Crown-Like Structures in Breast Adipose Tissue: Early Evidence and Current Issues in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Complex Relationship between Obesity and Breast Cancer
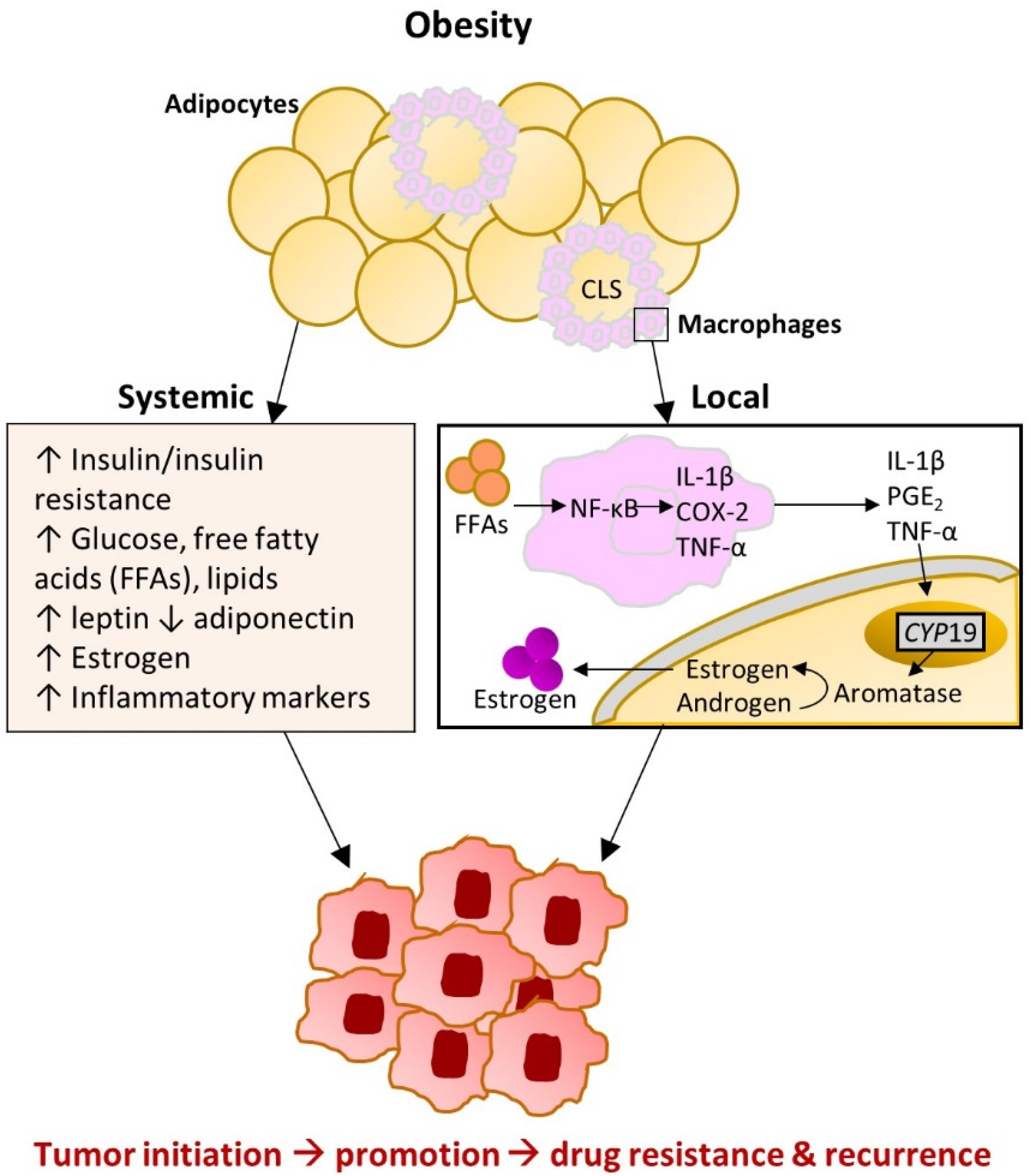
2.1. Obesity-Induced Changes in Adipose Tissue
2.2. Breast Adipose Tissue Microenvironment
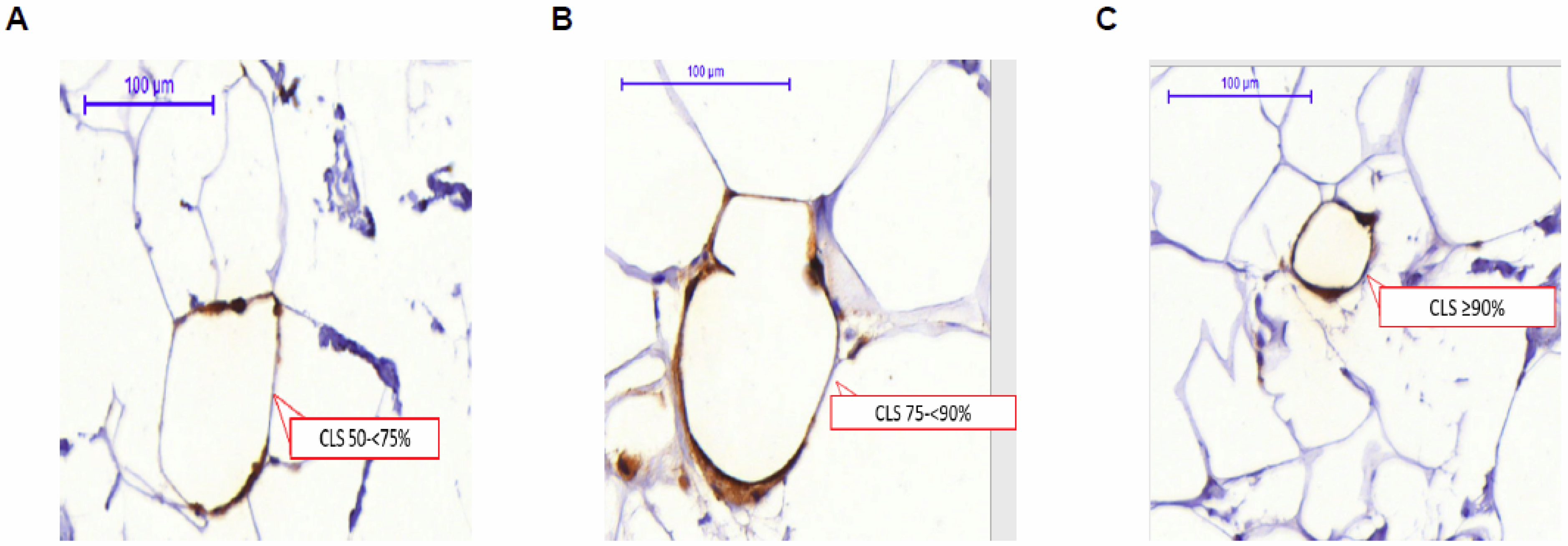
3. Crown-Like Structures of the Breast (CLS-B): Histologic Marker of Local Inflammation
4. Potential Etiologic Drivers of CLS-B
4.1. Obesity and CLS-B
4.2. Other Factors and CLS-B
5. CLS-B and Incident Breast Cancer
6. CLS-B as a Potential Driver of Prognosis
7. Future Directions
7.1. Methodology in CLS-B Assessment
7.2. The Role of CLS-B in the Incidence of Breast Cancer
7.3. The Role of CLS-B in Predicting Therapeutic Effectiveness and Breast Cancer Prognosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jiralerspong, S.; Goodwin, P.J. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Thun, M.J.; Linet, M.S.; Cerhan, J.R.; Haiman, C.; Schottenfeld, D. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, 4th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Koshiol, J.; Lin, S.-W. Can tissue-based immune markers be used for studying the natural history of cancer? Ann. Epidemiol. 2012, 22, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Berger, N.A. Crown-like structures in breast adipose tissue from normal weight women: Important impact. Cancer Prev. Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Saji, S.; Toi, M. Impact of body mass index on breast cancer in accordance with the life-stage of women. Front. Oncol. 2012, 2, 123. [Google Scholar] [CrossRef] [Green Version]
- James, F.; Wootton, S.; Jackson, A.; Wiseman, M.; Copson, E.; Cutress, R. Obesity in breast cancer–what is the risk factor? Eur. J. Cancer 2015, 51, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project—Systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Premenopausal Breast Cancer Collaborative Group. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J. Natl. Cancer Inst. 2011, 103, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Bandera, E.V.; Chandran, U.; Hong, C.-C.; Troester, M.A.; Bethea, T.N.; Adams-Campbell, L.L.; Haiman, C.A.; Park, S.-Y.; Olshan, A.F.; Ambrosone, C.B. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res. Treat. 2015, 150, 655–666. [Google Scholar] [CrossRef]
- Ma, H.; Ursin, G.; Xu, X.; Lee, E.; Togawa, K.; Malone, K.E.; Marchbanks, P.A.; McDonald, J.A.; Simon, M.S.; Folger, S.G. Body mass index at age 18 years and recent body mass index in relation to risk of breast cancer overall and ER/PR/HER2-defined subtypes in white women and African-American women: A pooled analysis. Breast Cancer Res. 2018, 20, 5. [Google Scholar] [CrossRef]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef]
