli, L. Multisession Radiosurgery for Optic Nerve Sheath Meningiomas—An Effective Option. Neurosurgery 2011, 69, 1116–1123. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Maltry, A.; McClelland, C. Waiting to deliver a final diagnosis. Surv. Ophthalmol. 2017, 62, 583–586. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Soltys, S.G.; Yorke, E.; Moiseenko, V.; Tomé, W.A.; Sahgal, A.; Xue, J.; Ma, L.; Solberg, T.D.; et al. Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways. Int. J. Radiat. Oncol. 2021, 110, 87–99. [Google Scholar] [CrossRef]
- Ratnayake, G.; Oh, T.; Mehta, R.; Hardy, T.; Woodford, K.; Haward, R.; Ruben, J.D.; Dally, M.J. Long-term treatment outcomes of patients with primary optic nerve sheath meningioma treated with stereotactic radiotherapy. J. Clin. Neurosci. 2019, 68, 162–167. [Google Scholar] [CrossRef]
- Kheir, V.; Faouzi, M.; Borruat, F.-X. Visual Outcomes of Fractionated Radiotherapy in Optic Nerve Sheath Meningioma: A Retrospective Study. Klin. Mon. Augenheilkd. 2019, 236, 526–529. [Google Scholar] [CrossRef]
- Hamilton, S.N.; Nichol, A.; Truong, P.; McKenzie, M.; Hsu, F.; Cheung, A.; Dolman, P.; Gete, E.; Ma, R. Visual Outcomes and Local Control after Fractionated Stereotactic Radiotherapy for Optic Nerve Sheath Meningioma. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 217–221. [Google Scholar] [CrossRef]
- Soldà, F.; Wharram, B.; Gunapala, R.; Brada, M. Fractionated Stereotactic Conformal Radiotherapy for Optic Nerve Sheath Meningiomas. Clin. Oncol. 2012, 24, e106–e112. [Google Scholar] [CrossRef]
- Lindegaard, J.; Heegaard, S.; Prause, J.U. Histopathologically verified non-vascular optic nerve lesions in Denmark 1940-99. Acta Ophthalmol. Scand. 2002, 80, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R. New Concepts in the Diagnosis and Management of Optic Nerve Sheath Meningioma. J. Neuro Ophthalmol. 2006, 26, 200–208. [Google Scholar] [CrossRef]
- Acker, G.; Kluge, A.; Lukas, M.; Conti, A.; Pasemann, D.; Meinert, F.; Nguyen, P.T.A.; Jelgersma, C.; Loebel, F.; Budach, V.; et al. Impact of 68Ga-DOTATOC PET/MRI on robotic radiosurgery treatment planning in meningioma patients: First experiences in a single institution. Neurosurg. Focus 2019, 46, E9. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Chan, C.; Wang, L.; Jani, K.; Holdsworth, S.J.; Iv, M.; Pollom, E.L.; Soltys, S.G. Physiological motion of the optic chiasm and its impact on stereotactic radiosurgery dose. Br. J. Radiol. 2019, 92, 20190170. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number of Patients/Lesions (% of 25/27) |
|---|---|
| Gender | |
| Male | 4 (16.0%) |
| Female | 21 (84.0%) |
| Side | |
| Left | 10 (37.0%) |
| Right | 17 (63.0%) |
| Growth Pattern | |
| Circularly | 25 (92.6%) |
| Peripherally | 2 (7.4%) |
| Involvement of optic canal | |
| Orbital | 11 (40.7%) |
| Canalicular | 2 (7.4%) |
| Both | 14 (51.9%) |
| Previous surgery | |
| Yes | 9 (33.3%) |
| No | 18 (66.7%) |
| Visual acuity (WHO category) | |
| 0 | 10 (37.0%) |
