Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
2.2. Treatment Plan
2.3. Evaluation Schedule
2.4. Disease Assessment—Response to Therapy
2.5. Quality of Life Scoring
3. Results
3.1. Patients’ Characteristics
3.2. Response to Therapy
3.3. Quality of Life Scoring
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, K.A.; Bavari, S.; Salazar, A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines 2015, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Salazar, A.M.; Celis, E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin. Immunol. 2020, 49, 101414. [Google Scholar] [CrossRef] [PubMed]
- Sammons, M.L.; Stephen, E.L.; Levy, H.B.; Baron, S.; Hilmas, D.E. Interferon induction in cynomolgus and rhesus monkey after repeated doses of a modified polyriboinosinic-polyribocytidylic acid complex. Antimicrob. Agents Chemother. 1977, 11, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Lefebvre, F.; Filali-Mouhim, A.; Cameron, M.J.; Goulet, J.P.; Haddad, E.K.; Breton, G.; Trumpfheller, C.; Pollak, S.; Shimeliovich, I.; et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 2011, 208, 2357–2366. [Google Scholar] [CrossRef]
- Levy, H.B.; Baer, G.; Baron, S.; Buckler, C.E.; Gibbs, C.J.; Iadarola, M.J.; London, W.T.; Rice, J. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J. Infect. Dis. 1975, 132, 434–439. [Google Scholar] [CrossRef]
- Sultan, H.; Wu, J.; Kumai, T.; Salazar, A.M.; Celis, E. Role of MDA5 and interferon-I in dendritic cells for T cell expansion by antitumor peptide vaccines in mice. Cancer Immunol. Immunother. 2018, 67, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Barral, P.M.; Sarkar, D.; Su, Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the cytoplasmic RNA sensors Rig-I And MDA-5: Key regulators of innate immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. Tlr3: Interferon induction by double-stranded RNA including poly (I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–115. [Google Scholar] [CrossRef]