- Houghton, S.C.; Eliassen, H.; Tamimi, R.M.; Willett, W.C.; Rosner, B.A.; Hankinson, S.E. Central adiposity and subsequent risk of breast cancer by menopause status. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- White, A.J.; Nichols, H.B.; Bradshaw, P.T.; Sandler, D.P. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer 2015, 121, 3700–3708. [Google Scholar] [CrossRef] [Green Version]
- Fagherazzi, G.; Chabbert-Buffet, N.; Fabre, A.; Guillas, G.; Boutron-Ruault, M.C.; Mesrine, S.; Clavel-Chapelon, F. Hip circumference is associated with the risk of premenopausal ER-/PR- breast cancer. Int. J. Obes. 2012, 36, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Canchola, A.J.; Anton-Culver, H.; Bernstein, L.; Clarke, C.A.; Henderson, K.; Ma, H.; Ursin, G.; Horn-Ross, P.L. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and inflammation: New insights into breast cancer development and progression. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 46–51. [Google Scholar] [CrossRef]
- Poloz, Y.; Stambolic, V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015, 6, e2037. [Google Scholar] [CrossRef] [Green Version]
- Agurs-Collins, T.; Ross, S.A.; Dunn, B.K. The Many Faces of Obesity and Its Influence on Breast Cancer Risk. Front. Oncol. 2019, 9, 765. [Google Scholar] [CrossRef] [Green Version]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Niraula, S.; Ocana, A.; Ennis, M.; Goodwin, P.J. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res. Treat. 2012, 134, 769–781. [Google Scholar] [CrossRef]
- Ewertz, M.; Gray, K.P.; Regan, M.M.; Ejlertsen, B.; Price, K.N.; Thurlimann, B.; Bonnefoi, H.; Forbes, J.F.; Paridaens, R.J.; Rabaglio, M.; et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1-98 trial. J. Clin. Oncol. 2012, 30, 3967–3975. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.B.; Gunnarsdottir, K.A.; Hojris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Taylor, S.K.; Hood, N. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J. Clin. Oncol. 2012, 30, 164–171. [Google Scholar] [CrossRef]
- Nechuta, S.; Chen, W.Y.; Cai, H.; Poole, E.M.; Kwan, M.L.; Flatt, S.W.; Patterson, R.E.; Pierce, J.P.; Caan, B.J.; Ou Shu, X. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int. J. Cancer 2016, 138, 2088–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestak, I.; Distler, W.; Forbes, J.F.; Dowsett, M.; Howell, A.; Cuzick, J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J. Clin. Oncol. 2010, 28, 3411–3415. [Google Scholar] [CrossRef] [PubMed]
- Norat, T.; Chan, D.; Vieira, A.R.; Aune, D.; Rosenblatt, D.N.; Vingeliene, S.; Abar, L.; Vieira, R. Systematic review on diet, nutrition, physical activity and survival and second cancers in breast cancer survivors. Contin. Update Proj. 2014, 222. Available online: https://www.aicr.org/wp-content/uploads/2020/01/breast-cancer-survivors-slr.pdf (accessed on 28 April 2021).
