YK-4-279 Attenuates Progression of Pre-Existing Pigmented Lesions to Nodular Melanoma in a Mouse Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
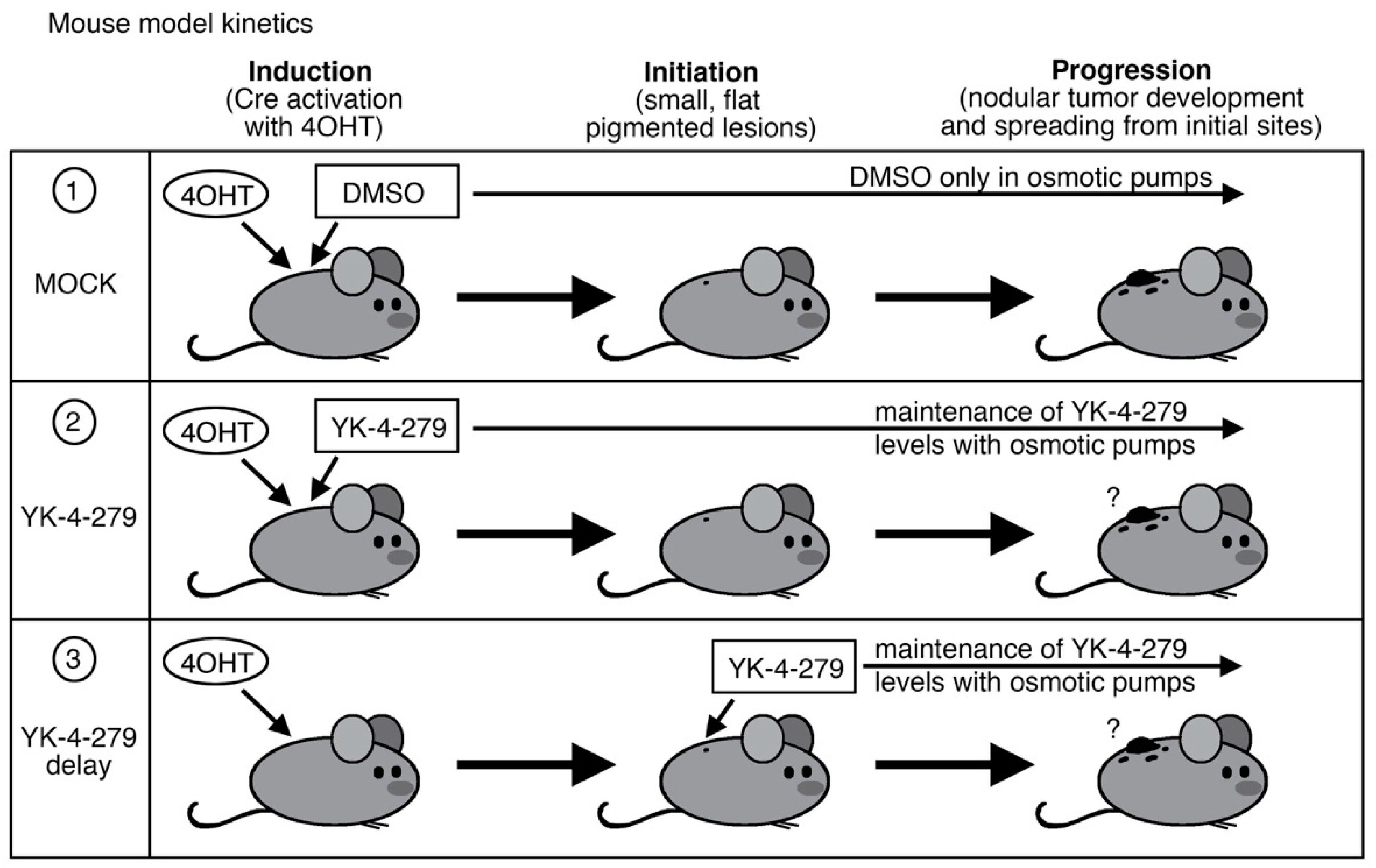
2.1. Melanoma Mouse Model
2.2. YK-4-279 Drug Treatment
2.3. Statistical Analysis
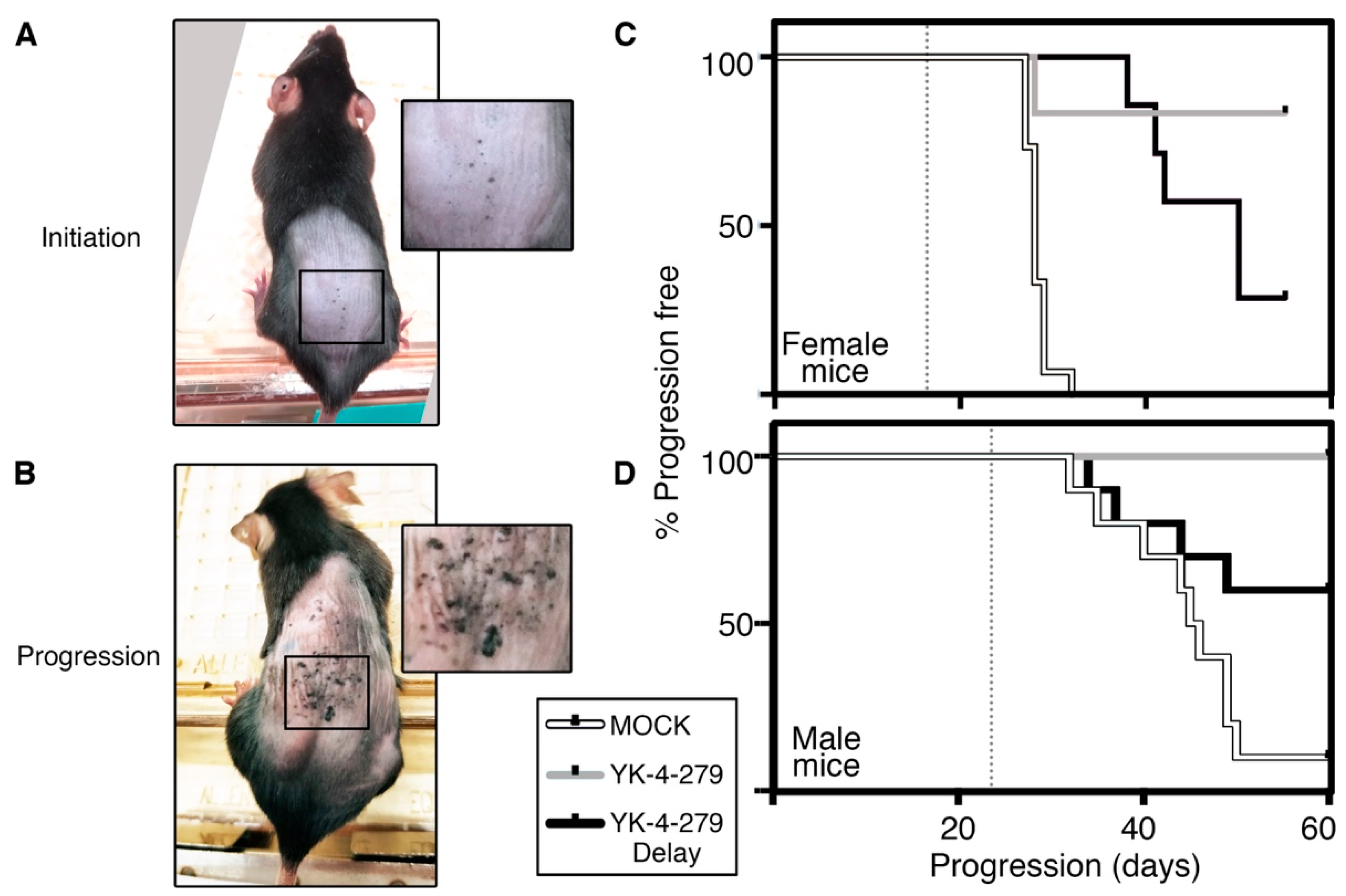
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Zhai, Y.; La, J.; Lui, J.W.; Moore, S.P.G.; Little, E.C.; Xiao, S.; Haresi, A.J.; Brem, C.; Bhawan, J.; et al. Targeting Pan-ETS Factors Inhibits Melanoma Progression. Cancer Res. 2021, 81, 2071–2085. [Google Scholar] [CrossRef]
- Erkizan, H.V.; Kong, Y.; Merchant, M.; Schlottmann, S.; Barber-Rotenberg, J.S.; Yuan, L.; Abaan, O.D.; Chou, T.H.; Dakshanamurthy, S.; Brown, M.L.; et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat. Med. 2009, 15, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Rahim, S.; Beauchamp, E.M.; Kong, Y.; Brown, M.L.; Toretsky, J.A.; Uren, A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PLoS ONE 2011, 6, e19343. [Google Scholar] [CrossRef] [Green Version]
- Rahim, S.; Minas, T.; Hong, S.H.; Justvig, S.; Celik, H.; Kont, Y.S.; Han, J.; Kallarakal, A.T.; Kong, Y.; Rudek, M.A.; et al. A small molecule inhibitor of ETV1, YK-4-279, prevents prostate cancer growth and metastasis in a mouse xenograft model. PLoS ONE 2014, 9, e114260. [Google Scholar] [CrossRef] [Green Version]
- Selvanathan, S.P.; Graham, G.T.; Erkizan, H.V.; Dirksen, U.; Natarajan, T.G.; Dakic, A.; Yu, S.; Liu, X.; Paulsen, M.T.; Ljungman, M.E.; et al. Oncogenic fusion protein EWS-FLI1 is a network hub that regulates alternative splicing. Proc. Natl. Acad. Sci. USA 2015, 112, E1307–E1316. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, J.A.; Federman, N.C.; Anderson, P.M.; Macy, M.E.; Riedel, R.F.; Davis, L.E.; Daw, N.C.; Wulff, J.; Kim, A.; Ratan, R.; et al. TK216 for relapsed/refractory Ewing sarcoma: Interim phase 1/2 results. J. Clin. Oncol. 2021, 39, 11500. [Google Scholar] [CrossRef]
