Using Process Indicators to Monitor Documentation of Patient-Centred Variables in an Integrated Oncology and Palliative Care Pathway—Results from a Cluster Randomized Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
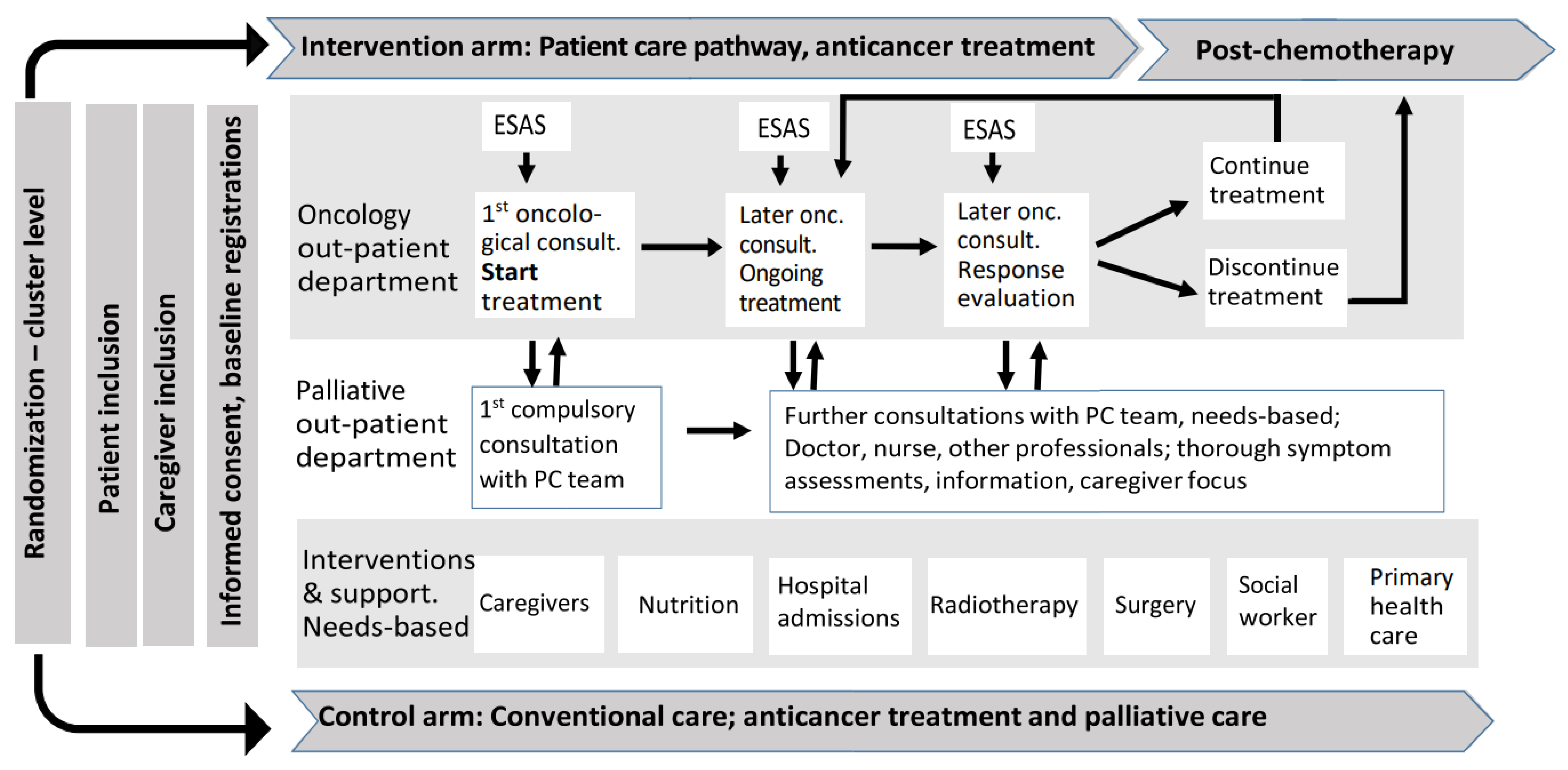
2.1. The PALLiON Intervention
2.2. The Monitoring Strategy and Procedures
2.3. Statistical Considerations
- Tables showing the documentation of each core indicator by time period for each of the three consultation types (Table 2)
- A figure showing program fulfilment for the core indicators by consultation type for each period (Figure 2)
- A figure showing program fulfilment by period, representing the summary proportion of all consultations and indicators (core and consultation specific) (Figure 3)
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.; Deliens, L.; et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef] [Green Version]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Li, Z.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T.; et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Groenvold, M.; Petersen, M.A.; Damkier, A.; Neergaard, M.A.; Nielsen, J.B.; Pedersen, L.; Sjøgren, P.; Strömgren, A.S.; Vejlgaard, T.B.; Gluud, C.; et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: The Danish Palliative Care Trial. Palliat. Med. 2017, 31, 814–824. [Google Scholar] [CrossRef]
- Jordhøy, M.S.; Fayers, P.; Saltnes, T.; Ahlner-Elmqvist, M.; Jannert, M.; Kaasa, S. A palliative-care intervention and death at home: A cluster randomised trial. Lancet 2000, 356, 888–893. [Google Scholar] [CrossRef]