- Takeoka, T.; Nagase, H.; Kurose, K.; Ohue, Y.; Yamasaki, M.; Takiguchi, S.; Sato, E.; Isobe, M.; Kanazawa, T.; Matsumoto, M.; et al. NY-ESO-1 Protein Cancer Vaccine with Poly-ICLC and OK-432: Rapid and Strong Induction of NY-ESO-1-specific Immune Responses by Poly-ICLC. J. Immunother. 2017, 40, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.M.; Petroni, G.R.; Smolkin, M.E.; Brenin, D.R.; Chianese-Bullock, K.A.; Smith, K.T.; Olson, W.C.; Fanous, I.S.; Nail, C.J.; Brenin, C.M.; et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 2017, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245, Erratum in 2019, 566, E13. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, D.; Dutoit, V.; Allard, M.; Grandjean Hallez, N.; Marinari, E.; Widmer, V.; Philippin, G.; Corlazzoli, F.; Gustave, R.; Kreutzfeldt, M.; et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro Oncol. 2019, 21, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in 2018, 555, 402. [Google Scholar] [CrossRef]
- Salazar, A.M.; Hilton, B.L.; Steven, O.; Meir, K.; Barbara, S.; Douglas, B.; Hernando, M.; Norman, M.; Karen, S.; Daniel, D.; et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: An open pilot study. Neurosurgery 1996, 38, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Hochster, H.; Blum, R.; Wiernik, P.H.; Hudes, G.R.; Kirkwood, J.; Trump, D.; Oken, M.M. Toxicity and response evaluation of the interferon inducer poly ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: A report of two clinical trials of the Eastern Cooperative Oncology Group. Invest. New Drugs. 2001, 19, 89–92. [Google Scholar] [CrossRef]