- Dankort, D.; Curley, D.P.; Cartlidge, R.A.; Nelson, B.; Karnezis, A.N.; Damsky, W.E., Jr.; You, M.J.; DePinho, R.A.; McMahon, M.; Bosenberg, M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009, 41, 544–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Haresi, A.J.; Huang, L.; Lang, D. Differences in tumor initiation and progression of melanoma in the Braf(CA);Tyr-CreERT2;Pten(f/f) model between male and female mice. Pigment Cell Melanoma Res. 2020, 33, 119–121. [Google Scholar] [CrossRef]
- Hooijkaas, A.I.; Gadiot, J.; van der Valk, M.; Mooi, W.J.; Blank, C.U. Targeting BRAFV600E in an inducible murine model of melanoma. Am. J. Pathol. 2012, 181, 785–794. [Google Scholar] [CrossRef]
- Keraliya, R.A.; Patel, C.; Patel, P.; Keraliya, V.; Soni, T.G.; Patel, R.C.; Patel, M.M. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm. 2012, 2012, 528079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamhamedi-Cherradi, S.E.; Menegaz, B.A.; Ramamoorthy, V.; Aiyer, R.A.; Maywald, R.L.; Buford, A.S.; Doolittle, D.K.; Culotta, K.S.; O’Dorisio, J.E.; Ludwig, J.A. An Oral Formulation of YK-4-279: Preclinical Efficacy and Acquired Resistance Patterns in Ewing Sarcoma. Mol. Cancer Ther. 2015, 14, 1591–1604. [Google Scholar] [CrossRef] [Green Version]
- Kollareddy, M.; Sherrard, A.; Park, J.H.; Szemes, M.; Gallacher, K.; Melegh, Z.; Oltean, S.; Michaelis, M.; Cinatl, J., Jr.; Kaidi, A.; et al. The small molecule inhibitor YK-4-279 disrupts mitotic progression of neuroblastoma cells, overcomes drug resistance and synergizes with inhibitors of mitosis. Cancer Lett. 2017, 403, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Povedano, J.M.; Li, V.; Lake, K.E.; Bai, X.; Rallabandi, R.; Kim, J.; Xie, Y.; De Brabander, J.K.; McFadden, D.G. TK216 targets microtubules in Ewing sarcoma cells. BioRxiv 2021. [Google Scholar] [CrossRef]