- Mei, L.; He, L.; Song, Y.; Lv, Y.; Zhang, L.; Hao, F.; Xu, M. Association between obesity with disease-free survival and overall survival in triple-negative breast cancer: A meta-analysis. Medicine 2018, 97, e0719. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 2016, 34, 4270. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, A.E.; Goodwin, P.J.; Chlebowski, R.T.; Pan, K.; Stambolic, V.; Dowling, R.J. Association of Obesity-Related Metabolic Disruptions with Cancer Risk and Outcome. J. Clin. Oncol. 2016, 34, 4249–4255. [Google Scholar] [CrossRef]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 27, 5760. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.; Dorgan, J.F.; Longcope, C.; Stanczyk, F.Z.; Stephenson, H.E., Jr.; Falk, R.T.; Miller, R.; et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003, 95, 1218–1226. [Google Scholar] [CrossRef]
- Dashti, S.G.; Simpson, J.A.; Karahalios, A.; Viallon, V.; Moreno-Betancur, M.; Gurrin, L.C.; MacInnis, R.J.; Lynch, B.M.; Baglietto, L.; Morris, H.A. Adiposity and estrogen receptor-positive, postmenopausal breast cancer risk: Quantification of the mediating effects of fasting insulin and free estradiol. Int. J. Cancer 2020, 146, 1541–1552. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Gunter, M.J.; Lange, T.; Chlebowski, R.T.; Lane, D.; Farhat, G.N.; Freiberg, M.S.; Keiding, N.; Lee, J.S.; Prentice, R.; et al. Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption, and breast cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The macrophage switch in obesity development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [Green Version]
- Bigornia, S.J.; Farb, M.G.; Mott, M.M.; Hess, D.T.; Carmine, B.; Fiscale, A.; Joseph, L.; Apovian, C.M.; Gokce, N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr. Diabetes 2012, 2, e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, K.A.; Mahurkar, S.; Alderete, T.L.; Hasson, R.E.; Adam, T.C.; Kim, J.S.; Beale, E.; Xie, C.; Greenberg, A.S.; Allayee, H.; et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes 2011, 60, 2802–2809. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Lehuede, C.; Laurent, V.; Dirat, B.; Dauvillier, S.; Bochet, L.; Le Gonidec, S.; Escourrou, G.; Valet, P.; Muller, C. Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer. Cancer Lett. 2012, 324, 142–151. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ren, G.S. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta-analysis. Medicine 2016, 95, e5602. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [PubMed]
- Bhandari, R.; Kelley, G.A.; Hartley, T.A.; Rockett, I.R. Metabolic syndrome is associated with increased breast cancer risk: A systematic review with meta-analysis. Int. J. Breast Cancer 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Ando, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Jia, J.; Dong, S.; Zhang, C.; Yu, S.; Li, L.; Mao, C.; Wang, D.; Chen, J.; Yuan, G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 158–165. [Google Scholar] [CrossRef]
- Dimou, N.L.; Papadimitriou, N.; Gill, D.; Christakoudi, S.; Murphy, N.; Gunter, M.J.; Travis, R.C.; Key, T.J.; Fortner, R.T.; Haycock, P.C. Sex hormone binding globulin and risk of breast cancer: A Mendelian randomization study. Int. J. Epidemiol. 2019, 48, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Renehan, A.G.; Harvie, M.; Howell, A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: Eight years on. Endocr. Relat. Cancer 2006, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- The Endogenous Hormones Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef] [Green Version]
- Autier, P.; Koechlin, A.; Boniol, M.; Mullie, P.; Bolli, G.; Rosenstock, J.; Boyle, P. Serum insulin and C-peptide concentration and breast cancer: A meta-analysis. Cancer Causes Control. 2013, 24, 873–883. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Zhang, S.; Chen, Q.; Zhang, M.; Quan, P.; Lu, J.; Sun, X. C-reactive protein and risk of breast cancer: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10508. [Google Scholar] [CrossRef]
- Kehm, R.D.; McDonald, J.A.; Fenton, S.E.; Kavanaugh-Lynch, M.; Leung, K.A.; McKenzie, K.E.; Mandelblatt, J.S.; Terry, M.B. Inflammatory Biomarkers and Breast Cancer Risk: A Systematic Review of the Evidence and Future Potential for Intervention Research. Int. J. Environ. Res. Public Health 2020, 17, 5445. [Google Scholar] [CrossRef]
- Pettersson, A.; Tamimi, R.M. Breast fat and breast cancer. Breast Cancer Res. Treat. 2012, 135, 321–323. [Google Scholar] [CrossRef]