- Butowski, N.; Chang, S.M.; Junck, L.; DeAngelis, L.M.; Abrey, L.; Fink, K.; Cloughesy, T.; Lamborn, K.R.; Salazar, A.M.; Prados, M.D. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: A North American Brain Tumor Consortium (NABTC01-05). J. Neurooncol. 2009, 91, 175–182. [Google Scholar] [CrossRef]
- Kyi, C.; Roudko, I.; Sabado, R.; Saenger, Y.; Loging, W.; Mandeli, J.; Thin, T.H.; Lehrer, D.; Donovan, M.; Posner, M.; et al. Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: A pilot trial. Clin. Cancer Res. 2018, 24, 4937–4948. [Google Scholar] [CrossRef]
- de la Torre, A.N.; Contractor, S.; Castaneda, I.; Cathcart, C.S.; Razdan, D.; Klyde, D.; Kisza, P.; Gonzales, S.F.; Salazar, A.M. A Phase I trial using local regional treatment, nonlethal irradiation, intratumoral and systemic polyinosinic-polycytidylic acid polylysine carboxymethylcellulose to treat liver cancer: In search of the abscopal effect. J. Hepatocell. Carcinoma. 2017, 4, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Marron, T.U.; Upadhyay, R.; Svensson-Arvelund, J.; Dhainaut, M.; Hussein, S.; Zhan, Y.; Ostrowski, D.; Yellin, M.; Marsh, H.; et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019, 25, 814–824. [Google Scholar] [CrossRef]
- Hartman, L.L.; Crawford, J.R.; Makale, M.T.; Milburn, M.; Joshi, S.; Salazar, A.M.; Hasenauer, B.; VandenBerg, S.R.; MacDonald, T.J.; Durden, D.L. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J. Pediatr. Hematol. Oncol. 2014, 36, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.J.; Evans, K.M.; Sampson, J.; Wood, J.L.N. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010, 51, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J. Putting the Immunologic Brakes on Cancer. Cell 2018, 175, 1452–1454. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290, Erratum in 2016, 13, 394. [Google Scholar] [CrossRef]