- Maltoni, M.; Scarpi, E.; Dall’Agata, M.; Schiavon, S.; Biasini, C.; Codecà, C.; Broglia, C.M.; Sansoni, E.; Bortolussi, R.; Garetto, F.; et al. Systematic versus on-demand early palliative care: A randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur. J. Cancer 2016, 69, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; et al. Effects of early integrated palliative care in patients with lung and gi cancer: A randomized clinical trial. J. Clin. Oncol. 2017, 35, 834–841. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Vanbutsele, G.; Pardon, K.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Cocquyt, V.; Geboes, K.; Deliens, L. Effect of early and systematic integration of palliative care in patients with advanced cancer: A randomised controlled trial. Lancet Oncol. 2018, 19, 394–404. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rodin, G.; Tannock, I.; Donner, A.; et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Bajwah, S.; Oluyase, A.O.; Yi, D.; Gao, W.; Evans, C.J.; Grande, G.; Todd, C.; Costantini, M.; Murtagh, F.E.; Higginson, I.J. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst. Rev. 2020, CD012780. [Google Scholar]
- Weeks, J.C.; Catalano, P.J.; Cronin, A.; Finkelman, M.D.; Mack, J.W.; Keating, N.L.; Schrag, D. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 2012, 367, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- WHO Definition of Palliative Care. Available online: http://www.who.int/cancer/palliative/definition/en/, (accessed on 21 December 2020).
- Baker, A. Crossing the Quality Chasm: A New Health System for the 21st Century; Institute of Medicine IOM, (US) Committee on Quality of Health Care in America; National Academies Press: Washington DC, USA, 2001; Volume 323, p. 1192. [Google Scholar]
- Cherny, N. ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann. Oncol. 2014, 25 (Suppl. 3), iii143–iii152. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care into Standard Oncology Care: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available online: https://www.fda.gov/media/77832/download. (accessed on 21 December 2020).