- Dowsett, M.; Folkerd, E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: A new hypothesis. Breast Cancer Res. Treat. 2015, 149, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Cohn, B.A.; Goldberg, M.; Flom, J.D.; Wei, Y.; Houghton, L.C.; Tehranifar, P.; McDonald, J.A.; Protacio, A.; Cirillo, P.; et al. Do Birth Weight and Weight Gain During Infancy and Early Childhood Explain Variation in Mammographic Density in Women in Midlife? Results From Cohort and Sibling Analyses. Am. J. Epidemiol. 2019, 188, 294–304. [Google Scholar] [CrossRef]
- Rice, M.S.; Bertrand, K.A.; VanderWeele, T.J.; Rosner, B.A.; Liao, X.; Adami, H.-O.; Tamimi, R.M. Mammographic density and breast cancer risk: A mediation analysis. Breast Cancer Res. 2016, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Morris, P.G.; Hudis, C.A.; Giri, D.; Morrow, M.; Falcone, D.J.; Zhou, X.K.; Du, B.; Brogi, E.; Crawford, C.B.; Kopelovich, L.; et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 2011, 4, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [Green Version]
- Maliniak, M.L.; Cheriyan, A.M.; Sherman, M.E.; Liu, Y.; Gogineni, K.; Liu, J.; He, J.; Krishnamurti, U.; Miller-Kleinhenz, J.; Ashiqueali, R.; et al. Detection of crown-like structures in breast adipose tissue and clinical outcomes among African-American and White women with breast cancer. Breast Cancer Res. 2020, 22, 65. [Google Scholar] [CrossRef]
- Greenlee, H.; Shi, Z.; Hibshoosh, H.; Giri, D.D.; Ahmed, A.; Williams, S.; Falcone, D.J.; Winston, L.A.; Zhou, X.K.; Hudis, C.A.; et al. Obesity-associated Breast Inflammation among Hispanic/Latina Breast Cancer Patients. Cancer Prev. Res. 2019, 12, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Brown, K.A.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Giri, D.D.; Zahid, H.; Bhardwaj, P.; Wendel, N.K.; Falcone, D.J.; et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev. Res. 2017, 10, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.A.; Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Wang, H.; Giri, D.D.; Morrow, M.; Falcone, D.J.; Wendel, N.K. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J. Clin. Endocrinol. Metab. 2017, 102, 1692–1701. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Morris, P.G.; Zhou, X.K.; Gucalp, A.; Giri, D.; Harbus, M.D.; Falcone, D.J.; Krasne, M.D.; Vahdat, L.T.; Subbaramaiah, K.; et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev. Res. 2015, 8, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and cancer: Local and systemic mechanisms. Annu. Rev. Med. 2015, 66, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Nag, S.A.; Zhang, R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015, 22, 264–289. [Google Scholar] [CrossRef]
- Rose, D.P.; Vona-Davis, L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors 2014, 40, 1–12. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A.; Gonul, I.I. The effect of adipocyte-macrophage crosstalk in obesity-related breast cancer. J. Mol. Endocrinol. 2019, 62, R201–R222. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev. Res. 2011, 4, 329–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullooly, M.; Yang, H.P.; Falk, R.T.; Nyante, S.J.; Cora, R.; Pfeiffer, R.M.; Radisky, D.C.; Visscher, D.W.; Hartmann, L.C.; Carter, J.M.; et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017, 19, 8. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Chen, I.C.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Winston, L.A.; Wang, H.; Williams, S.; Lu, Y.S.; Hsueh, T.H.; et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev. Res. 2018, 11, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Morris, P.G.; Howe, L.R.; Giri, D.D.; Morrow, M.; Wang, H.; Pollak, M.; Jones, L.W.; et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin. Cancer Res. 2016, 22, 2283–2289. [Google Scholar] [CrossRef] [Green Version]
- Vaysse, C.; Lomo, J.; Garred, O.; Fjeldheim, F.; Lofteroed, T.; Schlichting, E.; McTiernan, A.; Frydenberg, H.; Husoy, A.; Lundgren, S.; et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Shaik, A.N.; Kiavash, K.; Stark, K.; Boerner, J.L.; Ruterbusch, J.J.; Deirawan, H.; Bandyopadhyay, S.; Ali-Fehmi, R.; Dyson, G.; Cote, M.L. Inflammation markers on benign breast biopsy are associated with risk of invasive breast cancer in African American women. Breast Cancer Res. Treat. 2020, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.M.; Hoskin, T.L.; Pena, M.A.; Brahmbhatt, R.; Winham, S.J.; Frost, M.H.; Stallings-Mann, M.; Radisky, D.C.; Knutson, K.L.; Visscher, D.W.; et al. Macrophagic “Crown-like Structures” Are Associated with an Increased Risk of Breast Cancer in Benign Breast Disease. Cancer Prev. Res. 2018, 11, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.J.; Kim, E.S.; Koo, J.S. Tumor-associated macrophages and crown-like structures in adipose tissue in breast cancer. Breast Cancer Res. Treat. 2018, 170, 15–25. [Google Scholar] [CrossRef]
