Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Obtention of Cells over Expressing RECK
2.3. Western Blot
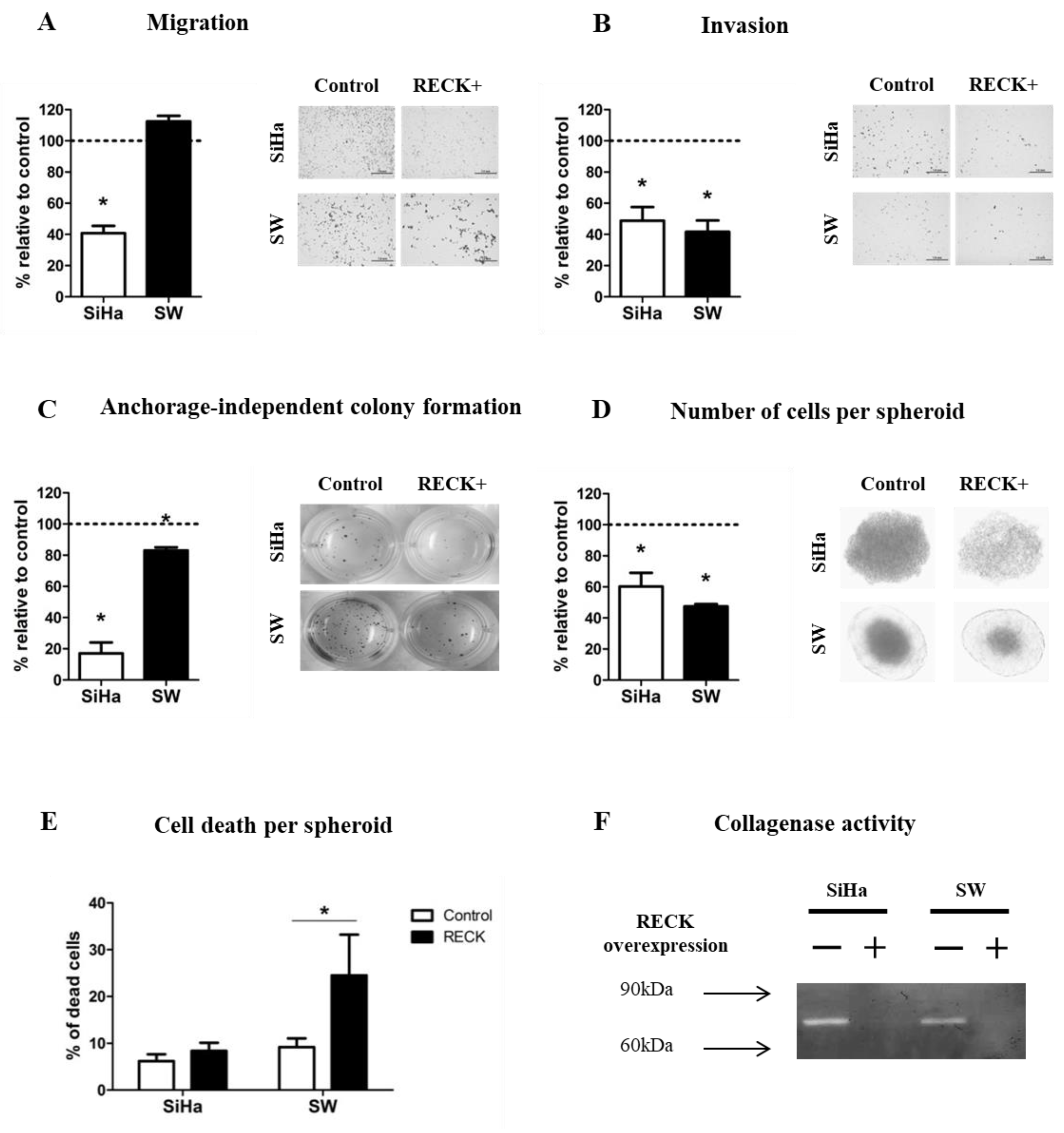
2.4. Clonogenic, Migration, Invasion and Anchorage Independent Growth Assays
2.5. Tumor Spheroids Formation
2.6. In Vitro Zymography
2.7. SDS-PAGE Gelatin Zymography
2.8. Tumor Growth Kinetics in Nude Mice
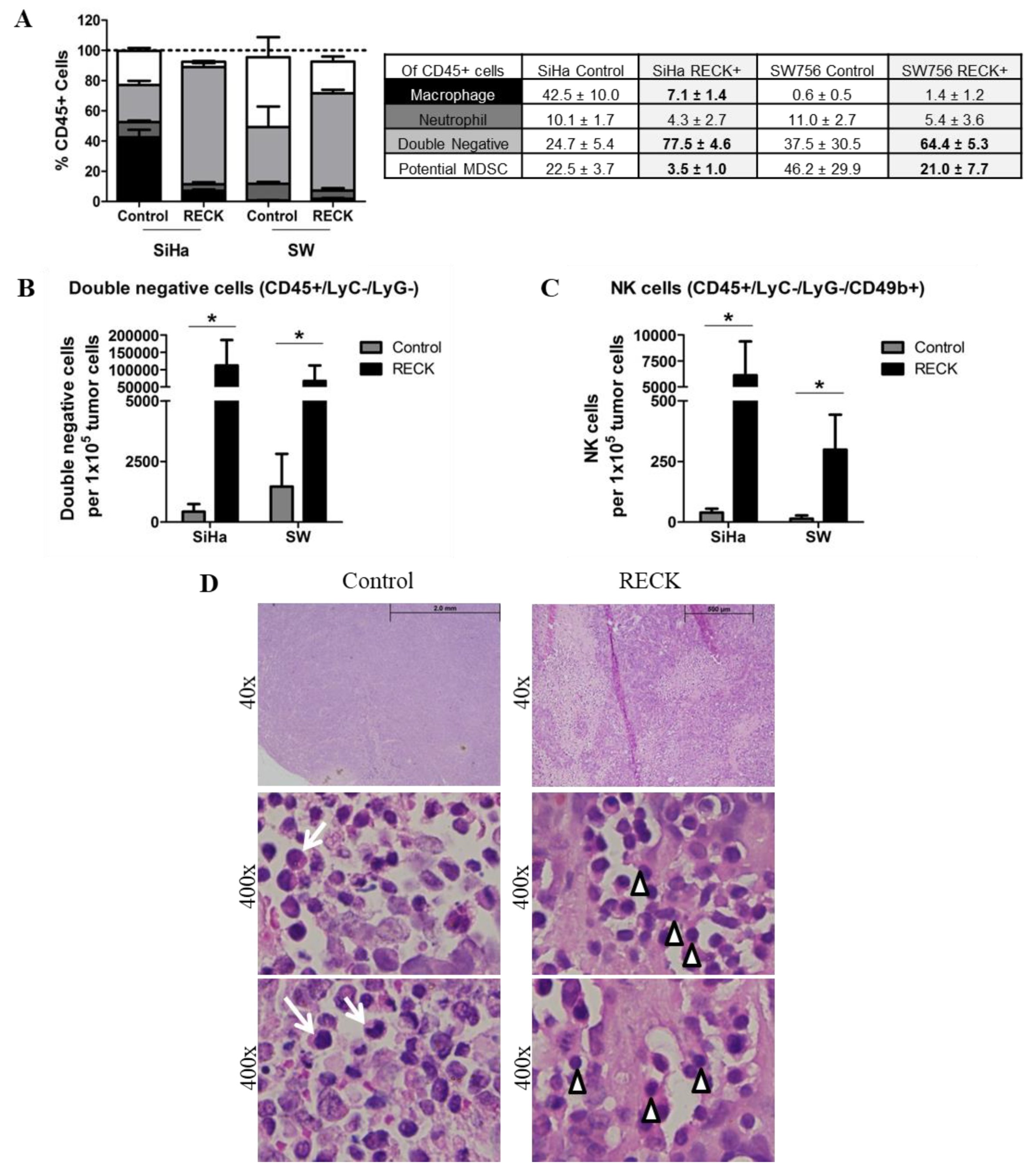
2.9. Analysis of Intratumoral Cell Populations
2.10. In Situ Zymography
2.11. Immunofluorescence
2.12. Protein Arrays
2.13. Global Gene Expression Analysis
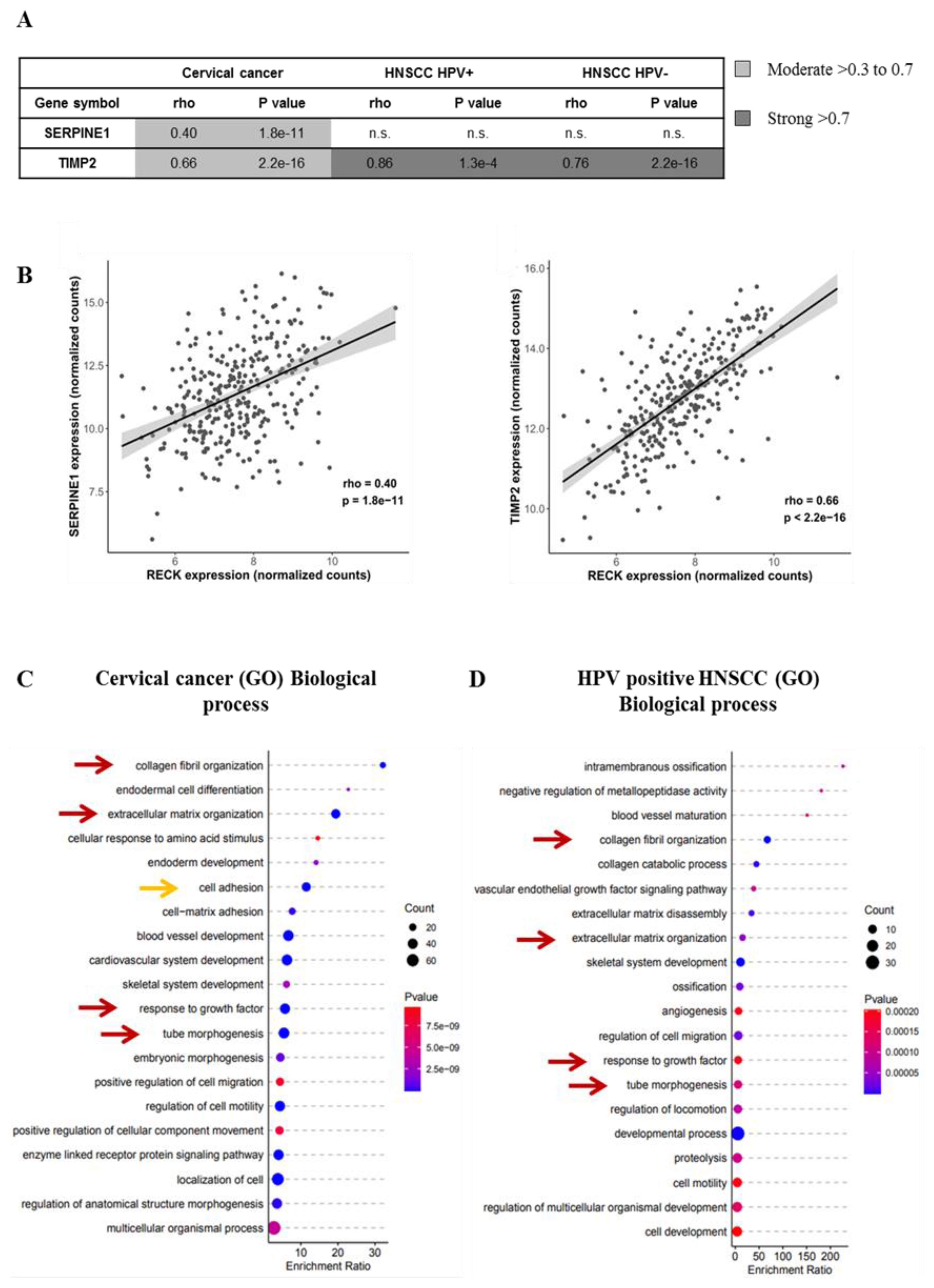
2.14. GO Pathway Enrichment Analysis
2.15. Statistical Analysis
3. Results
3.1. RECK Expression Delays HPV16- and HPV18-Positive Tumors Growth and Increases Overall Survival In Vivo
3.2. RECK Expression Reduces Characteristics Associated with the Tumorigenic Potential of HPV-Transformed Cell Lines
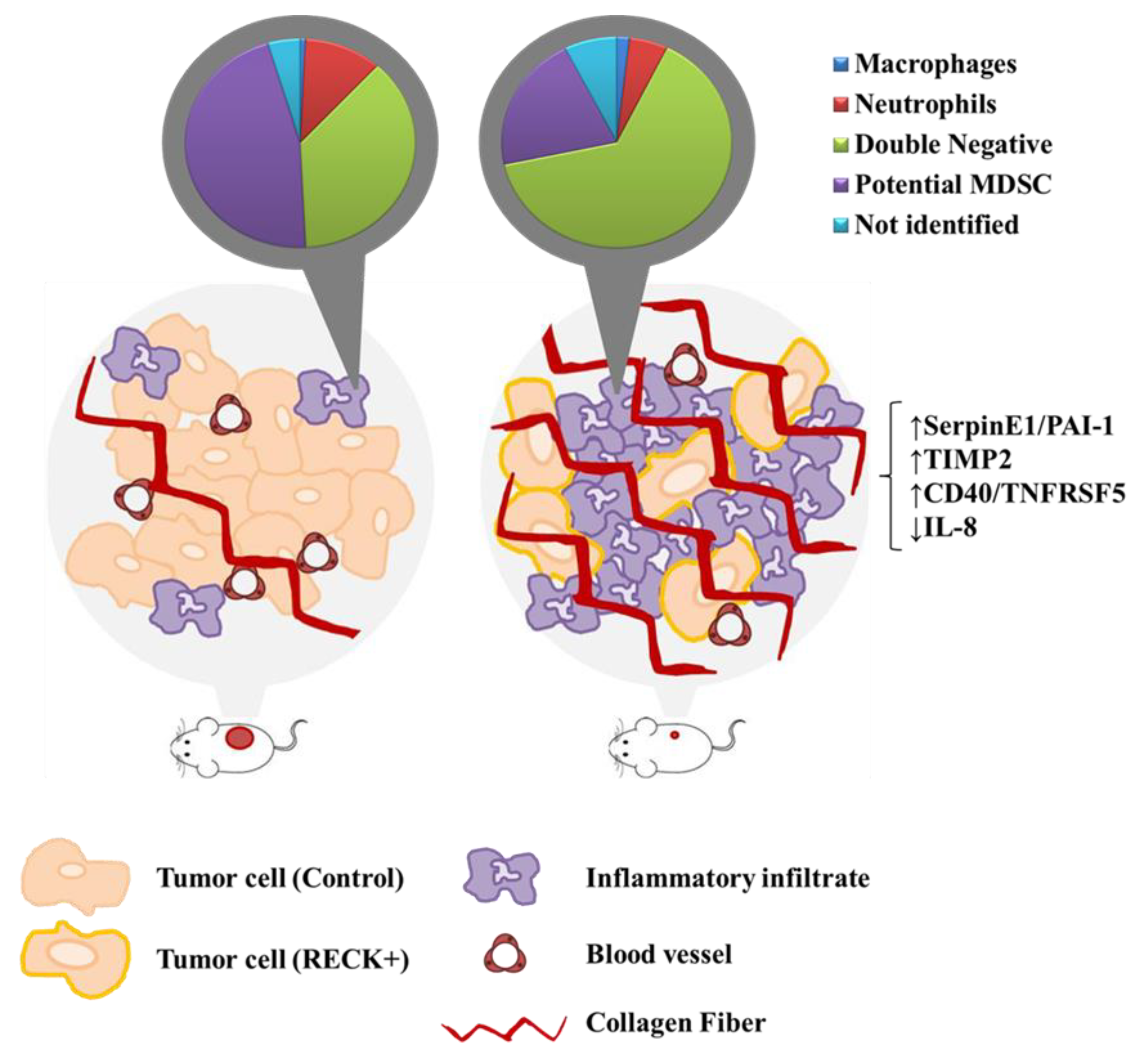
3.3. Tumors Established from RECK+ Cells Exhibited Less Tumor and Endothelial Cells and an Increased Inflammatory Infiltrate Population
3.4. RECK Expression Induces Alterations in the Intra-Tumoral Inflammatory Infiltrate Profile
3.5. RECK Expression Alters Collagenase Activity, Collagen Content and Expression of Protease Inhibitors In Vivo
3.6. RECK Expression Correlates with Higher Levels of Protease Inhibitors Both in Experimental Mouse Models and Cervical Cancer Clinical Samples
3.7. RECK down Regulation Is a Consistent Trait in Cervical Cancer History
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Schlecht, N.F.; Kulaga, S.; Robitaille, J.; Ferreira, S.; Santos, M.; Miyamura, R.A.; Duarte-Franco, E.; Rohan, T.E.; Ferenczy, A.; Villa, L.L.; et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001, 286, 3106–3114. [Google Scholar] [CrossRef] [Green Version]
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef]
- Herbster, S.; Paladino, A.; de Freitas, S.; Boccardo, E. Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics 2018, 73. [Google Scholar] [CrossRef] [PubMed]
- Prati, B.; Marangoni, B.; Boccardo, E. Human papillomavirus and genome instability: From productive infection to cancer. Clinics 2018, 73. [Google Scholar] [CrossRef]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2020, 1–19. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Fan, L.C.; Yang, S.C.; Tsao, C.H.; Lee, H.; Cheng, Y.W.; Lai, L.C.; Chang, Y.H. Human Papillomavirus Up-Regulates MMP-2 and MMP-9 Expression and Activity by Inducing Interleukin-8 in Lung Adenocarcinomas. PLoS ONE 2013, 8, e54423. [Google Scholar] [CrossRef]
