Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy
Abstract
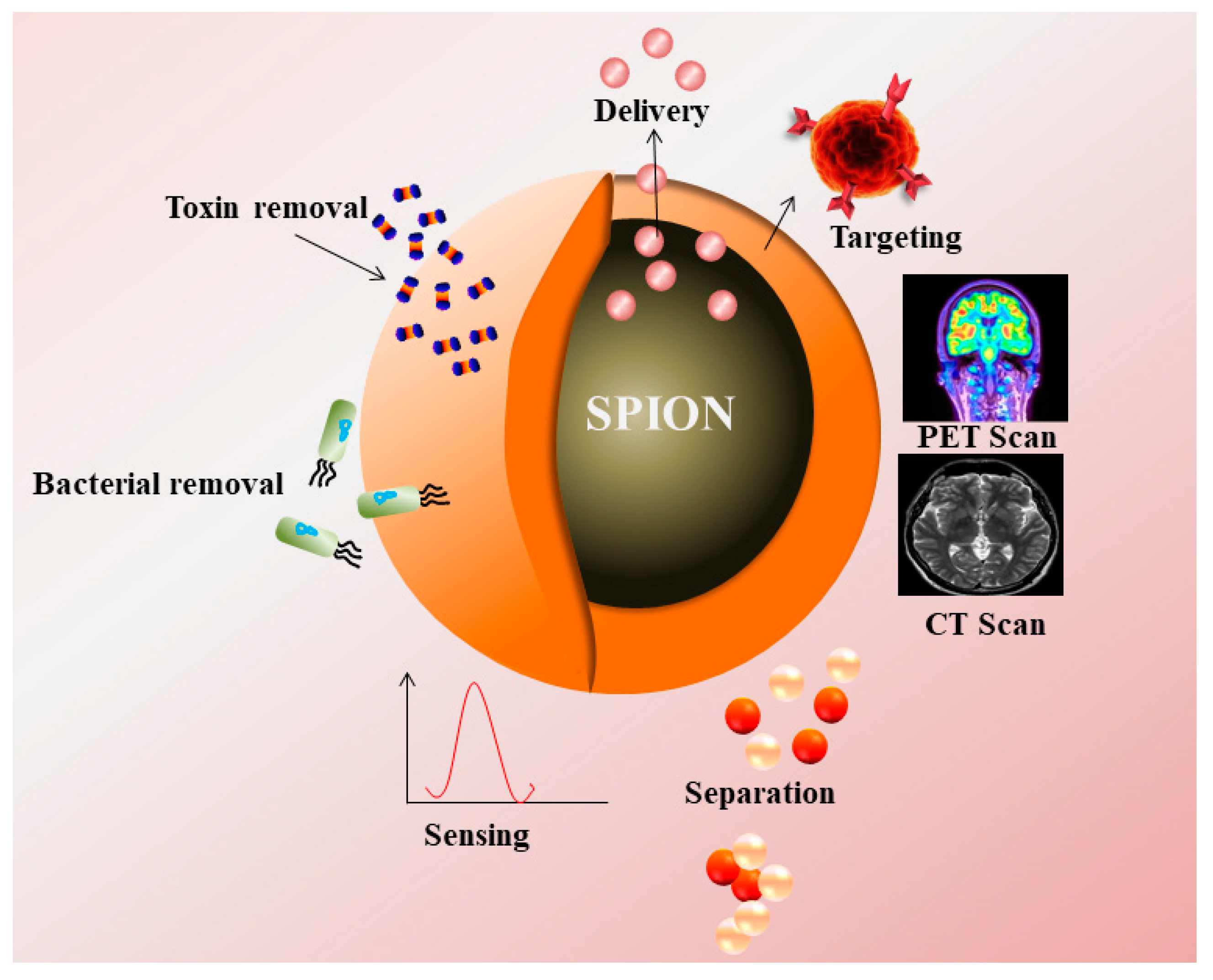
:Simple Summary
Abstract
1. Introduction
2. Biomedical and Clinical Applications
2.1. Magnetic Resonance Imaging (MRI)
2.2. Magnetic Particle Imaging (MPI)
2.3. Computed Tomography (CT)
2.4. PET (Positron Emission Tomography)
2.5. Nanozyme Applications for Biosensing
3. Gene and Drug Delivery Using IONPs
3.1. Gene Delivery
3.2. Drug Delivery
4. IONPs for Hyperthermia and Photothermal Therapy
5. IONPs for Broad Spectrum Antimicrobial Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bowles, J.F.W.; Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses. Weinheim and New York (VCH Verlagsgeseiischaft mbH). Mineralogical Magazine 1996, 61, 740–741. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mat. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chi. Phy. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—a review. Int. J. Eng. Sci. Technol. 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Bárcena, C.; Sra, A.K.; Gao, J. Applications of magnetic nanoparticles in biomedicine. Nanoscale Mag. Mat. and Appl. 2009. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, W.; Zhang, X.; Mei, X. Au Nanocage Functionalized with Ultra-small Fe3O4 Nanoparticles for Targeting T1-T2 Dual MRI and CT Imaging of Tumor. Sci Rep. 2016, 28258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, E.A.; Wickline, S.A. Contrast agents for MRI. Basic Res. Cardiol. 2008, 103, 114–121. [Google Scholar] [CrossRef]
- Skopalik, J.; Polakova, K.; Havrdova, M.; Justan, I.; Magro, M.; Milde, D.; Knopfova, L.; Jan Smarda, J.; Polakova, H.; Gabrielova, E.; et al. Mesenchymal stromal cell labeling by new uncoated superparamagnetic maghemite nanoparticles in comparison with commercial Resovist--an initial in vitro study. Int. J. Nanomed. 2014, 5355. [Google Scholar] [CrossRef] [Green Version]
- Aghighi, M.; Golovko, D.; Ansari, C.; Marina, N.M.; Pisani, L.; Kurlander, L.; Klenk, C.; Bhaumik, S.; Wendland, M.; Daldrup-Link, H.E. Imaging tumor necrosis with ferumoxytol. PLoS ONE 2015, 10, e0142665. [Google Scholar] [CrossRef]
- Jung, C.W. Surface properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Mag. Res. Imag. 1995, 13, 675–691. [Google Scholar] [CrossRef]