- Killick, D.R.; Stell, A.J.; Catchpole, B. Immunotherapy for canine cancer—Is it time to go back to the future? J. Small Anim. Pract. 2015, 56, 229–241. [Google Scholar] [CrossRef]
- Bergman, P.J. Cancer Immunotherapies. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 881–902. [Google Scholar] [CrossRef]
- Smith, J.S.; Yager, P.A.; Baer, G.M. Minimal interferon induction in dogs, using a modified polyriboinosinic-polyribocytidylic acid complex. Am. J. Vet. Res. 1980, 41, 1833–1835. [Google Scholar]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2013, 13, 176–183. [Google Scholar] [CrossRef]
- Veterinary Cooperative Oncology Group-Common Terminology. Criteria for Adverse Events (VCOG-CTCAE) following Chemotherapy or Biological Antineoplastic Therapy in Dogs and Cats V1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [Google Scholar] [CrossRef]
- Yazbek, K.V.; Fantoni, D.T. Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. J. Am. Vet. Med. Assoc. 2005, 226, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L. Immunomodulators, immunostimulants, and immunotherapies in small animal veterinary medicine. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.; Guth, A.; Coy, J.; Dow, S. Cancer immunotherapy in veterinary medicine: Current options and new developments. Vet. J. 2016, 207, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J. Veterinary oncology immunotherapies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Wu, J.; Fesenkova, V.I.; Fan, A.E.; Addis, D.; Salazar, A.M.; Celis, E. Poly-IC enhances the effectiveness of cancer immunotherapy by promoting T cell tumor infiltration. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
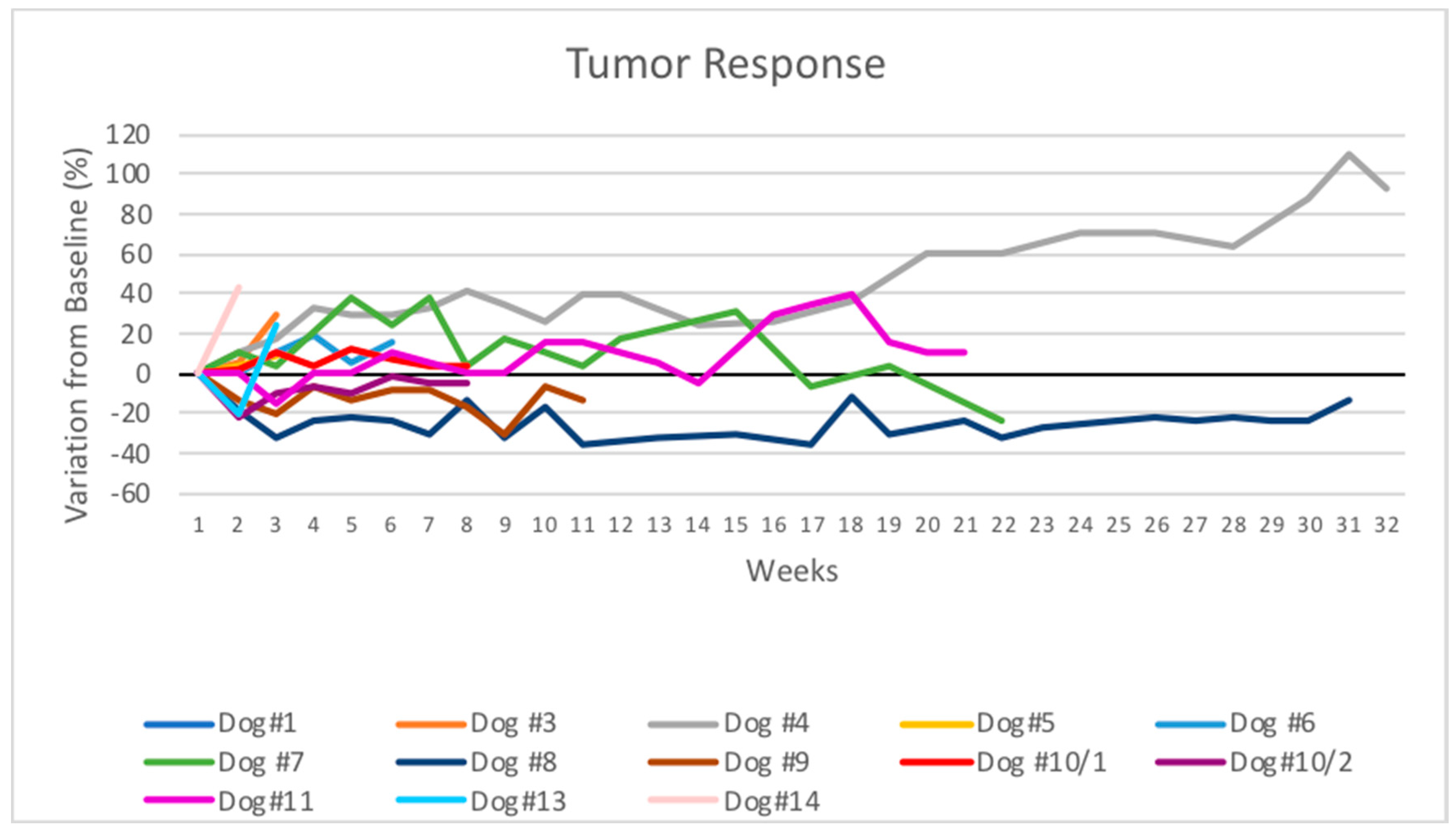
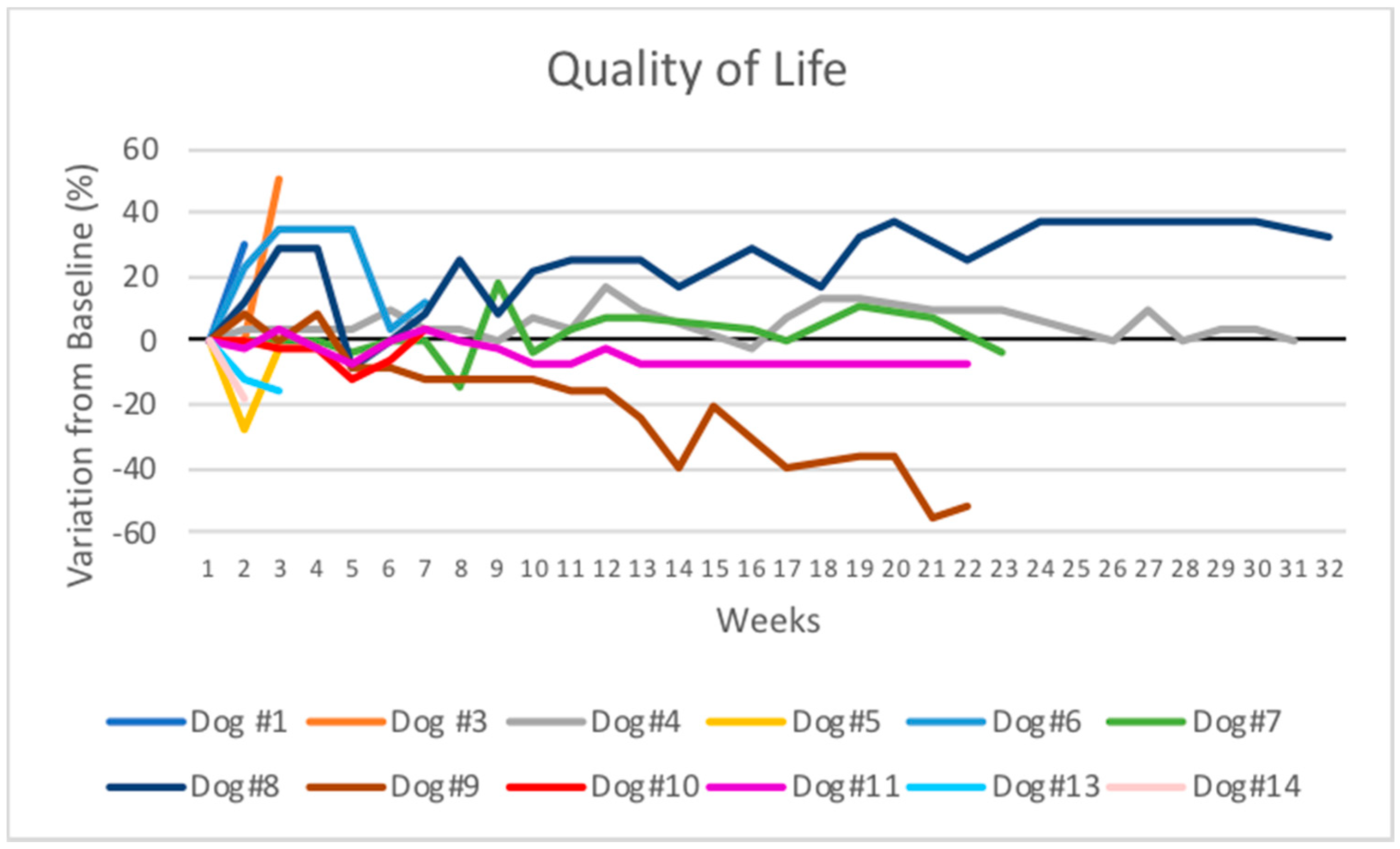
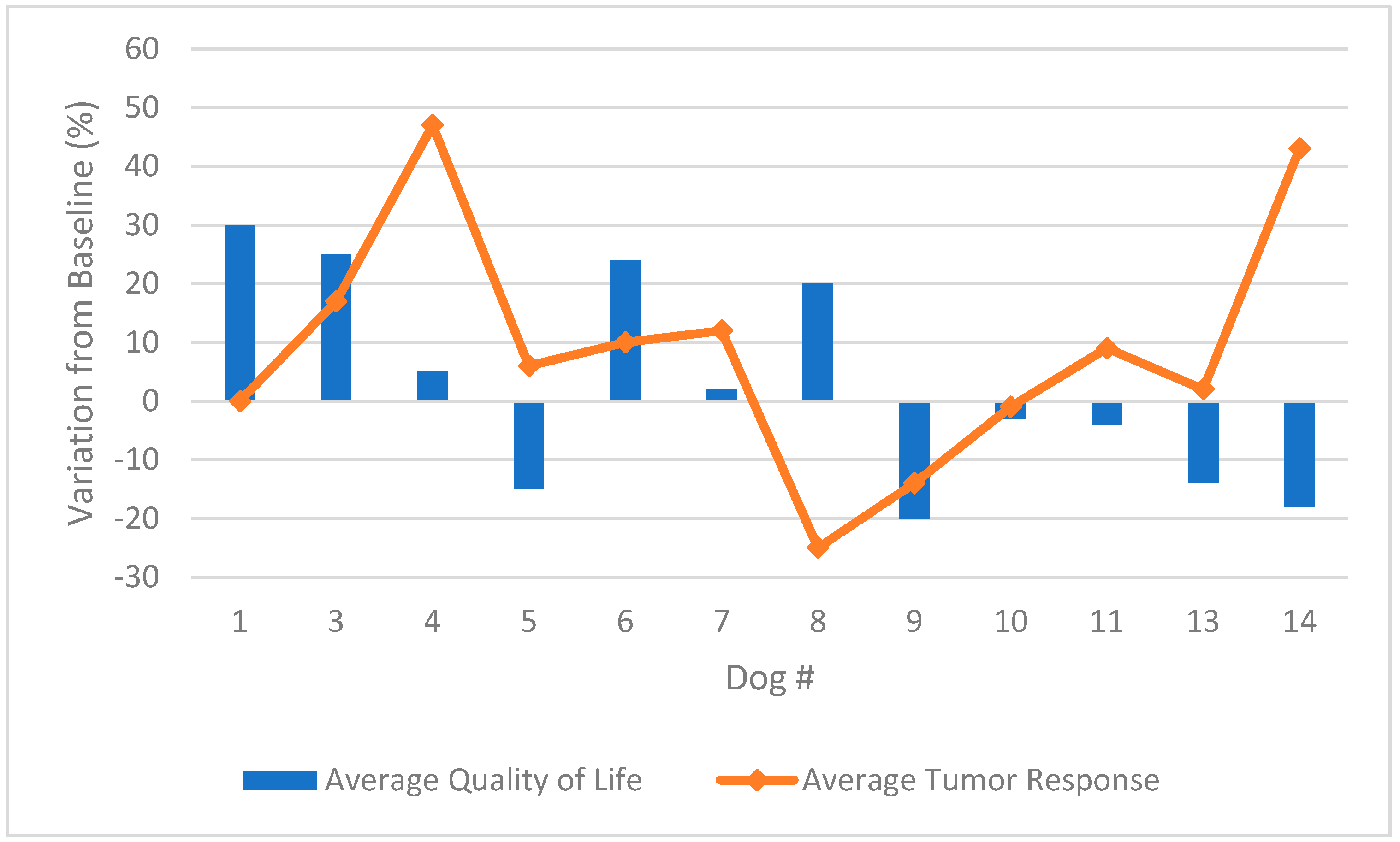