- Basukala, O.; Mittal, S.; Massimi, P.; Bestagno, M.; Banks, L. The HPV-18 E7 CKII phospho acceptor site is required for maintaining the transformed phenotype of cervical tumour-derived cells. PLoS Pathog. 2019, 15, e1007769. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.-C.; Lien, H.-C.; Ho, H.-N.; Lin, H.-H.; Chow, S.-N.; Huang, S.-C.; Hsu, S.-M. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003, 63, 6537–6542. [Google Scholar] [PubMed]
- Kaewprag, J.; Umnajvijit, W.; Ngamkham, J.; Ponglikitmongkol, M. HPV16 oncoproteins promote cervical cancer invasiveness by upregulating specific matrix metalloproteinases. PLoS ONE 2013, 8, e71611. [Google Scholar] [CrossRef] [PubMed]
- Discacciati, M.G.; Gimenes, F.; Pennacchi, P.C.; Faiao-Flores, F.; Zeferino, L.C.; Derchain, S.M.; Teixeira, J.C.; Costa, M.C.; Zonta, M.; Termini, L.; et al. MMP-9/RECK Imbalance: A Mechanism Associated with High-Grade Cervical Lesions and Genital Infection by Human Papillomavirus and Chlamydia trachomatis. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1539–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libra, M.; Scalisi, A.; Vella, N.; Clementi, S.; Sorio, R.; Stivala, F.; Spandidos, D.A.; Mazzarino, C. Uterine cervical carcinoma: Role of matrix metalloproteinases (review). Int. J. Oncol. 2009, 34, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cardeal, L.B.S.; Boccardo, E.; Termini, L.; Rabachini, T.; Andreoli, M.A.; di Loreto, C.; Filho, A.L.; Villa, L.L.; Maria-Engler, S.S. HPV16 Oncoproteins Induce MMPs/RECK-TIMP-2 Imbalance in Primary Keratinocytes: Possible Implications in Cervical Carcinogenesis. PLoS ONE 2012, 7, e33585. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Takahashi, R.; Kondo, S.; Mizoguchi, A.; Adachi, E.; Sasahara, R.M.; Nishimura, S.; Imamura, Y.; Kitayama, H.; Alexander, D.B.; et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001, 107, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Chandana, E.P.; Maeda, Y.; Ueda, A.; Kiyonari, H.; Oshima, N.; Yamamoto, M.; Kondo, S.; Oh, J.; Takahashi, R.; Yoshida, Y.; et al. Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice. BMC Dev. Biol. 2010, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Matsuzaki, T.; Takahashi, R.; Adachi, E.; Maeda, Y.; Yamaguchi, S.; Kitayama, H.; Echizenya, M.; Morioka, Y.; Alexander, D.B.; et al. The transformation suppressor gene Reck is required for postaxial patterning in mouse forelimbs. Biol. Open 2012, 1, 458–466. [Google Scholar] [CrossRef] [Green Version]
- van Lent, P.L.E.M.; Span, P.N.; Sloetjes, A.W.; Radstake, T.R.D.J.; van Lieshout, A.W.T.; Heuvel, J.J.T.M.; Sweep, C.G.J.; van den Berg, W.B. Expression and localisation of the new metalloproteinase inhibitor RECK (reversion inducing cysteine-rich protein with Kazal motifs) in inflamed synovial membranes of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, R.M.; Takahashi, C.; Sogayar, M.C.; Noda, M. Oncogene-mediated downregulation of RECK, a novel transformation suppressor gene. Braz. J. Med. Biol. Res. 1999, 32, 891–895. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Hisanaga, M.; Nagao, M.; Ikeda, N.; Fujii, H.; Koyama, F.; Mukogawa, T.; Matsumoto, H.; Kondo, S.; Takahashi, C.; et al. The membrane-anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin. Cancer Res. 2004, 10, 5572–5579. [Google Scholar] [CrossRef] [Green Version]
- Furumoto, K.; Arii, S.; Mori, A.; Furuyama, H.; Gorrin Rivas, M.J.; Nakao, T.; Isobe, N.; Murata, T.; Takahashi, C.; Noda, M.; et al. RECK gene expression in hepatocellular carcinoma: Correlation with invasion-related clinicopathological factors and its clinical significance. Reverse-inducing--cysteine-rich protein with Kazal motifs. Hepatology 2001, 33, 189–195. [Google Scholar] [CrossRef]
- Takenaka, K.; Ishikawa, S.; Yanagihara, K.; Miyahara, R.; Hasegawa, S.; Otake, Y.; Morioka, Y.; Takahashi, C.; Noda, M.; Ito, H.; et al. Prognostic significance of reversion-inducing cysteine-rich protein with Kazal motifs expression in resected pathologic stage IIIA N2 non-small-cell lung cancer. Ann. Surg. Oncol. 2005, 12, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Takahashi, C. Recklessness as a hallmark of aggressive cancer. Cancer Sci. 2007, 98, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cardeal, L.B.; Brohem, C.A.; Corrêa, T.C.S.; Winnischofer, S.M.B.; Nakano, F.; Boccardo, E.; Villa, L.L.; Sogayar, M.C.; Maria-Engler, S.S. Higher expression and activity of metalloproteinases in human cervical carcinoma cell lines is associated with HPV presence. Biochem. Cell Biol. 2006, 84, 713–719. [Google Scholar] [CrossRef]