- Weizenecker, J.; Gleich, B.; Rahmer, J.; Dahnke, H.; Borgert, J. Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol. 2009, 54, L1–L10. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Gandhi, S.; Krishnan, K.M. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale 2015, 7, 16890–16898. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.; Arami, H.; Krishnan, K.M. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett. 2016, 16, 3668–3674. [Google Scholar] [CrossRef]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast. Media Mol. Imag. 2014, 9, 37–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [Green Version]
- Naha, P.C.; Al Zaki, A.; Hecht, E.; Chorny, M.; Chhour, P.; Blankemeyer, E.; Yates, D.M.; Walter, W.R.; Litt, H.I. Dextran coated bismuth-iron oxide nanohybrid contrast agents for computed tomography and magnetic resonance imaging. J. Mat. Chem. B. 2014, 46, 8239–8248. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Wang, M.; Zhang, L.; Du, X.; Gu, H.; Zhang, C. Iodinated oil-loaded, fluorescent mesoporous silica-coated iron oxide nanoparticles for magnetic resonance imaging/computed tomography/fluorescence trimodal imaging. Int. J. Nanomed. 2014, 9, 2527–2534. [Google Scholar] [CrossRef] [Green Version]
- Reguera, J.; Jiménez, D.; Aberasturi, D.; Henriksen-Lacey, M.; Langer, J.; Espinosa, A.; Szczupak, B.; Wilhelm, C.; Liz-Marzán, L.M. Janus plasmonic-magnetic gold-iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale 2017, 9, 9467–9480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torresmartinderosales, R.; Tavare, R.; Paul, R.L.; Jauregui-Osoro, M.; Protti, A.; Glaria, A.; Varma, G.; Szanda, I.; Blower, P.J. Synthesis of 64CuII- bis(dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: In vivo evaluation as dual-modality PET-MRI agent. Angew. Chemie Int. Ed. 2011, 2, 8239–8248. [Google Scholar] [CrossRef] [Green Version]
- Nahrendorf, M.; Zhang, H.; Hembrador, S.; Panizzi, P.; Sosnovik, D.E.; Aikawa, E.; Libby, P.; Swirski, F.K.; Weissleder, R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2008, 117, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Stelter, L.; Pinkernelle, J.G.; Michel, R.; Schwartländer, R.; Raschzok, N.; Morgul, M.H.; Koch, M.; Denecke, T.; Ruf, J.; Bäumler, H.; et al. Modification of aminosilanized superparamagnetic nanoparticles: Feasibility of multimodal detection using 3T MRI, small animal PET, and fluorescence imaging. Mol. Imaging Biol. 2010, 12, 25–34. [Google Scholar] [CrossRef]
- Glaus, C.; Rossin, R.; Welch, M.J.; Bao, G. In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjug. Chem. 2010, 21, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Aryal, S.; Key, J.; Stigliano, C.; Landis, M.D.; Lee, D.Y.; Decuzzi, P. Positron emitting magnetic nanoconstructs for PET/MR imaging. Small 2014, 10, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Kernstine, K.H.; Stanford, W.; Mullan, B.F.; Rossi, N.P.; Thompson, B.H.; Bushnell, D.L.; McLaughlin, K.A.; Kern, J.A. PET, CT, and MRI with Combidex for mediastinal staging in non-small cell lung carcinoma. Ann. Thoracic Surg. 1999, 68, 1022–1028. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Saini, S.; Weissleder, R.; Hahn, P.F.; Yantiss, R.K.; Tempany, C.; Wood, B.J.; Mueller, P.R. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: Radiographic- pathologic correlation. Americ. J. Roentgenol. 1999, 172, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Europ. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef]
- Vogl, T.J.; Hammerstingl, R.; Schwarz, W.; Kümmel, S.; Müller, P.K.; Balzer, T.; Lauten, M.J.; Balzer, J.O.; Mack, M.G.; Schimpfky, C.; et al. Magnetic resonance imaging of focal liver lesions: Comparison of the superparamagnetic iron oxide resovist versus gadolinium-DTPA in the same patient. Invest. Radiol. 1996, 31, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Rummeny, E.J.; Daldrup, H.E.; Balzer, T.; Tombach, B.; Berns, T.; Peters, P.E. Clinical results with Resovist: A phase 2 clinical trial. Radiology 1995, 195, 489–496. [Google Scholar] [CrossRef]
- Kostura, L.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F.; Bulte, J.M.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004, 17, 513–517. [Google Scholar] [CrossRef]
- Briley-Saebo, K.C.; Mani, V.; Hyafil, F.; Cornily, J.C.; Fayad, Z.A. Fractionated Feridex and positive contrast: In vivo MR imaging of atherosclerosis. Mag. Res. Med. 2008, 59, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Pinder, S.E.; Douek, M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology 2013, 62, 481–486. [Google Scholar] [CrossRef]
- Bullivant, J.P.; Zhao, S.; Willenberg, B.J.; Kozissnik, B.; Batich, C.D.; Dobson, J. Materials characterization of feraheme/ferumoxytol and preliminary evaluation of its potential for magnetic fluid hyperthermia. Int. J. Mol. Sci. 2013, 14, 17501–17510. [Google Scholar] [CrossRef] [Green Version]
- Kellar, K.E.; Fujii, D.K.; Gunther, W.H.H.; Briley-Sæbø, K.; Bjørnerud, A.; Spiller, M.; Koenig, S.H. NC 100150 injection, a preparation of optimized iron oxide nanoparticles for positive-contrast MR angiography. J. Mag. Res. Imag. 2000, 11, 488–494. [Google Scholar] [CrossRef]
- Shan, L.; Leung, K.; Zhang, H.; Cheng, K.T.; Pagel, M. Molecular Imaging and Contrast Agent Database. Bethesda (MD) National Center for Biotechnology Information (US) 2004-2013. Available online: https://europepmc.org/books/n/micad/?extid=22220318&src=med (accessed on 20 April 2021).
