Evaluation of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Screening and Monitoring Biomarkers for Brain Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
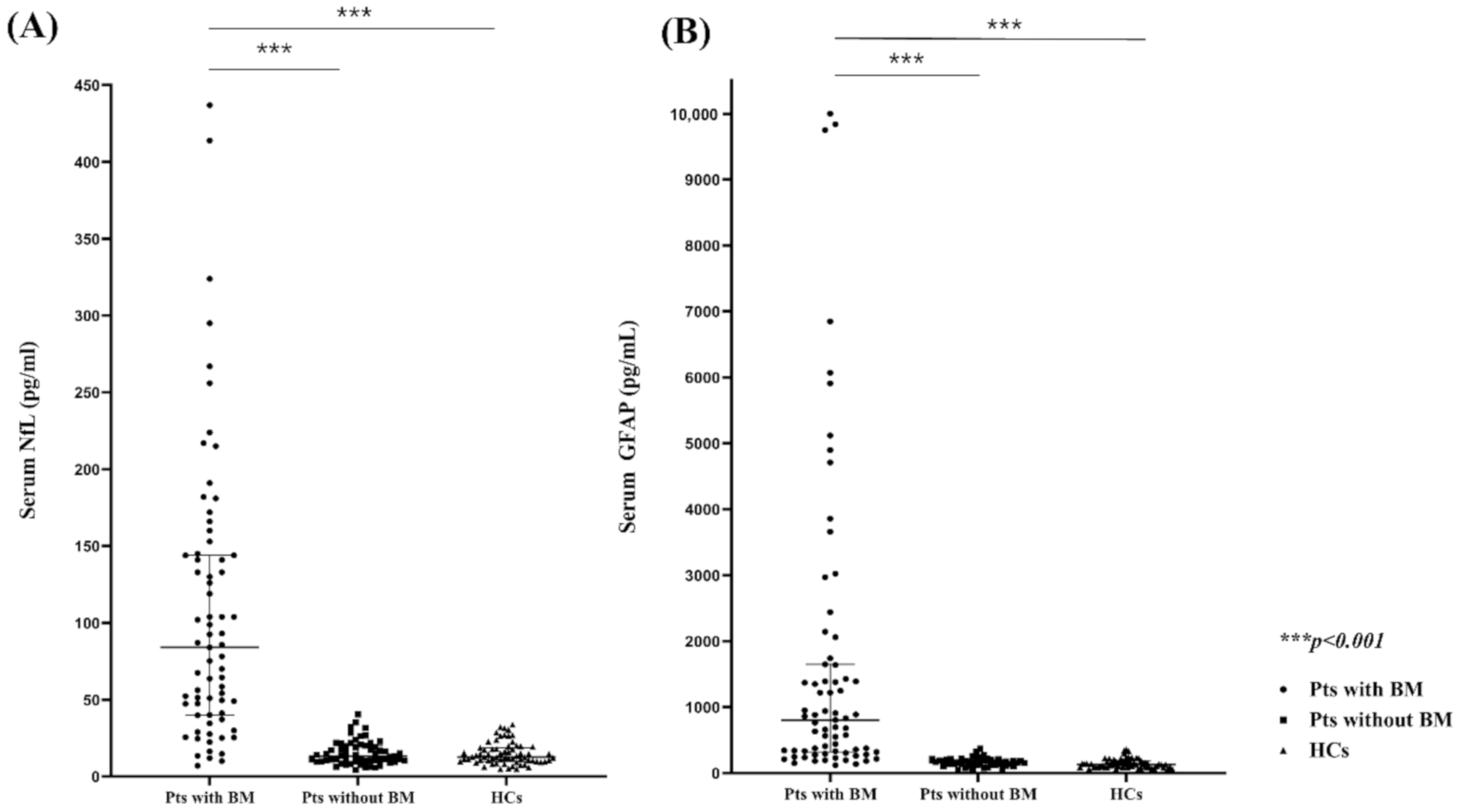
2.2. Comparison of the Serum NfL (sNfl) and GFAP (sGFAP) Levels in Patients with and without BMs and HCs
2.3. sNfL and sGFAP Level as BM Diagnostic Markers
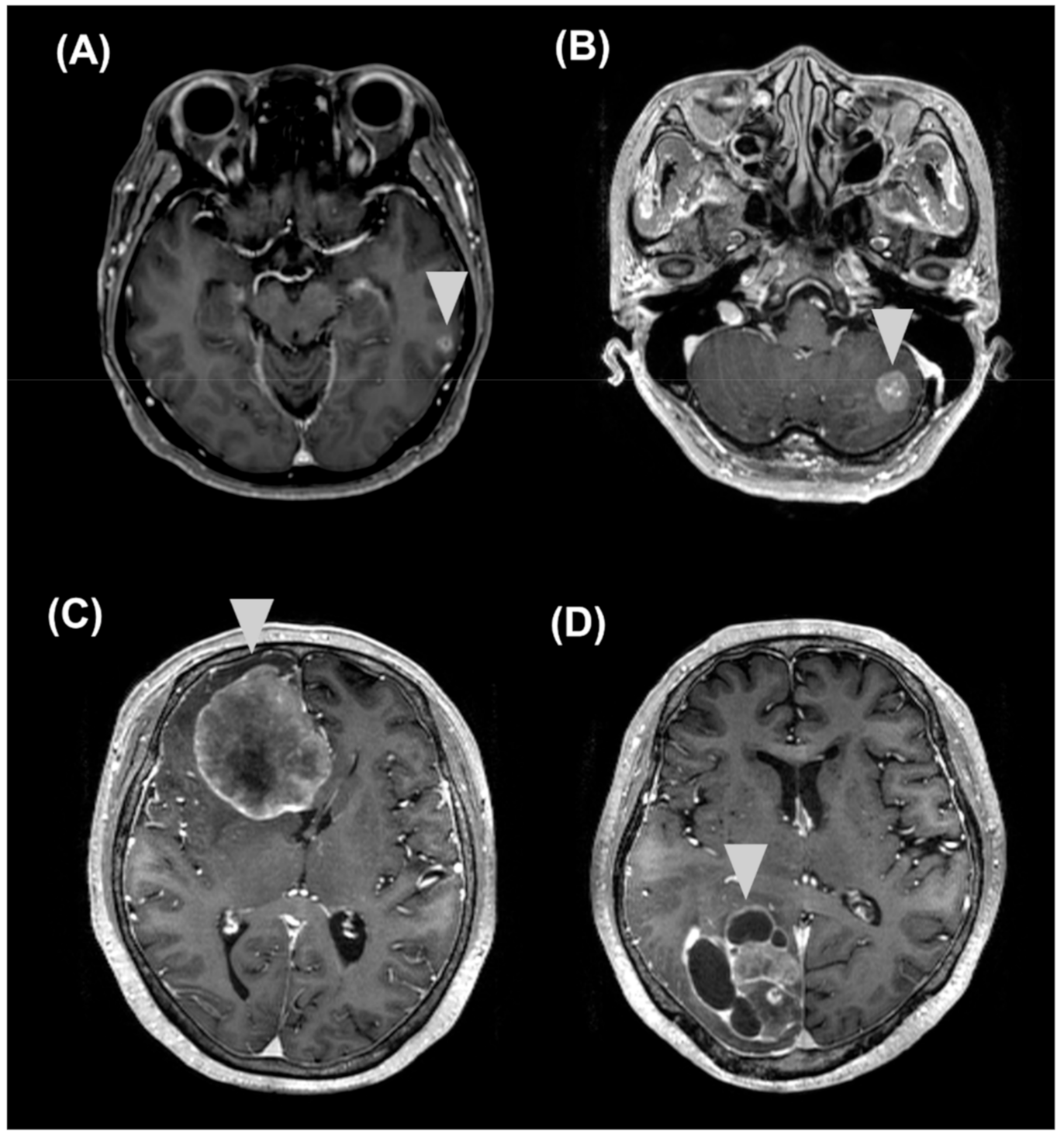
2.4. Correlations of sNFL and sGFAP Levels with Clinical and MRI Features
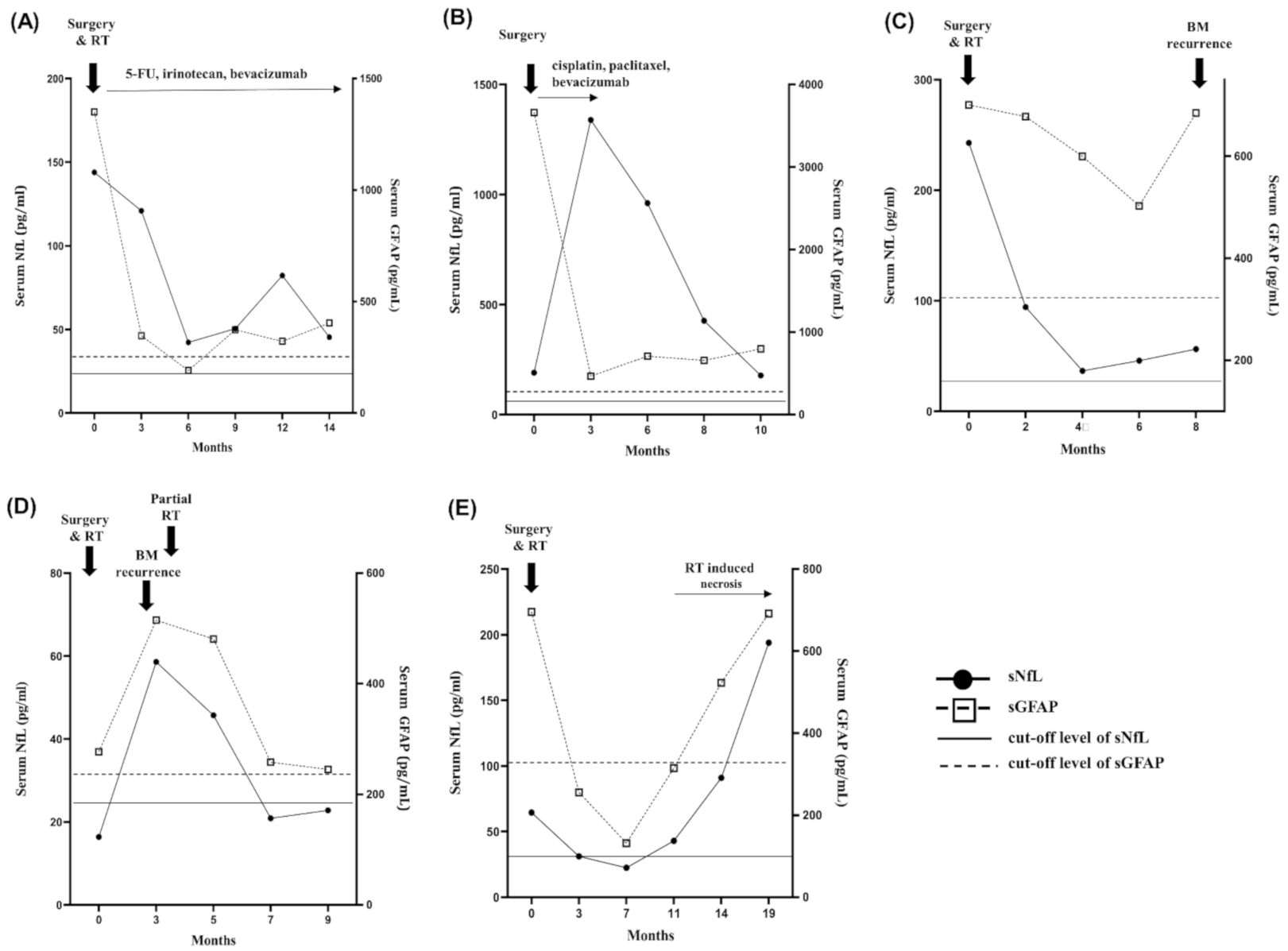
2.5. Longitudinal Follow-Up
3. Discussion
4. Materials and Methods
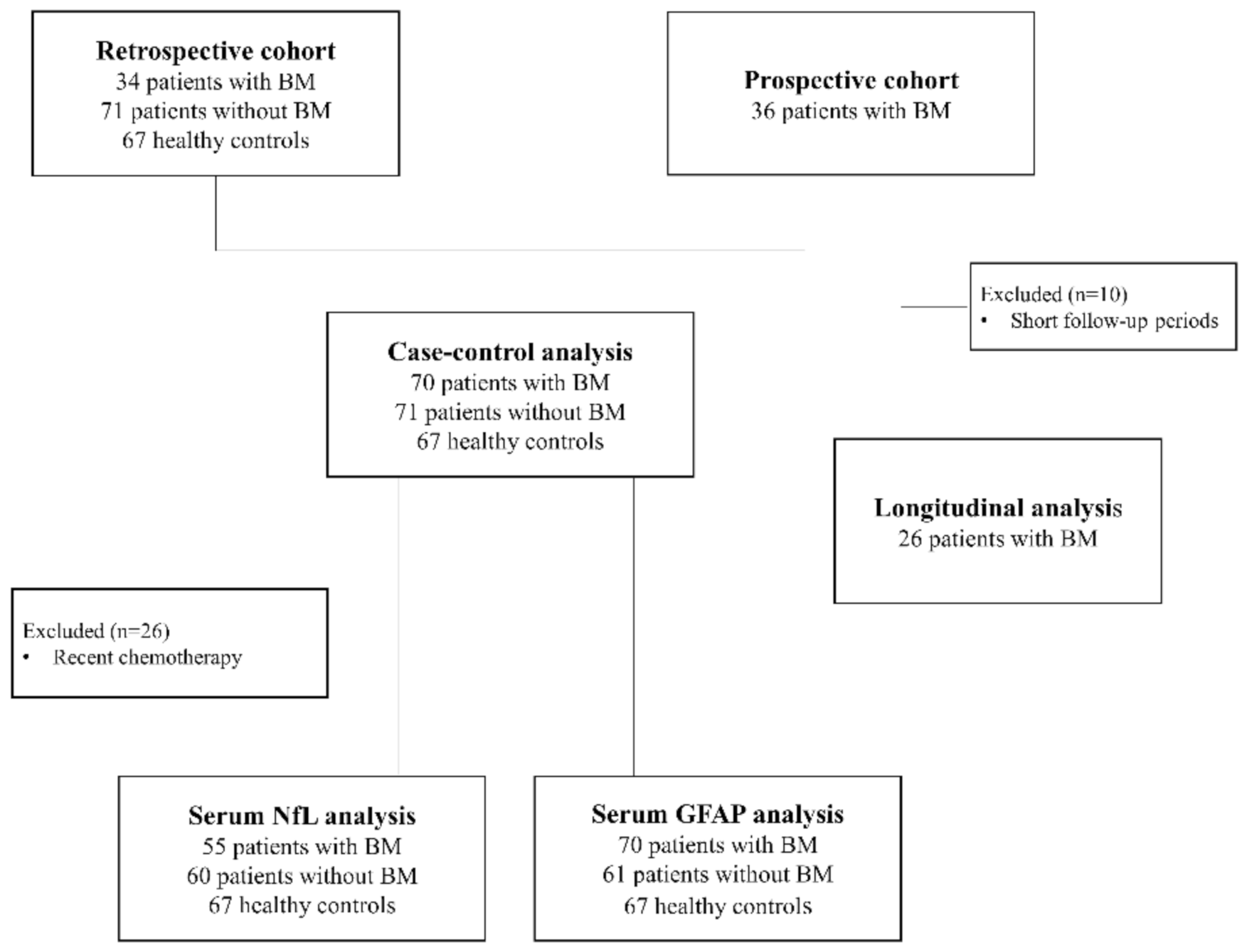
4.1. Study Design and Participants
4.2. sNfL and sGFAP Measurement
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Goncalves, A. Recent trends in epidemiology of brain metastases: Anove view. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar] [PubMed]
- Lin, X.; DeAngelis, L.M. Treatment of brain metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gil, M.J.; Martinez-Garcia, M.; Sierra, A.; Conesa, G.; Del Barco, S.; Gonzalez-Jimenez, S.; Villa, S. Breast cancer brain metastases: A review of the liaterature and a current multidisciplinary management guideline. Clin. Transl. Oncol. 2014, 16, 434–446. [Google Scholar] [CrossRef]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.S.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampton, J.H.; et al. Current multidisciplinary management of brain metastases. Cancer 2020, 126, 1309–1406. [Google Scholar] [CrossRef]