- Koru-Sengul, T.; Santander, A.M.; Miao, F.; Sanchez, L.G.; Jorda, M.; Gluck, S.; Ince, T.A.; Nadji, M.; Chen, Z.; Penichet, M.L.; et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res. Treat. 2016, 158, 113–126. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Pankratz, V.S.; Sellers, T.A.; Brandt, K.R.; Wang, A.; Janney, C.; Fredericksen, Z.S.; Cerhan, J.R.; Vachon, C.M. Age-specific trends in mammographic density: The Minnesota Breast Cancer Family Study. Am. J. Epidemiol. 2008, 167, 1027–1036. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Cossrow, N.; Falkner, B. Race/ethnic issues in obesity and obesity-related comorbidities. J. Clin. Endocrinol. Metab. 2004, 89, 2590–2594. [Google Scholar] [CrossRef] [Green Version]
- Wilk, J.B.; Lash, T.L. Risk factor studies of age-at-onset in a sample ascertained for Parkinson disease affected sibling pairs: A cautionary tale. Emerg. Themes Epidemiol. 2007, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Haka, A.S.; Sue, E.; Zhang, C.; Bhardwaj, P.; Sterling, J.; Carpenter, C.; Leonard, M.; Manzoor, M.; Walker, J.; Aleman, J.O.; et al. Noninvasive Detection of Inflammatory Changes in White Adipose Tissue by Label-Free Raman Spectroscopy. Anal. Chem. 2016, 88, 2140–2148. [Google Scholar] [CrossRef] [Green Version]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Zin, A.A.M.; Ang, K.C.; Ch’ng, E.S. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef] [Green Version]
- Springer, N.L.; Iyengar, N.M.; Bareja, R.; Verma, A.; Jochelson, M.S.; Giri, D.D.; Zhou, X.K.; Elemento, O.; Dannenberg, A.J.; Fischbach, C. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am. J. Pathol. 2019, 189. [Google Scholar] [CrossRef]
- Ossovskaya, V.; Wang, Y.; Budoff, A.; Xu, Q.; Lituev, A.; Potapova, O.; Vansant, G.; Monforte, J.; Daraselia, N. Exploring molecular pathways of triple-negative breast cancer. Genes Cancer 2011, 2, 870–879. [Google Scholar] [CrossRef]
- Blouin, R.A.; Warren, G.W. Pharmacokinetic considerations in obesity. J. Pharm. Sci. 1999, 88, 1–7. [Google Scholar] [CrossRef]
- de Azambuja, E.; McCaskill-Stevens, W.; Francis, P.; Quinaux, E.; Crown, J.P.; Vicente, M.; Giuliani, R.; Nordenskjold, B.; Gutierez, J.; Andersson, M.; et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: The experience of the BIG 02-98 trial. Breast Cancer Res. Treat. 2010, 119, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Desmedt, C.; Fornili, M.; Clatot, F.; Demicheli, R.; De Bortoli, D.; Di Leo, A.; Viale, G.; de Azambuja, E.; Crown, J.; Francis, P.A. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J. Clin. Oncol. 2020, 38, 2883–2891. [Google Scholar] [CrossRef]
- Fontanella, C.; Lederer, B.; Gade, S.; Vanoppen, M.; Blohmer, J.U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Gerber, B.; Hanusch, C.; et al. Impact of body mass index on neoadjuvant treatment outcome: A pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res. Treat. 2015, 150, 127–139. [Google Scholar] [CrossRef]
- Ladoire, S.; Dalban, C.; Roche, H.; Spielmann, M.; Fumoleau, P.; Levy, C.; Martin, A.L.; Ecarnot, F.; Bonnetain, F.; Ghiringhelli, F. Effect of obesity on disease-free and overall survival in node-positive breast cancer patients in a large French population: A pooled analysis of two randomised trials. Eur. J. Cancer 2014, 50, 506–516. [Google Scholar] [CrossRef]
- Litton, J.K.; Gonzalez-Angulo, A.M.; Warneke, C.L.; Buzdar, A.U.; Kau, S.W.; Bondy, M.; Mahabir, S.; Hortobagyi, G.N.; Brewster, A.M. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J. Clin. Oncol. 2008, 26, 4072–4077. [Google Scholar] [CrossRef]
- Pajares, B.; Pollan, M.; Martin, M.; Mackey, J.R.; Lluch, A.; Gavila, J.; Vogel, C.; Ruiz-Borrego, M.; Calvo, L.; Pienkowski, T.; et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: A pooled analysis. Breast Cancer Res. 2013, 15, R105. [Google Scholar] [CrossRef] [Green Version]
- Ross, K.H.; Gogineni, K.; Subhedar, P.D.; Lin, J.Y.; McCullough, L.E. Obesity and cancer treatment efficacy: Existing challenges and opportunities. Cancer 2019, 125, 1588–1592. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, M.; Zeng, N.; Xiong, M.; Hu, W.; Lv, W.; Yi, Y.; Zhang, Q.; Wu, Y. Cancer-associated adipocytes: Emerging supporters in breast cancer. J. Exp. Clin. Cancer Res. 2020, 39, 156. [Google Scholar] [CrossRef] [PubMed]