- Yu, F.; Huang, Y.; Cole, A.J.; Yang, V.C. Biomaterials The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.; Banga, I.; Maurya, P.K.; Eremin, S.A. Gold nanoparticles-single chain fragment variable antibody as immunoprobe for rapid detection of morphine by dipstick. RSC Adv. 2018, 8, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Mishra, P.; Banga, I.; Parmar, A.S.; Tripathi, P.P.; Gandhi, S. Chemiluminescence based immunoassay for the detection of heroin and its metabolites. Bioimpacts 2018, 8, 57–62. [Google Scholar] [CrossRef]
- Roberts, A.; Tripathi, P.P.; Gandhi, S. Graphene nanosheets as electric mediator for ultrafast sensing of urokinase plasminogen activator receptor-a biomarker of cancer. Biosens. Bioelectron. 2019, 141, 111398. [Google Scholar] [CrossRef]
- Kasoju, A.; Shahdeo, D.; Khan, A.A.; Shrikrishna, N.S.; Mahiri, S.; Alanazi, M.; Bhat, M.A.; Giri, J.; Gandhi, S. Fabrication of microfluidic device for Aflatoxin M1 detection in milk samples with specific aptamers. Sci. Rep. 2020, 10, 4627. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Yang, X.; Jiang, L.; Zhu, C.; Mao, H.; Wang, K. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine. Sens. Act. B Chem. 2014, 201, 160–166. [Google Scholar] [CrossRef]
- Kaçar, C.; Erden, P.E.; Pekyardimci, S.; Kiliç, E. An Fe3O4-nanoparticles-based amperometric biosensor for creatine determination. Art. Cells Nanomed. Biotechnol. 2013, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, J.; Lyle, S.; Zehr, K.; Cao, L.; Gao, D. Magnetite nanoparticle-linked immunosorbent assay. J. Phy. Chem. C. 2008, 112, 17357–17361. [Google Scholar] [CrossRef]
- Yang, M.; Guan, Y.; Yang, Y.; Xie, L.; Xia, T.; Xiong, W.; Guo, C. Immunological detection of hepatocellular carcinoma biomarker GP73 based on dissolved magnetic nanoparticles. Coll. Sur. A: Phys. Eng. Asp. 2014, 443, 280–285. [Google Scholar] [CrossRef]
- Yang, M.; Guan, Y.; Yang, Y.; Xia, T.; Xiong, W.; Wang, N.; Guo, C. Peroxidase-like activity of amino-functionalized magnetic nanoparticles and their applications in immunoassay. J. Coll. Int. Sci. 2013, 405, 291–295. [Google Scholar] [CrossRef]
- Kim, M.I.; Kim, M.S.; Woo, M.A.; Ye, Y.; Kang, K.S.; Lee, J.; Park, H.G. Highly efficient colorimetric detection of target cancer cells utilizing superior catalytic activity of graphene oxide-magnetic-platinum nanohybrids. Nanoscale 2014, 6, 1529–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, B.; Lagzi, I.; Teraji, S.; Nakanishi, H.; Cervenak, L.; Zámbó, D.; Deák, A.; Molnár, K.; Truszka, M.; Szekacs, I.; et al. Interaction of Positively Charged Gold Nanoparticles with Cancer Cells Monitored by an in Situ Label-Free Optical Biosensor and Transmission Electron Microscopy. ACS Appl. Mater. Interfaces 2018, 10, 26841–26850. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wang, Y.T.; Tseng, W.L. Amplified Peroxidase-Like Activity in Iron Oxide Nanoparticles Using Adenosine Monophosphate: Application to Urinary Protein Sensing. ACS Appl. Mat. Interf. 2017, 9, 10069–10077. [Google Scholar] [CrossRef]
- Ali, A.; Alsalhi, M.S.; Atif, M.; Ansari, A.A.; Israr, M.Q.; Sadaf, J.R.; Ahmed, E.; Nur, O.; Willander, M. Potentiometric urea biosensor utilizing nanobiocomposite of chitosan-iron oxide magnetic nanoparticles. J. Phy. Conf. Series 2013, 414, 012024. [Google Scholar] [CrossRef] [Green Version]