- Fureder, L.M.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Hainfellner, J.A.; Bartsch, R.; Zielinski, C.C.; Preusser, M.; Berghoff, A.S. Brain metastases as first manifestation of advanced cancer: Exploratory analysis of 459 patients at a tertiary care center. Clin. Exp. Metastasis 2018, 35, 727–738. [Google Scholar] [CrossRef]
- Levy, A.; Faivre-Finn, C.; Hasan, B.; De Maio, E.; Berghoff, A.S.; Girard, N.; Greillier, L.; Lantuéjoul, S.; O’Brien, M.; Reck, M.; et al. Diversity of brain metastases screening and management in non-small cell lung cancer in Europe: Results of the European Organ isation for Research and Treatment of Cancer Lung Cancer Group survey. Eur. J. Cancer 2018, 93, 37–46. [Google Scholar] [CrossRef]
- Schuette, W. Treatment of brain metastases from lung cancer: Chemotherapy. Lung. Cancer 2004, 45, S253–S257. [Google Scholar] [CrossRef]
- Mamon, H.J.; Yeap, B.Y.; Janne, P.A.; Reblando, J.; Shrager, S.; Jaklitsch, M.T.; Mentzer, S.; Lukanich, J.M.; Sugarbaker, D.J.; Baldini, E.; et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemo therapy, and radiation. J. Clin. Oncol. 2005, 23, 1530–1537. [Google Scholar] [CrossRef]
- Khali, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, M.K.; Park, N.Y.; Hyun, J.W.; Lee, M.Y.; Kim, H.J.; Jung, S.K.; Cha, Y. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci. Rep. 2020, 10, 7995. [Google Scholar] [CrossRef]