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennet, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J. Clin. Oncol. 2015, 34, 557–565. [Google Scholar] [CrossRef]
- Currow, D.C.; Allingham, S.; Yates, P.; Johnson, C.; Clark, K.; Eagar, K. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support. Care Cancer 2015, 23, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, F.; Lethrosne, C.; Pourel, N.; Molinier, O.; Pointreau, Y.; Domont, J.; Bourgeois, H.; Senellart, H.; Trémolières, P.; Lizée, T.; et al. Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudgeon, D. The Impact of Measuring Patient-Reported Outcome Measures on Quality of and Access to Palliative Care. J. Palliat. Med. 2018, 21 (Suppl. 1), S76–S80. [Google Scholar] [CrossRef] [Green Version]
- Etkind, S.N.; Daveson, B.A.; Kwok, W.; Witt, J.; Bausewein, C.; Higginson, I.J.; Murtagh, F.E. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: Does it make a difference? A systematic review. J. Pain Symptom Manag. 2015, 49, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar]
- Anatchkova, M.; Donelson, S.M.; Skalicky, A.M.; McHorney, C.A.; Jagun, D.; Whiteley, J. Exploring the implementation of patient-reported outcome measures in cancer care: Need for more real-world evidence results in the peer reviewed literature. J. Patient Rep. Outcomes 2018, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Bausewein, C.; Daveson, B.A.; Currow, D.C.; Downing, J.; Deliens, L.; Radbruch, L.; DeFilippi, K.; Fereira, P.L.; Harding, R.; Costantini, M.; et al. EAPC White Paper on outcome measurement in palliative care: Improving practice, attaining outcomes and delivering quality services—Recommendations from the European Association for Palliative Care (EAPC) Task Force on Outcome Measurement. Palliat. Med. 2016, 30, 6–22. [Google Scholar] [CrossRef] [Green Version]
- Graupner, C.; Kimman, M.L.; Mul, S.; Slok, A.H.M.; Claessens, D.; Kleijnen, J.; Dirksen, C.D.; Breukink, S.O. Patient outcomes, patient experiences and process indicators associated with the routine use of patient-reported outcome measures (PROMs) in cancer care: A systematic review. Support. Care Cancer 2021, 29, 573–593. [Google Scholar] [CrossRef]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol. 2014, 32, 4149–4154. [Google Scholar] [CrossRef] [Green Version]
- van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef] [Green Version]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Moore, G.F.; Audrey, S.; Barker, M.; Bond, L.; Bonell, C.; Hardeman, W.; Moore, L.; O’Catain, A.; Tinati, T.; Wight, D.; et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015, 350, h1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommerbakk, R.; Haugen, D.F.; Tjora, A.; Kaasa, S.; Hjermstad, M.J. Barriers to and facilitators for implementing quality improvements in palliative care—results from a qualitative interview study in Norway. BMC Palliat. Care 2016, 15, 61. [Google Scholar] [CrossRef] [Green Version]
- Hjermstad, M.J.; Aass, N.; Andersen, S.; Brunelli, C.; Dajani, O.; Garresori, H.; Hamre, H.; Haukland, E.C.; Holmberg, M.; Jordal, F.; et al. PALLiON—PALLiative care Integrated in ONcology: Study protocol for a Norwegian national cluster-randomized control trial with a complex intervention of early integration of palliative care. Trials 2020, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- European Pathway Association—EPA. E-P-A Definition of Care Pathway. Available online: http://e-p-a.org/care-pathways/. (accessed on 19 December 2020).
- Bradshaw, A.; Santarelli, M.; Mulderrig, M.; Khamis, A.; Sartain, K.; Boland, J.W.; Bennett, M.I.; Johnson, M.; Pearson, M.; Murtagh, F.E.M. Implementing person-centred outcome measures in palliative care: An exploratory qualitative study using Normalisation Process Theory to understand processes and context. Palliat. Med. 2021, 35, 397–407. [Google Scholar] [CrossRef]
- Nilsen, P. Making sense of implementation theories, models and frameworks. Implement. Sci. 2015, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Lundeby, T.; Wester, T.E.; Loge, J.H.; Kaasa, S.; Aass, N.K.; Grotmol, K.S.; Finset, A. Challenges and Learning Needs for Providers of Advanced Cancer Care: Focus Group Interviews with Physicians and Nurses. Palliat. Med. Rep. 2020, 1, 208–215. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Deming, W.E. Out of the Crisis; MIT Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Tague, N.R. “Plan–Do–Study–Act cycle”. In The Quality Toolbox, 2nd ed.; ASQ Quality Press: Milwaukee, WI, USA, 2005; Available online: https://docplayer.net/31433162-The-quality-toolbox-second-edition.html (accessed on 10 September 2020).
- Statistical process control (SPC), National Health Services NHS. Available online: https://improvement.nhs.uk/resources/statistical-process-control-spc/ (accessed on 15 October 2020).
- Moore, G.F.; Evans, R.E. What theory, for whom and in which context? Reflections on the application of theory in the development and evaluation of complex population health interventions. SSM Popul. Health 2017, 3, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Haun, M.W.; Estel, S.; Rucker, G.; Friederich, H.C.; Villalobos, M.; Thomas, M.; Hartmann, M. Early palliative care for adults with advanced cancer. Cochrane Database Syst. Rev. 2017, 6, CD011129. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D.; Guastaferro, K.; Azuero, A.; Rini, C.; Hendricks, B.A.; Dosse, C.; Taylor, R.; Williams, G.R.; Engler, S.; Smith, C.; et al. Applying the Multiphase Optimization Strategy for the Development of Optimized Interventions in Palliative Care. J. Pain Symptom Manag. 2020. [Google Scholar] [CrossRef] [PubMed]
- European Palliative Care Research Centre (PRC) 2019. Available online: https://bit.ly/PRCresearch. (accessed on 18 November 2020).