- Fornara, A.; Johansson, P.; Petersson, K.; Gustafsson, S.; Qin, J.; Olsson, E.; Ilver, D.; Krozer, A.; Muhammed, M.; Johansson, C. Tailored magnetic nanoparticles for direct and sensitive detection of biomolecules in biological samples. Nano Lett. 2008, 8, 3423–3428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.-Y.; Xu, X.; Yang, Y.; Huang, L.-H.; He, Z.-Y.; Xu, Y.-H.; Chen, J.-J.; Feng, Z.-S. Preparation and characterization of reduced graphene oxide/Fe3O4 nanocomposite by a facile in-situ deposition method for glucose biosensor applications. Mat. Res. Bull. 2018, 101, 340–346. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, W.; Zhang, X.; Chai, H.; Huang, Y. FeNPs@Co3O4 hollow nanocages hybrids as effective peroxidase mimics for glucose biosensing. Sens. Act. B Chem. 2018, 263, 575–584. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Borroni, E.; Miola, M.; Ferraris, S.; Ricci, G.; Rožman, K.Z.; Kostevšek, N.; Catizone, A.; Rimondini, L.; Prata, M.; Verne, E.; et al. Tumor targeting by lentiviral vectors combined with magnetic nanoparticles in mice. Acta Biomat. 2017, 59, 303–316. [Google Scholar] [CrossRef]
- Cheong, S.J.; Lee, C.M.; Kim, S.L.; Jeong, H.J.; Kim, E.M.; Park, E.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H. Superparamagnetic iron oxide nanoparticles-loaded chitosan-linoleic acid nanoparticles as an effective hepatocyte-targeted gene delivery system. Int. J. Pharm. 2009, 372, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, B. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, U.M.; Teller, S.; Sendler, M.; Palankar, R.; van den Brandt, C.; Schwaiger, T.; Kühn, J.P.; Ribback, S.; Glöckl, G.; Evert, M.; et al. Tumour-specific delivery of siRNA-coupled superparamagnetic iron oxide nanoparticles, targeted against PLK1, stops progression of pancreatic cancer. Gut 2016, 65, 1838–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mat. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Campos, R.; Baratella, D.; Lima, G.; Holà, K.; Divoky, C.; Stollberger, R.; Malina, O.; Aparicio, C.; Zoppellaro, G.; et al. A magnetically drivable nanovehicle for curcumin with antioxidant capacity and MRI relaxation properties. Chem. A Eur. J. 2014, 20, 11913–11920. [Google Scholar] [CrossRef] [PubMed]
- Lübbe, A.S.; Bergemann, C.; Huhnt, W.; Riess, H.; Brock, J.W.; Huhn, D. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy. Can. Res. 1996, 56, 4694–4701. [Google Scholar]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R.; et al. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar]
- Lübbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Kerlan, R.K.; Fidelman, N.A.; Venook, A.P.; LaBerge, J.M.; Koda, J.; Gordon, R.L. Hepatocellular Carcinoma: Regional Therapy with a Magnetic Targeted Carrier Bound to Doxorubicin in a Dual MR Imaging/Conventional Angiography Suite-Initial Experience with Four Patients. Radiology 2004, 230, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Europ. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.J.; Guo, M.; Yan, H.S.; Wang, C.H.; Liu, K.L. Cisplatin-loaded polymer/magnetite composite nanoparticles as multifunctional therapeutic nanomedicine. Chinese J Poly. Sci. Eng. Ed. 2014, 32, 1329–1337. [Google Scholar] [CrossRef]
- Islamian, J.P.; Hatamian, M.; Aval, N.A.; Rashidi, M.R.; Mesbahi, A.; Mohammadzadeh, M.; Jafarabadi, M.A. Targeted superparamagnetic nanoparticles coated with 2-deoxy-D-gloucose and doxorubicin more sensitize breast cancer cells to ionizing radiation. Breast 2017, 33, 97–103. [Google Scholar] [CrossRef]