| Dog | Breed | Age (Years) | Gender | Primary Tumor | Location | TNM | WHO Stage | Metastasis | Prior Therapies |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mongrel | 16 | Male * | STS | Jaw | T3N1M1 | IV | Olfactory bulb | Surgery, chemotherapy (4 Dx) |
| 2 | Mongrel | 3 | Male * | STS | Thorax | T4N1M1 | IV | LN, lungs | None |
| 3 | Cocker spaniel | 13 | Female * | STS | Abdominal region | T4N1M1 | IV | LN, lungs, liver, spleen | Surgery, ECT |
| 4 | Mongrel | 8 | Female * | STS | Mandible | T3N1M0 | III | LN | Surgery, chemotherapy (5 Dx) |
| 5 | Mongrel | 16 | Female * | STS | Thorax | T4N1M1 | IV | Lungs, liver | None |
| 6 | Labrador | 11 | Female | Histiocytic sarcoma | Thorax | T3N1M1 | IV | LN, lungs, skin, liver, spleen, intestinal serosa, myocardium, esophagus | None |
| 7 | Teckel | 12 | Male * | Carcinoma | Dorsal to the left eye | T2N0M0 | II | none | Radiotherapy |
| 8 | Maltese | 13 | Female * | Carcinoma | Left perianal region | T3N1M0 | III | LN | Chemotherapy) (RTKi) |
| 9 | Mongrel | 15 | Female * | Adenocarcinoma | Breast, inguinal region and inner face of the right pelvic limb | T4N2M1 | IV | LN, skin, liver | Surgery, chemotherapy (4 Dx, 1 Cb) |
| 10 | Mongrel | 12 | Female * | Adenocarcinoma | Paravertebral | T3N2M1 | IV | Subcutaneous, lungs | None |
| 11 | Shih-tzu | 15 | Female * | Adenocarcinoma | Breast, vulva | T3N1M1 | IV | LN, liver, skin | Surgery, chemotherapy (4 Cb) |
| 12 | Mongrel | 9 | Female * | Adenocarcinoma | Breast | T4N2M1 | IV | LN, lungs, skin, liver, spleen | Surgery, chemotherapy (5 Dx, 6 Cb) |
| 13 | Bulldog | 11 | Male * | Multicentric NHL | Lymphoid tissue | stage IV | IV | Liver, Spleen | Chemotherapy (Vc, Cc, Dx, Pr) |
| 14 | Mongrel | 10 | Male * | Multicentric NHL | Lymphoid tissue | stage IV | IV | Liver, Spleen | Chemotherapy (1 Vc, 1 Cc, 1 Dx) |