- Lehuede, C.; Li, X.; Dauvillier, S.; Vaysse, C.; Franchet, C.; Clement, E.; Esteve, D.; Longue, M.; Chaltiel, L.; Le Gonidec, S.; et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: Role of the major vault protein (MVP). Breast Cancer Res. 2019, 21, 7. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Olson, O.C.; Kim, H.; Quail, D.F.; Foley, E.A.; Joyce, J.A. Tumor-Associated Macrophages Suppress the Cytotoxic Activity of Antimitotic Agents. Cell Rep. 2017, 19, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef] [Green Version]
- Ioannides, S.J.; Barlow, P.L.; Elwood, J.M.; Porter, D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: A systematic review. Breast Cancer Res. Treat. 2014, 147, 237–248. [Google Scholar] [CrossRef]
- Kwan, M.L.; Habel, L.A.; Slattery, M.L.; Caan, B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control. 2007, 18, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Ahern, T.P.; Lash, T.L.; Damkier, P.; Christiansen, P.M.; Cronin-Fenton, D.P. Statins and breast cancer prognosis: Evidence and opportunities. Lancet Oncol. 2014, 15, e461–e468. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Aragaki, A.K.; Chlebowski, R.T.; Manson, J.E.; Rohan, T.E.; Chen, C.; Vitolins, M.Z.; Tinker, L.F.; LeBlanc, E.S.; Kuller, L.H.; et al. Diabetes, metformin and incidence of and death from invasive cancer in postmenopausal women: Results from the women’s health initiative. Int. J. Cancer 2016, 138, 1915–1927. [Google Scholar] [CrossRef] [Green Version]
- Giles, E.D.; Jindal, S.; Wellberg, E.A.; Schedin, T.; Anderson, S.M.; Thor, A.D.; Edwards, D.P.; MacLean, P.S.; Schedin, P. Metformin inhibits stromal aromatase expression and tumor progression in a rodent model of postmenopausal breast cancer. Breast Cancer Res. 2018, 20, 50. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-κB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Gazzerro, P.; Proto, M.C.; Gangemi, G.; Malfitano, A.M.; Ciaglia, E.; Pisanti, S.; Santoro, A.; Laezza, C.; Bifulco, M. Pharmacological actions of statins: A critical appraisal in the management of cancer. Pharmacol. Rev. 2012, 64, 102–146. [Google Scholar] [CrossRef]
| First Author (Year) | Study Design | Institutions/ Affiliations | Country, Race/ Ethnicity Distribution | Study Population | Study Years | CLS-B Analyses Conducted |
|---|---|---|---|---|---|---|
| Breast cancer incidence studies (n = 2) | ||||||
| Shaik (2020) [84] | Nested case–control + cross-sectional analysis | Detroit BBD cohort and KTB | USA 100% AA | n = 84 BBD cases n = 47 BBD controls n = 50 KTB volunteers without BBD or breast cancer | BBD diagnosis: 1997–2010 Follow up for breast cancer through 2016 |
|
| Carter (2017) [85] | Nested case–control + cross-sectional analysis | Mayo BBD cohort and KTB | USA Unknown | n = 86 BBD cases n = 86 BBD controls n = 86 KTB volunteers without clinical breast abnormalities | BBD diagnosis: 1967–2001 Follow up for breast cancer: Unknown |
|
| Breast cancer prognosis studies (n = 4) | ||||||
| Maliniak (2020) [69] | Cohort + cross-sectional analysis | Emory University-affiliated tumor registries | USA 51% AA 49% White | n = 342 breast cancer patients
| Breast cancer diagnosis: 2007–2012 Follow up for breast cancer outcomes: 2018 |
|
| Cha (2018) [86] | Cohort + cross-sectional analysis | Yonsei University | South Korea | Group 1: n = 56 non-breast cancer patients
| Unknown |
|
| Koru-Sengul (2016) [87] | Cohort + cross-sectional analysis | University of Miami/Jackson Memorial Hospital tumor registry | USA 33% Black 33% non-Black Latina 33% Caucasian | n = 150 breast cancer patients
| Cases obtained: 1978–1997 Followed for at least 5 years |
|
| Iyengar (2016) [82] | Cohort + cross-sectional analysis | MSKCC | USA 83% White 13% Black 3% Asian | Cohort 1: n = 100 patients (mostly breast cancer)
| Mastectomy: 2011–2013 (Cohort 1); 2001–2006 (Cohort 2) Cohort 2: Follow up for breast cancer outcomes: 2014 | Cohort 1:
|
| Cross-sectional studies of CLS-B (n = 8) | ||||||
| Greenlee (2018) [70] | Cross-sectional | Columbia University Medical Center | USA 100% Hispanic a | n = 91 breast cancer patients
| Mastectomy: 2007–2012 |
|
| Iyengar (2018) [81] | Cross-sectional | National Taiwan University Hospital and MSKCC | Taiwan USA 100% Caucasian | n = 72 Taiwanese breast cancer patients
| Mastectomy: 2011–2016 (Taiwanese); 2011–2013 (US Caucasian) |
|
| Iyengar (2017) [71] | Cross-sectional | MSKCC | USA 76% Caucasian 9% Black, Asian, or Other 14% Unknown | n = 72 patients (mostly breast cancer)
| Mastectomy: 2011–2013 |
|
| Mullooly (2017) [80] | Cross-sectional | PBCS | Poland | n = 83 breast cancer patients
| Study recruitment: 2000–2003 |
|