- Arachchige, M.P.; Laha, S.S.; Naik, A.R.; Lewis, K.T.; Naik, R.; Jena, B.P. Functionalized nanoparticles enable tracking the rapid entry and release of doxorubicin in human pancreatic cancer cells. Micron 2017, 92, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Ren, Z.; Huang, B.; Maomao, P.; Haojie, L.; Yina, D.; Qiufan, X.; Yihua, Z.; Changliang, Z.; Zhe, L.; et al. Daunorubicin loaded Fe3O4 nanoparticles induce apoptosis of glioma cells and disrupt tight junction at blood-brain barrier. J. Nanosci. Nanotechnol. 2016, 16, 12356–12361. [Google Scholar] [CrossRef]
- Gupta, J.; Prakash, A.; Jaiswal, M.K.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Mag. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, F.; Ma, L.; Yao, H.; Wang, Q.; Wen, L.; Wang, Q.; Gu, N.; Chen, S. Inhibitory effect of magnetic Fe3o4 nanoparticles coloaded with homoharringtonine on human leukemia cells in vivo and in vitro. Int. J. Nanomed. 2016, 11, 4413–4422. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.; Kong, Y.; Chen, X.; Weijie, L.; Dejun, L.; Xie, Y.; Yan, Z.; Hanbing, Z.; Zhaohua, C.; Huili, D.; et al. Fe3O4 nanoparticles and cryoablation enhance ice crystal formation to improve the efficiency of killing breast cancer cells. Oncotarget 2017, 8, 11389–11399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crile, G. The Effects of Heat and Radiation on Cancers Implanted on the Feet of Mice. Cancer Res. 1963, 23, 372–380. [Google Scholar]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mat. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Gabudean, A.M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.C.; Wang, X.; Wang, Z.; Liu, G.; Zhou, W.; Wen, L.; Li, Q.; Wang, X.; Chen, X.; et al. Facile synthesis of pentacle gold-copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007, 2, 47–52. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mat. 2009, 8, 935–939. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, K.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano. 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Voltairas, P.A.; Fotiadis, D.I.; Michalis, L.K. Hydrodynamics of magnetic drug targeting. J. Biomech. 2002, 35, 813–821. [Google Scholar] [CrossRef]
- Niu, C.; Xu, Y.; An, S.; Zhang, M.; Hu, Y.; Wang, L.; Peng, Q. Near-infrared induced phase-shifted ICG/Fe3O4 loaded PLGA nanoparticles for photothermal tumor ablation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Maity, D. Systematic investigations on heating effects of carboxyl-amine functionalized superparamagnetic iron oxide nanoparticles (SPIONs) based ferrofluids for in vitro cancer hyperthermia therapy. J. Mol. Liq. 2018, 256, 224–237. [Google Scholar] [CrossRef]
- Mazario, E.; Forget, A.; Belkahla, H.; Lomas, J.S.; Decorse, P.; Chevillot-Biraud, A.; Verbeke, P.; Wilhelm, C.; Ammar, S.; Chahine, J.E.H.; et al. Functionalization of Iron Oxide Nanoparticles with HSA Protein for Thermal Therapy. IEEE Trans Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Shen, S.; Kong, F.; Guo, X.; Wu, L.; Shen, H.; Xie, M.; Wang, X.; Jin, Y.; Ge, Y. CMCTS stabilized Fe3O4 particles with extremely low toxicity as highly efficient near-infrared photothermal agents for in vivo tumor ablation. Nanoscale 2013, 5, 8056–8066. [Google Scholar] [CrossRef]
- Tomitaka, A.; Koshi, T.; Hatsugai, S.; Yamada, T.; Takemura, Y. Magnetic characterization of surface-coated magnetic nanoparticles for biomedical application. J. Mag. Magn. Mater. 2011, 323, 1398–1403. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Novoselova, O.S.; Filatova, A.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Plasmonics for treatment of atherosclerosis: Results of NANOM-FIM trial. J. Nanomed. Nanotechnol. 2013, 2, 4. [Google Scholar] [CrossRef]