- Antunes, B.; Harding, R.; Higginson, I.J.; Euroimpact. Implementing patient-reported outcome measures in palliative care clinical practice: A systematic review of facilitators and barriers. Palliat. Med. 2014, 28, 158–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Basch, E.; Dueck, A.C.; Rogak, L.J.; Minasian, L.M.; Kelly, W.K.; O’Mara, A.M.; Denicoff, A.M.; Seisler, D.; Atherton, P.J.; Paskett, E.; et al. Feasibility Assessment of Patient Reporting of Symptomatic Adverse Events in Multicenter Cancer Clinical Trials. JAMA Oncol. 2017, 3, 1043–1050. [Google Scholar] [CrossRef]
- Ediebah, D.E.; Quinten, C.; Coens, C.; Ringash, J.; Dancey, J.; Zikos, E.; Gotay, C.; Brundage, M.; Tu, D.; Flechtner, H.H.; et al. Quality of life as a prognostic indicator of survival: A pooled analysis of individual patient data from Canadian Cancer Trials Group clinical trials. Cancer 2018, 124, 3409–3416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhalgh, J.; Gooding, K.; Gibbons, E.; Dalkin, S.; Wright, J.; Valderas, J.; Black, N. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J. Patient Rep. Outcomes 2018, 2, 42. [Google Scholar] [CrossRef]
- Forst, D.A.; Quain, K.; Landay, S.L.; Anand, M.; Kaslow-Zieve, E.; Mesa, M.M.; Jacobs, J.M.; Dietrich, J.; Parsons, M.W.; Horick, N.; et al. Perceptions of prognosis and goal of treatment in patients with malignant gliomas and their caregivers. Neurooncol. Pract. 2020, 7, 490–497. [Google Scholar] [CrossRef]
- Epstein, A.S.; Prigerson, H.G.; O’Reilly, E.M.; Maciejewski, P.K. Discussions of Life Expectancy and Changes in Illness Understanding in Patients With Advanced Cancer. J. Clin. Oncol. 2016, 34, 2398–2403. [Google Scholar] [CrossRef]
- Hannon, B.; Swami, N.; Rodin, G.; Pope, A.; Zimmermann, C. Experiences of patients and caregivers with early palliative care: A qualitative study. Palliat. Med. 2017, 31, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Mo, L.; Paiva, C.E. The Importance of Prognostication: Impact of Prognostic Predictions, Disclosures, Awareness, and Acceptance on Patient Outcomes. Curr. Treat. Options Oncol. 2021, 22, 12. [Google Scholar] [CrossRef]
- Mack, J.W.; Jacobson, J.; Frank, D.; Cronin, A.M.; Horvath, K.; Allen, V.; Wind, J.; Schrag, D. Evaluation of Patient and Family Outpatient Complaints as a Strategy to Prioritize Efforts to Improve Cancer Care Delivery. Jt. Comm. J. Qual. Patient Saf. 2017, 43, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Back, A.L. Patient-Clinician Communication Issues in Palliative Care for Patients With Advanced Cancer. J. Clin. Oncol. 2020, 38, 866–876. [Google Scholar] [CrossRef]
- Abernethy, E.R.; Campbell, G.P.; Pentz, R.D. Why many oncologists fail to share accurate prognoses: They care deeply for their patients. Cancer. 2020, 126, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Butow, P.N.; Clayton, J.M.; Epstein, R.M. Prognostic Awareness in Adult Oncology and Palliative Care. J. Clin. Oncol. 2020, 38, 877–884. [Google Scholar] [CrossRef]
- Tulsky, J.A.; Beach, M.C.; Butow, P.N.; Hickman, S.E.; Mack, J.W.; Morrison, R.S.; Street, R.L.; Sudore, R.L.; White, D.B.; Pollack, K.I. A research agenda for communication between health care professionals and patients living with serious illness. JAMA Intern. Med. 2017, 177, 1361–1366. [Google Scholar] [CrossRef]