- Trombetta-Lima, M.; Maria, S.; Winnischofer, B.; Angelo, M.; Demasi, A.; Filho, R.A.; Carreira, A.C.O.; Wei, B.; De Assis Ribas, T.; Konig, M.S.; et al. Isolation and characterization of novel RECK tumor suppressor gene splice variants. Oncotarget 2015, 6, 33120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naldini, L.; Blömer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni-Cardoso, A.; Vilamaior, P.S.L.; Taboga, S.R.; Carvalho, H.F. Localized matrix metalloproteinase (MMP)-2 and MMP-9 activity in the rat ventral prostate during the first week of postnatal development. Histochem. Cell Biol. 2008, 129, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.M.; Duckett, L.; Daul, B.; Petrie, H.T. A simple method for detecting up to five immunofluorescent parameters together with DNA staining for cell cycle or viability on a benchtop flow cytometer. J. Immunol. Methods 2003, 275, 113–121. [Google Scholar] [CrossRef]
- Falcon, S.; Gentleman, R. BIOINFORMATICS APPLICATIONS NOTE Systems biology Using GOstats to test gene lists for GO term association. Bioinformatics 2007, 23, 257–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tcyganov, E.; Mastio, J.; Chen, E.; Gabrilovich, D.I. Plasticity of myeloid-derived suppressor cells in cancer. Curr. Opin. Immunol. 2018, 51, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, N.; Baba, T.; Matsumura, N.; Murakami, R.; Abiko, K.; Hamanishi, J.; Yamaguchi, K.; Koshiyama, M.; Yoshioka, Y.; Konishi, I. Genomic profile predicts the efficacy of neoadjuvant chemotherapy for cervical cancer patients. BMC Cancer 2015, 15, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Imamura, Y.; Ishibashi, R.; Chandana, E.P.S.; Yamamoto, M.; Noda, M. The Reck tumor suppressor protein alleviates tissue damage and promotes functional recovery after transient cerebral ischemia in mice. J. Neurochem. 2010, 115, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Ishikawa, S.; Kawano, Y.; Yanagihara, K.; Miyahara, R.; Otake, Y.; Morioka, Y.; Takahashi, C.; Noda, M.; Wada, H.; et al. Expression of a novel matrix metalloproteinase regulator, RECK, and its clinical significance in resected non-small cell lung cancer. Eur. J. Cancer 2004, 40, 1617–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tseng, S.-H. The potential of RECK inducers as antitumor agents for glioma. Anticancer Res. 2012, 32, 2991–2998. [Google Scholar] [PubMed]