- Varhaug, K.N.; Torkildsen, O.; Myhr, K.M.; Vedeler, C.A. Neurofilament light chain as a biomarker in multiple Sclerosis. Front. Neurol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Uphaus, T.; Bittner, S.; Groschel, S.; Steffen, F.; Muthuraman, M.; Wasser, K.; Weber-Kruger, M.; Zipp, F.; Wachter, R.; Groschel, K. NfL (Neurofilament Light Chain) Levels as a Predictive Marker for Long-Term Outcome After Ischemic. Stroke 2019, 50, 3077–3084. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Y.; Meng, S.; He, Z.; Hu, W. Neurofilament light chain protein in neurodegenerative dementia: A systematic re view and network meta-analysis. Neurosci. Biobehv. Rev. 2019, 102, 123–138. [Google Scholar] [CrossRef]
- Quattrini, A.; Previtali, S.; Feltri, M.L.; Canal, N.; Nemni, R.; Wrabetz, L. Beta 4 integrin and other Schwann cell markers in axonal neuropathy. Glia 1996, 17, 294–306. [Google Scholar] [CrossRef]
- Oeckl, P.; Halbgebauer, S.; Anderl-Straub, S.; Steinacker, P.; Huss, A.M.; Neugebauer, H.; von Arnim, C.A.F.; Diehl-Schmid, J.; Grimmer, T.; Kornhuber, J.; et al. Glial Fibrillary Acidic Protein in Serum is Increased in Alzheimer’s Disease and Correlates with Cognitive Impairment. J. Alzheimers Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef]