- Kharlamov, A. Frontiers of plasmonic photothermal and stem cell therapy of atherosclerosis: Nanotoxicity in NANOM-PCI trial. Eur. Heart J. 2015, 36, 384. [Google Scholar]
- Sudjarwo, S.A.; Sudjarwo, G.W.; Koerniasari. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017, 12, 381–390. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [Green Version]
- Nehra, P.; Chauhan, R.P.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef]
- Rafi, M.M.; Ahmed, K.S.Z.; Nazeer, K.P.; Kumar, D.S.; Thamilselvan, M. Synthesis, characterization and magnetic properties of hematite (α-Fe2O3) nanoparticles on polysaccharide templates and their antibacterial activity. App. Nanosci. 2015, 5, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hughes, J.; Chen, Y. Impacts of hematite nanoparticle exposure on biomechanical, adhesive, and surface electrical properties of escherichia coli cells. App. Environ. Microbiol. 2012, 78, 3905–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Ali, M.S.; Oh, I.G.; Baek, K.H. Proteasome inhibitory, antioxidant, and synergistic antibacterial and anticandidal activity of green biosynthesized magnetic Fe3O4 nanoparticles using the aqueous extract of corn (Zea mays L.) ear leaves. Artif. Cells Nanomed. Biotechnol. 2017, 45, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, N.; Bozeman, E.N.; Qian, W.; Wang, L.; Chen, H.; Lipowska, M.; Staley, C.A.; Wang, Y.A.; Mao, H.; Yang, L. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics 2017, 7, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Saravanan, M.; Udaya Prakash, N.K.; Valan Arasu, M.; Vijayakumar, B.; Vincent, S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mat. Res. Bulletin. 2013, 48, 3323–3327. [Google Scholar] [CrossRef]



| Serial No. | Compound Name | Coating | Applications | Clinically Approved | References |
|---|---|---|---|---|---|
| 1 | Ferumoxtran (Combidex®) | Dextran | Lymph node imaging, macrophage imaging, blood pool agent, cell labelling, CNS imaging, MRI | In clinical trials | [27,28] |
| 2 | Ferucarbotran (Resovist®) | Carboxydextran | Liver imaging, cell labelling, CNS imaging, MRI | Approved | [29,30,31] |
| 3 | Ferumoxide (Feridex®) | Dextran | Liver imaging, cell labelling, CNS imaging, MRI | Withdrawn from market | [32,33,34] |
| 4 | Ferumoxytol (Feraheme®) | Carboxymethyl-dextran | Iron replacement therapy in patients with chronic kidney diseases | Approved | [35] |
| 5 | Feruglose (Clariscan™) | PEGylated starch | Blood pool agent, MRI | In clinical trials | [36] |
| 6 | Ferumoxsil (Gastromark®) | Siloxane | Oral GI imaging | Approved | [13,37] |
| Serial No. | IONPs/Conjugated IONPs | Applications | References |
|---|---|---|---|
| 1 | Fe3O4 NPs | Exhibit peroxidase enzyme like activity. Used for fluorescent turn off system for detection of protein in urine | [50] |
| 2 | Chitosan coated IONPs with urease IONPs | Used for detection of urea | [51] |
| 3 | IONPs | Detection of Brucella antibodies with a LOD of 0.05 µg/mL | [52] |
| 4 | Fe3O4 nanocomposites/graphene oxide | Biosensor synthesis for glucose detection with the range 0.5–10 mM | [53] |
| 5 | Fe3O4 NPs loaded in Co3O4 nanocages | Used for glucose detection with the range of 0.5–30 µM with an LOD of 0.05 µM | [54] |
| Serial No. | IONPs/Conjugated IONPs | Applications | References |
|---|---|---|---|