| Vaysse (2017) [83] | Cross-sectional | Energy Balance and Breast Cancer Aspects-II | Norway | n = 107 breast cancer patients
| Unknown |
|
| Brown (2017) [72] | Cross-sectional | MSKCC | USA | n = 161 patients (mostly breast cancer)
| Unknown |
|
| Iyengar (2015) [73] | Cross-sectional | MSKCC | USA 86% White 7% Black 6% Asian | n = 237 patients (mostly breast cancer)
| Mastectomy: 2011–2013 |
|
| Morris (2011) [67] | Cross-sectional (pilot study) | MSKCC | USA | n = 30 patients (mostly breast cancer)
| Enrolled: 2010 |
|
| First Author (Year) | Patient Study Population | % CLS-B+ by BMI (kg/m2) Group | Association between BMI and CLS-B aOR (95%CI) if Presented | Association with Other Adiposity Measures | Direction of Association: -/Null/+ | |
|---|---|---|---|---|---|---|
| Shaik (2020) [84] | BBD + Komen Normal Tissue Bank | NR | Not associated (p > 0.1) | Null | ||
| Carter (2017) [85] | BBD + Komen Normal Tissue Bank | BMI < 25: BMI 25–< 30: BMI ≥ 30: | 7% 13% 29% | + | ||
| Maliniak (2020) [69] | Breast cancer | BMI < 25: BMI 25–< 30: BMI ≥ 30: | 16% 29% 45% | Reference 2.34 (1.17 to 4.70) 4.73 (2.48 to 9.01) | + | |
| Cha (2018) a [86] | Breast cancer | BMI < 25: BMI ≥ 25: | 15% 27% | + | ||
| Greenlee (2018) [70] | Breast cancer | BMI 18.5–< 25: BMI 25–< 30: BMI 30–< 35: BMI ≥ 35: | 24% 34% 57% 65% | + | ||
| Iyengar (2018) b [81] | Breast cancer | BMI < 23: BMI 23–< 27.5: BMI ≥ 27.5: | 24% 48% 76% | Body fat, VAT, and SAT (all p < 0.01) | + | |
| Iyengar (2017) [71] | Mostly breast cancer | All BMI < 25: | 39% | CLS-B- vs. CLS-B + median BMI: 21.8 vs. 23.0, p = 0.04 | + | |
| Mullooly (2017) [80] | Breast cancer | BMI < 25: BMI 25–< 30: BMI ≥ 30: | 17% 36% 54% | Reference 1.93 (0.50 to 7.40) 4.63 (1.08 to 19.83) | + | |
| Vaysse (2017) [83] | Breast cancer | BMI < 25: BMI 25–< 30: BMI ≥30: | NR | Reference 3.2 (1.28 to 8.15) 6.9 (1.35 to 35.0) | WHR and % truncal fat (all p < 0.05) | + |
| Iyengar (2016) c [82] | Breast cancer | BMI < 25: BMI 25–< 30: BMI ≥ 30: | 23% 33% 67% | + | ||
| Iyengar (2015) [73] | Mostly breast cancer | BMI < 25 BMI 25–< 30: BMI ≥ 30: | 34% 53% 90% | CLS concordance between breast and abdominal SAT (p = 0.12) | + | |
| First Author (Year) | N Studies | Summary of Evidence |
|---|---|---|
| Patient characteristics | ||
| Obesity | 11 studies [69,70,71,73,80,81,82,83,84,85,86] | Strong positive association in studies of breast cancer patients (see Table 2); inconclusive evidence for BBD patients and women without BBD or breast cancer |
| Age | 8 studies [69,70,71,73,80,81,85,86] | Positive trend with age in studies of breast cancer patients although majority of associations were not statistically significant; no association observed between age and CLS-B among BBD patients [85] |
| Menopausal status | 6 studies [69,70,71,72,73,81] | Positive trend with postmenopausal status among breast cancer patients although majority of associations were not statistically significant |
| Race/ethnicity | 4 studies [69,70,81,87] | Evidence of greater CLS-B density among Black breast cancer patients in n = 2 studies [69,83] (no association when adjusting for BMI in the one study [69] with this information); No strong evidence of differences in CLS-B detection by country of origin among Hispanic/Latina patients [70] or when comparing Taiwanese to US Caucasian patients [80] |
| Smoking status | 2 studies [69,70] | Positive trend with current smoking status in breast cancer patients but inconclusive (very few current smokers in both studies) |
| Age at menopause | 2 studies [69,80] | Inconclusive evidence |
| Reproductive factors | 2 studies [69,80] | Inconclusive evidence |
| Family history of breast cancer | 2 studies [69,80] | Inconclusive evidence |
| Tumor characteristics | ||
| Molecular subtype | 6 studies [69,70,71,73,80,86] | No/little evidence for differences by ER status, PR status, or other tumor subtypes observed |
| Nodal status | 4 studies [69,80,82,86] | Some evidence suggesting association with lymph node-negative disease but all together inconclusive |
| Grade | 4 studies [69,80,82,86] | Inconclusive evidence |
| Stage | 3 studies [69,70,86] | Inconclusive evidence |
| First Author (Year) | N Total | Study Design | Antibody: % CLS-B+ | Outcome | N Outcomes | Adjusted Estimate (95%CI) if Reported | Summary of Results
|
|---|---|---|---|---|---|---|---|
| Breast cancer incidence studies (n = 2) | |||||||
| Shaik (2020) [84] | 55 cases/ 47 controls | Nested case–control | CD68: Cases: 67% Controls: 40% | Invasive breast cancer | - | Any CLS-B vs. none: 3.98 (1.40 to 11.3) ≥5 CLS-B/sample vs. none: 4.99 (1.32 to 18.9) | Positive association between CLS-B and breast cancer among BBD patients