- Aktas, O.; Smith, M.A.; Rees, W.A.; Bennett, J.L.; She, D.; Katz, E.; Cree, B.A.C.; N-MOmentum scientific group and N-MOmentum study investigators. Serum Glial Fibrillary Acidic Protein: A Neuromyelitis Optica Spectrum Disorder Biomarker. Ann. Neurol. 2021, 5, 895–910. [Google Scholar] [CrossRef]
- Choi, H.; Puvenna, V.; Brennan, C.; Mahmoud, S.; Wang, X.; Phillips, M.; Janigro, D.; Mazzone, P. S100B and S100B autoantibody as biomarkers for early detection of brain metastases in lung cancer. Transl. Lung Cancer Res. 2016, 5, 413–419. [Google Scholar] [CrossRef]
- Pang, X.; Min, J.; Liu., L.; Liu., Y.; Ma., N.; Zhang., H. S100B protein as a possible participant in the brain metastasis of NSCLC. Med. Oncol. 2012, 29, 2626–2632. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Wu, H.; Jia, Y.; Liu, J.; Mu, X.; Wu, H.; Zhao, Y. Over-expression of S100B protein as a serum marker of brain metastasis in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2019, 215, 427–432. [Google Scholar] [CrossRef]
- Mu, S.; Ma, H.; Shi, J.; Zhen, D. The expression of S100B protein in serum of patients with brain metastases from small-cell lung cancer and its clinical significance. Oncol. Lett. 2017, 14, 7107–7110. [Google Scholar] [CrossRef]
- Kondrup, M.; Nygaard, A.D.; Madsen, J.S.; Bechmann, T. S100B as a biomarker for brain metastases in patients with non-small cell lung cancer. Biomed. Rep. 2020, 12, 204–208. [Google Scholar] [CrossRef]