| 1 | IONP coated with doxorubicin (DOX) and 2-deoxy- D glucose | NPs when combined with doxorubicin and 2-deoxy-D-glucose showed enhanced chemotherapeutic actions in breast cancer cells via targeting | [69] |
| 2 | Dextran coated IONP conjugated with FITC and DOX | This nanoconjugate has various applications they are used for drug delivery, MRI, FITC fluorescence imaging, pancreatic cancer treatment via hyperthermia | [70] |
| 3 | Daunorubicin loaded IONPs | Used for treatment of brain glioma, it was observed that these NPs have the capacity to cross the blood brain barrier and act as a drug to treat blood cancer | [71] |
| 4 | DOX loaded in reduced graphene oxide coated IONPs | Caused inhibition of growth in HeLa cells when assisted with hyperthermia treatment | [72] |
| 5 | IONP conjugates with Homoharringtonine | Used for hematological anomalies, drug conjugated with IONPs were more effective in reducing tumor growth in case of leukemia in mice compared to only drug treatment | [73] |
| 6 | Fe3O4 nanoparticles | Used for tumor treatment using cryoablation therapy, extreme cold temperature is provided to destroy cells and tissues. Cryoprobes (thermally conductive fluids) are injected intravenously to the targeted regions | [74] |
| Serial No | IONPs/Conjugated IONPs | Applications | References |
|---|---|---|---|
| 1 | Fe3O4/ICG/PFP encapsulated in PLGA | In vitro treatment of MCF-7 breast cancer cells via PTT, where the nanoconjugate is used an as agent for tumor termination | [87] |
| 2 | SPIONs | Hyperthermia based theraphy for liver cancer treatment | [88] |
| 3 | Carboxyl amine functionalized SPIONs | Terapthalic acid and amino terapthalic acid coated SPIONs caused in vitro hyperthermia and induces cell death in MCF-7 breast cancer cells | [89] |
| 4 | IONP functionalized with HAS protein | Used for magnetic thermal therapy where the MNPs at 36 °C produce a localized heat in presence of alternating magnetic field | [90] |
| 5 | CMCT functionalized Fe3O4 NPs | Used for photothermal therapy where the NPs found to be accumulated at the tumour region and due to PTT, there is an increase in temperature up to 52 °C | [91] |
| 6 | Fe3O4/NiFe2O4 NPs coated with oleic acid | Used for magnetic hyperthermia therapy where oleic acid coated NP clusters were targeted in vitro in HeLa cells and in presence of external magnetic field an increase in temperature was observed. | [92] |
| 7 | NanoTherm™ Aqueous dispersion of superparamagnetic iron oxide nanoparticles | Used for hyperthermia and currently in clinical trial phase | [76] |
| 8 | NCT01270139 Iron-bearing nanoparticles | Used for hyperthermia and currently in clinical trial phase | [93] |
| 9 | NCT01436123 Gold nanoparticles with iron oxide-silica shells | Used for hyperthermia and currently in clinical trial phase | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy. Cancers 2021, 13, 2213. https://doi.org/10.3390/cancers13092213
Chouhan RS, Horvat M, Ahmed J, Alhokbany N, Alshehri SM, Gandhi S. Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy. Cancers. 2021; 13(9):2213. https://doi.org/10.3390/cancers13092213
Chicago/Turabian StyleChouhan, Raghuraj Singh, Milena Horvat, Jahangeer Ahmed, Norah Alhokbany, Saad M. Alshehri, and Sonu Gandhi. 2021. "Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy" Cancers 13, no. 9: 2213. https://doi.org/10.3390/cancers13092213
APA StyleChouhan, R. S., Horvat, M., Ahmed, J., Alhokbany, N., Alshehri, S. M., & Gandhi, S. (2021). Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy. Cancers, 13(9), 2213. https://doi.org/10.3390/cancers13092213