- Chung, T.-T.; Yeh, C.-B.; Li, Y.-C.; Su, S.-C.; Chien, M.-H.; Yang, S.-F.; Hsieh, Y.-H. Effect of RECK gene polymorphisms on hepatocellular carcinoma susceptibility and clinicopathologic features. PLoS ONE 2012, 7, e33517. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zhang, G.; Ma, W.; Liu, Y.; Zhao, R.; Zhang, Q.; Pang, D. Low expression of RECK indicates a shorter survival for patients with invasive breast cancer. Cancer Sci. 2012, 103, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, S.; Jiang, L.; Zhang, S.; Li, W.; Chen, Z.; Zhang, D. Expression of RECK and MMP-2 in salivary adenoid cystic carcinoma: Correlation with tumor progression and patient prognosis. Oncol. Lett. 2014, 7, 1549–1555. [Google Scholar] [CrossRef]
- Takahashi, C.; Sheng, Z.; Horan, T.P.; Kitayama, H.; Maki, M.; Hitomi, K.; Kitaura, Y.; Takai, S.; Sasahara, R.M.; Horimoto, A.; et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc. Natl. Acad. Sci. USA 1998, 95, 13221–13226. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, R.M.; Brochado, S.M.; Takahashi, C.; Oh, J.; Maria-Engler, S.S.; Granjeiro, J.M.; Noda, M.; Sogayar, M.C. Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect. Prev. 2002, 26, 435–443. [Google Scholar] [CrossRef]
- Takagi, S.; Simizu, S.; Osada, H. RECK Negatively Regulates Matrix Metalloproteinase-9 Transcription. Cancer Res. 2009, 69, 1502–1508. [Google Scholar] [CrossRef] [Green Version]
- Alexius-Lindgren, M.; Andersson, E.; Lindstedt, I.; Engström, W. The RECK gene and biological malignancy--its significance in angiogenesis and inhibition of matrix metalloproteinases. Anticancer Res. 2014, 34, 3867–3873. [Google Scholar]
- Zhao, H.; Wang, Y.; Yang, L.; Jiang, R.; Li, W. MiR-25 promotes gastric cancer cells growth and motility by targeting RECK. Mol. Cell. Biochem. 2014, 385, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.A.; Roy, D.M.; Reyngold, M.; Giri, D.; Snyder, A.; Turcan, S.; Badwe, C.R.; Lyman, J.; Bromberg, J.; King, T.A.; et al. RECK controls breast cancer metastasis by modulating a convergent, STAT3-dependent neoangiogenic switch. Oncogene 2015, 34, 2189–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masui, T.; Doi, R.; Koshiba, T.; Fujimoto, K.; Tsuji, S.; Nakajima, S.; Koizumi, M.; Toyoda, E.; Tulachan, S.; Ito, D.; et al. RECK expression in pancreatic cancer: Its correlation with lower invasiveness and better prognosis. Clin. Cancer Res. 2003, 9, 1779–1784. [Google Scholar] [PubMed]