- Bechmann, T.; Madsen, J.S.; Brandslund, I.; Lund, E.D.; Ormstrup, T.; Jakobsen, E.H.; Jylling, A.M.B.; Steffensen, K.D.; Jakobsen, A. Predicting brain metastases of breast cancer based on serum S100B and serum HER. Oncol. Lett. 2013, 6, 1265–1270. [Google Scholar] [CrossRef]
- Fitzgerald, D.P.; Palmieri, D.; Hua, E.; Hargrave, E.; Herring, J.M.; Qian, Y.; Vega-Valle, E.; Weil, R.J.; Stark, A.M.; Vortmeyer, A.O.; et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis 2008, 25, 799–810. [Google Scholar] [CrossRef]
- He, B.P.; Wang, J.J.; Zhang, X.; Wu, Y.; Wang, M.; Bay, B.H.; Chang, A.Y. Differential reactions of microglia to brain metastasis of lung cancer. Mol. Med. 2006, 12, 161–170. [Google Scholar] [CrossRef]
- Brunkhorst, R.; Pfeilschifter, W.; Foerch, C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-path physiological background and clinical findings. Transl. Stroke Res. 2010, 1, 246–251. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Hepner, A.; Porter, J.; Hare, F.; Nasir, S.S.; Zetterberg, H.; Blennow, K.; Martin, M.G. Serum neurofilament light, glial fibrillary acidic protein and tau are possible serum biomarkers for activity of brain metastases and gliomas. World J. Oncol. 2019, 10, 169–175. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Hviid, C.V.B.; Meldgaard, P.; Sorensen, B.S.; Sandfeld-Paulsen, B. neurofilament light chain as a biomarker for brain metastases. Cancers 2020, 12, 2852. [Google Scholar] [CrossRef]