| Dog | Treatment Duration (Weeks) | Dose of poly-ICLC (mg) | BOR | Survival (Days) | Survival after poly-ICLC (Days) | Concurrent Disease | Status |
|---|---|---|---|---|---|---|---|
| 4 | 30 | 1.00 | PD | 411 | 259 | None | alive |
| 7 | 22 | 0.50 | SD | 346 | 206 | MVI | alive |
| 8 | 31 | 0.25 | PR | 520 | 252 | CKD, MVI, TVI | dead (kidney and heart failure) |
| 9 | 21 | 1.00 | SD | 470 | 181 | None | Dead (euthanasia) |
| 11 | 21 | 0.50 | SD | 2108 | 178 | MVI, TVI | alive |
| 10 | 8 | 1.00 | SD | 123 | 133 | None | dead (euthanasia) |
| 6 | 6 | 1.00 | PD | 133 | 112 | None | dead (euthanasia) |
| 5 | 3 | 0.50 | NE | 555 | 77 | MVI | alive |
| 13 | 3 | 1.00 | PD | 218 | 24 | MVI, TVI | Dead (respiratory failure) |
| 1 | 2 | 0.50 | NE | 607 | 46 | MVI, TVI | Dead (seizure) |
| 14 | 2 | 1.00 | PD | 100 | 24 | None | Dead (respiratory failure) |
| 2 | 1 | 0.25 | PD | 127 | 13 | None | Dead (probable cause?) |
| 12 | 1 | 0.25 | PD | 443 | 6 | MVI | Dead (euthanasia) |
| Dog | Treatment Duration (Weeks) | Baseline Tumor Burden Nontarget Lesions | Final Tumor Burden Nontarget Lesions | BOR Nontarget Lesions | BOR Target and Nontarget Lesions |
|---|---|---|---|---|---|
| 4 | 30 | LN: 1 enlarged (3.8 cm) | LN: 1 enlarged (3.9 cm) | SD | SD |
| 8 | 31 | LN: 2 enlarged (4.6 cm and 1.9 cm) | LN: 3 enlarged (4.5, 2.1, 1.4 cm) | PD | PD |
| 9 | 21 | Liver: 2 nodules (4.6 cm and 2.9 cm) | Liver: 2 nodules (4.7 and 3,3 cm) + another Spleen: 2 nodules (0.9 and 2.2 cm) | PD | PD |
| 10 | 6 | Lungs: unquantifiable nodules | Lungs: unquantifiable nodules | PD | PD |
| 11 | 21 | Liver: 1 nodule (5.1 cm) LN: 3 enlarged (1.7, 0.9, 0.5 cm) | Liver: 4 nodules (7.0, 1.0, 0.3, 0.7 cm) LNs: 4 enlarged (2.1, 1.0, 1.5, 1.2 cm) Stomach: 1 nodule (1.2 cm) | PD | PD |
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | TOTAL |
|---|---|---|---|---|---|---|