- Liu, Y.; Li, L.; Liu, Y.; Geng, P.; Li, G.; Yang, Y.; Song, H. RECK inhibits cervical cancer cell migration and invasion by promoting p53 signaling pathway. J. Cell. Biochem. 2018, 119, 3058–3066. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. “Sprouting angiogenesis”, a reappraisal. Dev. Biol. 2012, 372, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Siekmann, A.F.; Affolter, M.; Belting, H.-G. The tip cell concept 10 years after: New players tune in for a common theme. Exp. Cell Res. 2013, 319, 1255–1263. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef]
- Le Maux Chansac, B.; Missé, D.; Richon, C.; Vergnon, I.; Kubin, M.; Soria, J.-C.; Moretta, A.; Chouaib, S.; Mami-Chouaib, F. Potentiation of NK cell-mediated cytotoxicity in human lung adenocarcinoma: Role of NKG2D-dependent pathway. Int. Immunol. 2008, 20, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Wang, X.; Zhang, H.; Deng, L.; Zhang, Y. MMP9 mediates MICA shedding in human osteosarcomas. Cell Biol. Int. 2011, 35, 569–574. [Google Scholar] [CrossRef]
- Shiraishi, K.; Mimura, K.; Kua, L.F.; Koh, V.; Siang, L.K.; Nakajima, S.; Fujii, H.; Shabbir, A.; Yong, W.P.; So, J.; et al. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J. Gastroenterol. 2016, 51, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Sparmann, A.; Bar-Sagi, D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004, 6, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Houghton, A.M.; Mariani, T.J.; Perera, S.; Kim, C.B.; Padera, R.; Tonon, G.; McNamara, K.; Marconcini, L.A.; Hezel, A.; et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006, 25, 2105–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freisinger, C.M.; Huttenlocher, A. Live Imaging and Gene Expression Analysis in Zebrafish Identifies a Link between Neutrophils and Epithelial to Mesenchymal Transition. PLoS ONE 2014, 9, e112183. [Google Scholar] [CrossRef]