- Holländer, N.; Sauerbrei, W.; Schumacher, M. Confidence intervals for the effect of a prognostic factor after selection of an’optimal’ cutpoint. Stat. Med. 2004, 23, 1701–1713. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proprssal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]

| Clinical Characteristics | Patients with BMs (n = 70) | Patients without BMs (n = 71) | Healthy Controls (n = 67) | p |
|---|---|---|---|---|
| Age, years, median (IQR) | 60 (51–69) | 60 (54–59) | 60 (54–69) | 0.953 |
| Sex, female (%) | 69 | 56 | 71 | 0.162 |
| Primary cancer, (n) | Lung cancer (39) Breast cancer (26) Colon cancer (2) Ovary cancer (2) Cervical cancer (1) | Lung cancer (34) Breast cancer (30) Colon cancer (3) Ovary cancer (2) Cervical cancer (2) | N/A | 0.797 |
| Time from initial cancer diagnosis to BMs, months, median (IQR) | 22.5 (8–50) | N/A | N/A | N/A |
| sNfL | sGFAP | ||||
|---|---|---|---|---|---|
| AUC | Cut-Off (pg/mL) | AUC | Cut-Off (pg/mL) | ||
| 31–50 years (n = 41) | 0.88 | 11.8 | 31–50 years (n = 44) | 0.986 | 185 |
| 51–60 years (n = 49) | 1.0 | 21.8 | 51–60 years (n = 58) | 0.961 | 189 |
| 61–70 years (n = 63) | 0.936 | 23.9 | 61–70 years (n = 72) | 0.964 | 246 |
| 71–80 years (n = 29) | 0.958 | 35.2 | 71–80 years (n = 34) | 0.939 | 371 |
| Protein | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | Accuracy, % (95% CI) |
|---|---|---|---|---|---|
| sNfL | 91 (0.8–0.96) | 91 (0.84–0.95) | 81 (0.68–0.89) | 95 (0.91–0.99) | 91 (0.85–0.94) |
| sGFAP | 91 (0.82–0.97) | 97 (0.93–0.99) | 94 (0.86–0.98) | 96 (0.91–0.98) | 95 (0.91–0.98) |