| Related to poly-ICLC | ||||||
| Lethargy/fatigue | 7/14 | 0 | 0 | 0 | 0 | 7/14 |
| Allergic reaction/ hypersensitivity | 1/14 | 2/14 | 0 | 0 | 0 | 3/14 |
| Not related to poly-ICLC | ||||||
| Skin ulceration | 0 | 2/14 | 2/14 | 0 | 0 | 4/14 |
| Seizure | 0 | 0 | 0 | 1/14 | 1/14 | 2/14 |
| Dyspnea | 0 | 0 | 0 | 0 | 3/14 | 3/14 |
| Increased alkaline phosphatase | 1/6 | 2/6 | 2/6 | 0 | 0 | 5/6 |
| Increased ALT | 0 | 2/6 | 2/6 | 0 | 0 | 4/6 |
| Increased BUN | 2/6 | 1/6 | 0 | 0 | 0 | 3/6 |
| Increased creatinine | 1/6 | 0 | 0 | 0 | 0 | 1/6 |
| Decreased hemoglobin | 5/6 | 1/6 | 0 | 0 | 0 | 6/6 |
| Cystitis | 1/6 | 0 | 0 | 0 | 0 | 1/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voges, A.R.N.; Ubukata, R.; Yazbek, K.V.B.; Caballero, O.L.; Salazar, A.M.; Massoco, C.d.O.; Dagli, M.L.Z. Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events. Cancers 2021, 13, 2237. https://doi.org/10.3390/cancers13092237
Voges ARN, Ubukata R, Yazbek KVB, Caballero OL, Salazar AM, Massoco CdO, Dagli MLZ. Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events. Cancers. 2021; 13(9):2237. https://doi.org/10.3390/cancers13092237
Chicago/Turabian StyleVoges, Alessandra Rinah Nogueira, Rodrigo Ubukata, Karina Velloso Braga Yazbek, Otávia Luisa Caballero, Andres Mario Salazar, Cristina de Oliveira Massoco, and Maria Lucia Zaidan Dagli. 2021. "Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events" Cancers 13, no. 9: 2237. https://doi.org/10.3390/cancers13092237
APA StyleVoges, A. R. N., Ubukata, R., Yazbek, K. V. B., Caballero, O. L., Salazar, A. M., Massoco, C. d. O., & Dagli, M. L. Z. (2021). Intratumoral (Poly-ICLC) Therapy for Dogs with Advanced Cancers: First Report on Clinical Effectiveness, Quality of Life, and Adverse Events. Cancers, 13(9), 2237. https://doi.org/10.3390/cancers13092237