| 1 | 3 (11.1%) |
| 2 | 3 (11.1%) |
| 3 | 0 (0.0%) |
| 4 | 4 (14.8%) |
| 5 | 7 (25.9%) |
| Exophthalmos | |
| Yes | 8 (29.6%) |
| No | 19 (70.4%) |
| Restricted mobility | |
| Yes | 8 (29.6%) |
| No | 19 (70.4%) |
| Visual field restriction | |
| Yes | 12 (44.5%) |
| No | 5 (18.5%) |
| Blind/dark-bright | 10 (37.0%) |
| Follow-Up Time (Month) | Visual Acuity (WHO) before CK | Visual Acuity (WHO) after CK | PTV (cm2) | Estimated Mobility of Ipsilateral Optic Nerve (mm) | |
|---|---|---|---|---|---|
| Previous surgery | |||||
| yes (n = 9) | 23.4 (6.4–78.7) | 4 (1–5) | 4 (0–5) | 1.6 (0.7–14.1) | 3.6 (1.7–11.8) * |
| no (n = 18) | 30.5 (8.0–83.7) | 0 (0–5) | 0 (0–5) | 0.8 (0.1–3.6) | 2.5 (0.8–6.4) |
| Fraction scheme | |||||
| 1 (n = 4) | 49.1 (8.2–74.7) | 5 (5–5) | 5 (5–5) | 2.8 (1.7–14.1) | 3.7 (2.3–5.1) * |
| 4–5 (n = 23) | 25.8 (6.4–83.7) | 1 (0–5) | 0 (0–5) | 0.9 (0.1–3.6) | 3.0 (0.8–11.8) |
| EQD2 in Gy (n = 16, only WHO Category < 5) | ||
|---|---|---|
| Organ | Mean | Median (Min–Max) |
| PTV | ||
| Dmin | 35.2 | 34.0 (30.5–46.8) |
| Dmean | 41.7 | 39.7 (36.1–53.0) |
| Dmax | 49.6 | 48.0 (40.7–70.9) |
| Ipsilateral optic nerve * | ||
| Dmax | 43.8 | 43.1 (26.5–62.0) |
| Dmean | 20.8 | 18.6 (5.5–44.1) |
| Optic chiasm | ||
| Dmax | 3.9 | 1.4 (0.1–27.9) |
| Contralateral optic nerve ** | ||
| Dmax | 1.8 | 1.3 (0.5–5.3) |
| Ipsilateral Retina/eye | ||
| Dmax | 15.7 | 5.3 (0.6–48.0) |
| Ipsilateral lens | ||
| Dmax | 0.5 | 0.4 (0.2–1.3) |
| First Author/Study Type | Year | Number of Patients | Radiation Device | Number of Fractions | Total Dose in Gy | Follow up in months | Local Control | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Senger C./Retrospective (actual study) | 2021 | 25 [27 lesions] | Cyberknife | 1 4–5 | 14–15 (70% isodose) 20–25 (70–85% isodose) | 37 (range: 6–84) | 96% (11% remission, 85% stable, 4% progression) | mild headache 4%, transient diplopia 4% |
| Marchetti M. [27]/Prospective | 2011 | 21 | Cyberknife | 5 | 25.0 (75%–85% isodose) | 30 (range: 11–68) | 100% (10% showed tumor shrinkage) | abnormal lacrimation 10% *, temporary diplopia with mild optic neuropathy 5% *, dizziness 5% * |
| Romanelli P. [18]/Retrospective | 2011 | 5 | Cyberknife | 4 | 20.0 (70% isodose) | 36–74 | 100% | n/a |
| Liu, D. [26]/Retrospective | 2010 | 13 pONSM [17 sONSM; total: 30] | Gammaknife | 1–2 | 13.3 (range 10.0–17.0) | 56 | 93,3% at 5 years (66% regression 27% stable, 7% progression) | reversible conjunctival oedema 13% *, transient orbital pain 3% *, transient headache 3% * |
| Romanelli P. [25]/Retrospective | 2007 | 3 | Cyberknife | 4 | 20.0 (80% isodose) | 37 | 100% at 3 years | n/a |
| First Author/Study Type | Year | Number of Patients | Radiation Device | Total Dose in Gy | Follow-Up in Months | Local Control | Acute Side Effects | Long Term Side Effects |
|---|---|---|---|---|---|---|---|---|
| Kheir V. [31]/Retrospective | 2019 | 16 | n/a | 50.4 | 31 (range: 2–156) | 100% (79% * stable, 21% * reduced) (radiological reports of 88% * of pat.) | none | none |