- Gabellini, C.; Trisciuoglio, D.; Desideri, M.; Candiloro, A.; Ragazzoni, Y.; Orlandi, A.; Zupi, G.; Del Bufalo, D. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur. J. Cancer 2009, 45, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef]
- Singer, M.; Sansonetti, P.J. IL-8 Is a Key Chemokine Regulating Neutrophil Recruitment in a New Mouse Model of Shigella- Induced Colitis. J. Immunol. 2004, 173, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 155, 1428–1433. [Google Scholar] [PubMed]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Sakaguchi, H.; Aoki, I.; Tamaya, T. Clinical Implications of Expression of Interleukin 8 Related to Angiogenesis in Uterine Cervical Cancers. Cancer Res. 2000, 60, 2632–2635. [Google Scholar]
- Kemp, T.J.; Hildesheim, A.; García-Piñeres, A.; Williams, M.C.; Shearer, G.M.; Rodriguez, A.C.; Schiffman, M.; Burk, R.; Freer, E.; Bonilla, J.; et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1954–1959. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.E.; Shvetsov, Y.B.; Thompson, P.J.; Hernandez, B.Y.; Zhu, X.; Wilkens, L.R.; Killeen, J.; Vo, D.D.; Moscicki, A.-B.; Goodman, M.T. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study. Int. J. Cancer 2013, 133, 1187–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Shang, H.; Cui, L.; Zhang, Z.; Zhang, Y.; Li, Y.; Wu, J.; Li, R.K.; Xie, J. Targeted blockade of interleukin-8 abrogates its promotion of cervical cancer growth and metastasis. Mol. Cell. Biochem. 2013, 375, 69–79. [Google Scholar] [CrossRef]
- Jia, L.; Li, F.; Shao, M.; Zhang, W.; Zhang, C.; Zhao, X.; Luan, H.; Qi, Y.; Zhang, P.; Liang, L.; et al. IL-8 is upregulated in cervical cancer tissues and is associated with the proliferation and migration of HeLa cervical cancer cells. Oncol. Lett. 2018, 15, 1350–1356. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [Green Version]
- Mian, B.M.; Dinney, C.P.N.; Bermejo, C.E.; Sweeney, P.; Tellez, C.; Yang, X.D.; Gudas, J.M.; McConkey, D.J.; Bar-Eli, M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 2003, 9, 3167–3175. [Google Scholar]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Proost, P.; Wuyts, A.; Van Damme, J.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood 2000, 96, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Guedez, L.; Jensen-Taubman, S.; Bourboulia, D.; Kwityn, C.J.; Wei, B.; Caterina, J.; Stetler-Stevenson, W.G. TIMP-2 targets tumor-associated myeloid suppressor cells with effects in cancer immune dysfunction and angiogenesis. J. Immunother. 