- Gilligan, T.; Bohlke, K.; Baile, W.F. Patient-Clinician Communication: American Society of Clinical Oncology Consensus Guideline Summary. J. Oncol. Pract. 2018, 14, 42–46. [Google Scholar] [CrossRef]
- Grol, R.; Grimshaw, J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet 2003, 362, 1225–1230. [Google Scholar] [CrossRef]
- Hausner, D.; Tricou, C.; Mathews, J.; Wadhwa, D.; Pope, A.; Swami, N.; Hannon, B.; Rodin, G.; Krzyzanowska, M.K.; Le, L.W.; et al. Timing of Palliative Care Referral Before and After Evidence from Trials Supporting Early Palliative Care. Oncologist 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Grol, R.; Wensing, M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med. J. Aust. 2004, 180 (Suppl. 6), S57–S60. [Google Scholar] [CrossRef]
- Moore, G.F.; Evans, R.E.; Hawkins, J.; Littlecott, H.; Melendez-Torres, G.J.; Bonell, C.; Murphy, S. From complex social interventions to interventions in complex social systems: Future directions and unresolved questions for intervention development and evaluation. Evaluation 2019, 25, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, S.; Palla, S.; Hui, D.; Nguyen, L.; Chacko, R.; Li, Z.; Fadul, N.; Scott, C.; Thornton, V.; Coldman, B.; et al. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist 2011, 16, 105–111. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, T.W.; O’Donnell, J.D.; Crowley-Matoka, M.; Rabow, M.W.; Smith, C.B.; White, D.B.; Tiver, G.A.; Arnold, R.M.; Schenker, Y. Perceptions of palliative care among hematologic malignancy specialists: A mixed-methods study. J. Oncol. Pract. 2015, 11, e230–e238. [Google Scholar] [CrossRef]
- Salins, N.; Ghoshal, A.; Hughes, S.; Preston, N. How views of oncologists and haematologists impacts palliative care referral: A systematic review. BMC Palliat. Care 2020, 19, 175. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Leighl, N.; Rydall, A.; Rodin, G.; Tannock, I.; Hannon, B. Perceptions of palliative care among patients with advanced cancer and their caregivers. Can. Med. Assoc. J. 2016, 188, E217–E227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, S.L.; Daly, B.J.; Lipson, A.R.; Blackstone, E. Association between strong patient-oncologist agreement regarding goals of care and aggressive care at end-of-life for patients with advanced cancer. Support. Care Cancer 2020, 28, 5139–5146. [Google Scholar] [CrossRef] [PubMed]
- Jang, R.W.; Krzyzanowska, M.K.; Zimmermann, C.; Taback, N.; Alibhai, S.M. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J. Natl. Cancer Inst. 2015, 107, dju424. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Process Indicators |
|---|
| Core process indicators in all consultation types (n = 4) 1 |
|
| Consultation specific process indicators; first oncology outpatient consultation (n = 6) 4 |
|
| Consultation specific process indicators; palliative care consultations during chemotherapy (n = 5) 4 |
|
| Consultation specific process indicators; subsequent oncology outpatient consultations during chemotherapy (n = 4) 4 |
|
| First Oncological Consultations | Period I | Period II | Period III | Period IV | Period VI | Total |
| Number of consultations | N = 7 | N = 8 | N = 9 | N = 32 | N = 20 | N = 76 |
| Core indicators | n (%) | n (%) | n (%) | n (%) | n (%) | |
| ESAS a | 5 (71.4) | 4 (50.0) | 2 (22.2) | 4 (12.5) | 1 (5.0) | |
| ECOG b | 7 (100) | 6 (75.0) | 8 (88.9) | 24 (75.0) | 18 (90.0) | |
| Weight | 6 (85.7) | 5 (62.5) | 8 (88.9) | 26 (81.2) | 15 (75.0) | |