| sNfL or sGFAP | 98 (0.90–0.99) | 88 (0.81–0.93) | 78 (0.67–0.87) | 99 (0.95–0.99) | 91 (0.82–0.95) |
| sNfL and sGFAP | 87 (0.75–0.95) | 99 (0.96–0.99) | 98 (0.89–0.99) | 95 (0.92–0.98) | 96 (0.92–0.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Gwak, H.-S.; Lee, Y.; Park, N.-Y.; Han, M.; Kim, Y.; Kim, S.-Y.; Kim, H.J. Evaluation of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Screening and Monitoring Biomarkers for Brain Metastases. Cancers 2021, 13, 2227. https://doi.org/10.3390/cancers13092227
Kim S-H, Gwak H-S, Lee Y, Park N-Y, Han M, Kim Y, Kim S-Y, Kim HJ. Evaluation of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Screening and Monitoring Biomarkers for Brain Metastases. Cancers. 2021; 13(9):2227. https://doi.org/10.3390/cancers13092227
Chicago/Turabian StyleKim, Su-Hyun, Ho-Shin Gwak, Youngjoo Lee, Na-Young Park, Mira Han, Yeseul Kim, So-Yeon Kim, and Ho Jin Kim. 2021. "Evaluation of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Screening and Monitoring Biomarkers for Brain Metastases" Cancers 13, no. 9: 2227. https://doi.org/10.3390/cancers13092227
APA StyleKim, S.-H., Gwak, H.-S., Lee, Y., Park, N.-Y., Han, M., Kim, Y., Kim, S.-Y., & Kim, H. J. (2021). Evaluation of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Screening and Monitoring Biomarkers for Brain Metastases. Cancers, 13(9), 2227. https://doi.org/10.3390/cancers13092227