| Ratnayake G. [30]/Retrospective | 2019 | 26 | Novalis TX (ExacTrac) | 50.4 (range 50.4–54.0; 37.5 in 5–10%) | 68 (range 20–134) | 100% (96.1% stable, 3.8% mild reduction) | overall 53.8% of pat., fatigue 23.1%, headache 19.2%, alopecia 3.8%, dizziness 3.8% | dry eye 11.6%, retinopathy 4% *, multiple intracranial meningiomas 4% * |
| Hamilton S. N. [32]/Retrospective | 2018 | 23 pONSM (18 sONSM; total: 41) | 6 MV linear accelerator | 50.0 and 50.4 | 45.6 visual assessment, 52.8 MRI | 100% at 5 years for pONSM | headache 32%, nausea 15%, conjunctivitis 7%, dry eye 5%, eye discomfort 2% | hypopituitarism 13% of pONSM, retinopathy 7%, ocular pain 5%, cataract 2% (no differentiation of p/sONSM) |
| Paulsen F. [1]/Retrospective | 2012 | 37 pONSM (76 sONSM; total: 109) | 6 MV linear accelerator (Philips SL 25, Elekta Precise) | 54.0 (range: 50.4–54.0) | 30.2 clinical, 42.7 radio–graphic, 53.7 ophthalmologic | 100% at 3 years and 98% at 5 years | alopecia 58% *, erythema 34% *, pain 28% *, vertigo 12% *, nausea 10% *, moderate increased cranial pressure 10% * | obstructive hydrocephalus 3% * with sONSM |
| Soldà F. [33]/Retrospective | 2012 | 45 | 6 MV linear accelerator | 50.0 | 30 (range: 12–156) | 100% at 5 years | tiredness, small patches of transient alopecia | retinopathy 4% |
| Adeberg S. [15]/Prospective | 2011 | 40 | 6 MV linear accelerator (Siemens) | 54.0 (range: 25–66) | 60 (range: 4–228) | 100% | local alopecia (most pat.), fatigue 20% *, xerophthalmia 5% *, conjunctivitis 3% * | new headaches 3% *, hyperlacrimation 8% *, changed taste perception 3% *, scotoma and visual disorder 3% * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senger, C.; Kluge, A.; Kord, M.; Zimmermann, Z.; Conti, A.; Kufeld, M.; Kreimeier, A.; Loebel, F.; Stromberger, C.; Budach, V.; et al. Effectiveness and Safety of Robotic Radiosurgery for Optic Nerve Sheath Meningiomas: A Single Institution Series. Cancers 2021, 13, 2165. https://doi.org/10.3390/cancers13092165
Senger C, Kluge A, Kord M, Zimmermann Z, Conti A, Kufeld M, Kreimeier A, Loebel F, Stromberger C, Budach V, et al. Effectiveness and Safety of Robotic Radiosurgery for Optic Nerve Sheath Meningiomas: A Single Institution Series. Cancers. 2021; 13(9):2165. https://doi.org/10.3390/cancers13092165
Chicago/Turabian StyleSenger, Carolin, Anne Kluge, Melina Kord, Zoe Zimmermann, Alfredo Conti, Markus Kufeld, Anita Kreimeier, Franziska Loebel, Carmen Stromberger, Volker Budach, and et al. 2021. "Effectiveness and Safety of Robotic Radiosurgery for Optic Nerve Sheath Meningiomas: A Single Institution Series" Cancers 13, no. 9: 2165. https://doi.org/10.3390/cancers13092165
APA StyleSenger, C., Kluge, A., Kord, M., Zimmermann, Z., Conti, A., Kufeld, M., Kreimeier, A., Loebel, F., Stromberger, C., Budach, V., Vajkoczy, P., & Acker, G. (2021). Effectiveness and Safety of Robotic Radiosurgery for Optic Nerve Sheath Meningiomas: A Single Institution Series. Cancers, 13(9), 2165. https://doi.org/10.3390/cancers13092165