| Report sent to GP c | 1 (14.3) | 8 (100) | 9 (100) | 26 (81.2) | 17 (85.0) | |
| Documented core indicators e | 19 (67.9) | 23 (71.9) | 27 (75.0) | 80 (62.5) | 51 (63.8) | 200 |
| No. of core indicators for documentation d | 28 | 32 | 36 | 128 | 80 | 304 |
| Program fulfilment, first oncological consultations f | 65.8 | |||||
| Palliative care consultations | Period I | Period II | Period III | Period IV | Period VI | Total |
| Number of consultations | n = 9 | n = 11 | n = 11 | n = 38 | n = 18 | N = 87 |
| Core indicators | n (%) | n (%) | n (%) | n (%) | n (%) | |
| ESAS a | 8 (88.9) | 11 (100) | 11 (100) | 37 (97.3) | 17 (94.4) | |
| ECOG b | 6 (66.7) | 10 (90.9) | 11 (100) | 37 (97.3) | 17 (94.4) | |
| Weight | 8 (88.9) | 10 (90.9) | 11 (100) | 36 (94.7) | 17 (94.4) | |
| Report sent to GP c | 8 (88.9) | 11 (100) | 11 (100) | 36 (94.7) | 17 (94.4) | |
| Documented core indicators e | 30 (83.3) | 42 (95.4) | 44 (100.0) | 146 (96.1) | 68 (94.4) | 330 |
| No. of core indicators for documentation d | 36 | 44 | 44 | 152 | 72 | 348 |
| Program fulfilment, palliative care consultations f | 94.8% | |||||
| Oncological consultations during chemotherapy. | Period I | Period II | Period III | Period IV | Period VI | Total |
| Number of consultations | n = 31 | n = 64 | n = 53 | n = 79 | n = 45 | N = 272 |
| Core indicators | n (%) | n (%) | n (%) | n (%) | n (%) | |
| ESAS a | 25 (80.6) | 53 (82.9) | 38 (71.7) | 3 (3.8) | 4 (8.8) | |
| ECOG b | 22 (71.0) | 54 (84.3) | 39 (73.6) | 58 (73.4) | 43 (95.6) | |
| Weight | 22 (71.0) | 45 (70.3) | 38 (71.7) | 52 (65.9) | 28 (62.2) | |
| Report sent to GP c | 10 (32.2) | 64 (100) | 52 (98.1) | 67 (84.8) | 36 (80.0) | |
| Documented core indicators e | 79 (63.7) | 216 (84.3) | 167 (78.8) | 180 (57.0) | 111 (61.7) | 753 |
| No. of core indicators for documentation d | 124 | 256 | 212 | 316 | 180 | 1088 |
| Program fulfilment, Oncological consultations during chemotherapy f | 69.20% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hjermstad, M.J.; Hamfjord, J.; Aass, N.; Dajani, O.; Lundeby, T.; Wester, T.; Kaasa, S. Using Process Indicators to Monitor Documentation of Patient-Centred Variables in an Integrated Oncology and Palliative Care Pathway—Results from a Cluster Randomized Trial. Cancers 2021, 13, 2194. https://doi.org/10.3390/cancers13092194
Hjermstad MJ, Hamfjord J, Aass N, Dajani O, Lundeby T, Wester T, Kaasa S. Using Process Indicators to Monitor Documentation of Patient-Centred Variables in an Integrated Oncology and Palliative Care Pathway—Results from a Cluster Randomized Trial. Cancers. 2021; 13(9):2194. https://doi.org/10.3390/cancers13092194
Chicago/Turabian StyleHjermstad, Marianne Jensen, Julian Hamfjord, Nina Aass, Olav Dajani, Tonje Lundeby, Torunn Wester, and Stein Kaasa. 2021. "Using Process Indicators to Monitor Documentation of Patient-Centred Variables in an Integrated Oncology and Palliative Care Pathway—Results from a Cluster Randomized Trial" Cancers 13, no. 9: 2194. https://doi.org/10.3390/cancers13092194
APA StyleHjermstad, M. J., Hamfjord, J., Aass, N., Dajani, O., Lundeby, T., Wester, T., & Kaasa, S. (2021). Using Process Indicators to Monitor Documentation of Patient-Centred Variables in an Integrated Oncology and Palliative Care Pathway—Results from a Cluster Randomized Trial. Cancers, 13(9), 2194. https://doi.org/10.3390/cancers13092194