|
| Carter (2017) [85] | 86 cases / 86 controls | Nested case–control | CD68: Cases: 24% Controls: 19% | Invasive or in situ breast cancer | - | Any CLS-B vs. none: NR >5 CLS-B/sample vs. none: 6.8 (1.4 to 32.4) | Positive association between CLS-B and breast cancer among BBD patients
|
| Breast cancer prognosis studies (n = 4) | |||||||
| Maliniak (2020) [69] | 319 | Cohort | CD68: 30% | OS PFS | 46 recurrences 52 deaths | OS (Any CLS-B vs. none): 1.02 (0.55 to 1.87) PFS (Any CLS-B vs. none): 0.99 (0.59 to 1.67) | Null association between CLS-B and breast cancer prognosis in a diverse population of breast cancer patients
|
| Cha (2018) [86] | 140 a | Cohort | CD68: 18% CD163: 13% | OS DFS | 18 recurrences 11 deaths | OS (CLS-B present vs. absent): CD68: univariate p = 0.390 CD163 univariate p = 0.492 DFS (CLS-B present vs. absent): CD68: univariate p = 0.899 CD163: univariate p = 0.883 | Not enough breast cancer outcomes to draw conclusions |
| Koru-Sengul (2016) [87] | 150 | Cohort | CD163: NR CD40: NR CD206: NR | OS PFS | 83 recurrences 88 deaths | OS (density of CLS): CD163: 2.14 (0.46 to 9.96) b CD40: 9.14 (1.00 to 83.60) b CD206: 0.65 (0.03 to 12.58) b PFS (density of CLS): CD163: 2.30 (0.66 to 8.03) b CD40: 4.12 (0.49 to 34.92) b CD206: 1.16 (0.09 to 14.28) b | Positive association between CLS-B and breast cancer prognosis
|
| Iyengar (2016) [82] | 127 | Case-only analysis | CD68: 41% | Average time to distant recurrence | 127 recurrences 99 deaths | Any CLS-B vs. none: 1.83 (1.07 to 3.13) | Positive association between CLS-B and breast cancer prognosis
|
| First Author (Year) | Tissue Specimen | Tissue Specimens per Subject | Antibody | % CLS-B+ |
|---|---|---|---|---|
| Breast cancer incidence studies (n = 2) | ||||
| Shaik (2020) [84] | BBD: FFPE BBD biopsy tissue KTB donors: FFPE percutaneous needle biopsy tissue | 1 | CD68 | BBD Cases: 67% BBD Controls: 40% KTB donors: 18% |
| Carter (2017) [85] | BBD: FFPE BBD biopsy tissue KTB donors: FFPE normal breast tissue | 1 | CD68 | BBD Cases: 24% BBD Controls: 19% KTB donors: 3% |
| Breast cancer prognosis studies (n = 4) | ||||
| Maliniak (2020) [69] | FFPE non-tumor tissue | 1 | CD68 | Overall: 30% AA: 32% White: 29% |
| Cha (2018) [86] | Group 1: FFPE reduction mammoplasty Group 2: FFPE non-tumor tissue Group 3: FFPE tumor tissue | Unknown | CD68 CD163 | CD68, CD163 Group 1: 2%, 2% Group 2: 0%, 0% Group 3: 18%, 13% |
| Koru-Sengul (2016) [87] | FFPE tumor tissue | 1 | CD163 CD206 CD40 | Density of CLS: CD163, CD206, CD40 Mean (SD) All: 0.06 (0.14); 0.03 (0.07); 0.01 (0.07) Black: 0.11 (0.22); 0.04 (0.09); 0.02 (0.11) NBLA: 0.05 (0.08); 0.03 (0.05); 0 (0) CA: 0.03 (0.07); 0.02 (0.06); 0 (0.02) |
| Iyengar (2016) [82] | FFPE non-tumor tissue | 5 | CD68 | 41% |
| Cross-sectional studies of CLS-B (n = 8) | ||||
| Greenlee (2018) [70] | FFPE non-tumor tissue | 5 | CD68 | 45% |
| Iyengar (2018) [81] | FFPE non-tumor tissue | 5 | CD68 | Taiwanese: 43% US Caucasian: 55% |
| Iyengar (2017) [71] | FFPE non-tumor tissue | 5 | CD68 | 39% |
| Mullooly (2017) [80] | FFPE non-tumor tissue | 1 | CD68 | 36% |
| Vaysse (2017) [83] | FFPE tumor tissue | Unknown | CD68 | 54% |
| Brown (2017) [72] | FFPE non-tumor tissue | 5 | CD68 | 57% |
| Iyengar (2015) [73] | FFPE non-tumor tissue | 5 | CD68 | 51% |
| Morris (2011) [67] | FFPE non-tumor tissue | 4–5 | CD68 | 47% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliniak, M.L.; Miller-Kleinhenz, J.; Cronin-Fenton, D.P.; Lash, T.L.; Gogineni, K.; Janssen, E.A.M.; McCullough, L.E. Crown-Like Structures in Breast Adipose Tissue: Early Evidence and Current Issues in Breast Cancer. Cancers 2021, 13, 2222. https://doi.org/10.3390/cancers13092222
Maliniak ML, Miller-Kleinhenz J, Cronin-Fenton DP, Lash TL, Gogineni K, Janssen EAM, McCullough LE. Crown-Like Structures in Breast Adipose Tissue: Early Evidence and Current Issues in Breast Cancer. Cancers. 2021; 13(9):2222. https://doi.org/10.3390/cancers13092222
Chicago/Turabian StyleMaliniak, Maret L., Jasmine Miller-Kleinhenz, Deirdre P. Cronin-Fenton, Timothy L. Lash, Keerthi Gogineni, Emiel A. M. Janssen, and Lauren E. McCullough. 2021. "Crown-Like Structures in Breast Adipose Tissue: Early Evidence and Current Issues in Breast Cancer" Cancers 13, no. 9: 2222. https://doi.org/10.3390/cancers13092222
APA StyleMaliniak, M. L., Miller-Kleinhenz, J., Cronin-Fenton, D. P., Lash, T. L., Gogineni, K., Janssen, E. A. M., & McCullough, L. E. (2021). Crown-Like Structures in Breast Adipose Tissue: Early Evidence and Current Issues in Breast Cancer. Cancers, 13(9), 2222. https://doi.org/10.3390/cancers13092222