2012, 35, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagima, Y.; Avigdor, A.; Goichberg, P.; Shivtiel, S.; Tesio, M.; Kalinkovich, A.; Golan, K.; Dar, A.; Kollet, O.; Petit, I.; et al. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. J. Clin. Investig. 2009, 119, 492–503. [Google Scholar] [CrossRef]
- Golan, K.; Vagima, Y.; Goichberg, P.; Gur-Cohen, S.; Lapidot, T. MT1-MMP and RECK: Opposite and essential roles in hematopoietic stem and progenitor cell retention and migration. J. Mol. Med. 2011, 89, 1167–1174. [Google Scholar] [CrossRef]
- de Almeida, G.M.; Yamamoto, M.; Morioka, Y.; Ogawa, S.; Matsuzaki, T.; Noda, M. Critical roles for murine Reck in the regulation of vascular patterning and stabilization. Sci. Rep. 2015, 5, 17860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, S.; Miki, T.; Takegami, Y.; Kido, Y.; Noda, M.; Hara, E.; Shamma, A.; Takahashi, C. Reversion-inducing cysteine-rich protein with Kazal motifs interferes with epidermal growth factor receptor signaling. Oncogene 2011, 30, 737–750. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbster, S.; Trombetta-Lima, M.; de Souza-Santos, P.T.; Paladino, A.; Silveira, C.R.F.; Sogayar, M.C.; Villa, L.L.; Lepique, A.P.; Boccardo, E. Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms. Cancers 2021, 13, 2217. https://doi.org/10.3390/cancers13092217
Herbster S, Trombetta-Lima M, de Souza-Santos PT, Paladino A, Silveira CRF, Sogayar MC, Villa LL, Lepique AP, Boccardo E. Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms. Cancers. 2021; 13(9):2217. https://doi.org/10.3390/cancers13092217
Chicago/Turabian StyleHerbster, Suellen, Marina Trombetta-Lima, Paulo Thiago de Souza-Santos, Andressa Paladino, Caio Raony Farina Silveira, Mari Cleide Sogayar, Luisa Lina Villa, Ana Paula Lepique, and Enrique Boccardo. 2021. "Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms" Cancers 13, no. 9: 2217. https://doi.org/10.3390/cancers13092217
APA StyleHerbster, S., Trombetta-Lima, M., de Souza-Santos, P. T., Paladino, A., Silveira, C. R. F., Sogayar, M. C., Villa, L. L., Lepique, A. P., & Boccardo, E. (2021). Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms. Cancers, 13(9), 2217. https://doi.org/10.3390/cancers13092217