- De Magalhaes, J.P. How ageing processes influence cancer. Nat. Rev. Cancer 2013, 13, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic significance of BRAF and NRAS mutations in melanoma: A German study from routine care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.E.; Edmiston, S.N.; Alexander, A.; Groben, P.A.; Parrish, E.; Kricker, A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; From, L.; et al. Association Between NRAS and BRAF Mutational Status and Melanoma-Specific Survival Among Patients With Higher-Risk Primary Melanoma. JAMA Oncol. 2015, 1, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlot, C.; Dubois-Pot, H.; Serchov, T.; Tourrette, Y.; Wasylyk, B. A review of post-translational modifications and subcellular localization of Ets transcription factors: Possible connection with cancer and involvement in the hypoxic response. Methods Mol. Biol. 2010, 647, 3–30. [Google Scholar] [CrossRef]
- Hollenhorst, P.C.; Ferris, M.W.; Hull, M.A.; Chae, H.; Kim, S.; Graves, B.J. Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 2011, 25, 2147–2157. [Google Scholar] [CrossRef] [Green Version]
- Zöllner, S.K.; Selvanathan, S.P.; Graham, G.T.; Commins, R.M.T.; Hong, S.H.; Moseley, E.; Parks, S.; Haladyna, J.N.; Erkizan, H.V.; Dirksen, U.; et al. Inhibition of the oncogenic fusion protein EWS-FLI1 causes G2-M cell cycle arrest and enhanced vincristine sensitivity in Ewing’s sarcoma. Sci. Signal. 2017, 10, eaam8429. [Google Scholar] [CrossRef] [Green Version]
- Luther, C.; Swami, U.; Zhang, J.; Milhem, M.; Zakharia, Y. Advanced stage melanoma therapies: Detailing the present and exploring the future. Crit Rev. Oncol. Hematol. 2019, 133, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, S.; Shi, P.; Chen, M.; Yu, S.; Hong, S.; Li, Y.; Liu, R.; Xiao, H. The ETS Inhibitor YK-4-279 Suppresses Thyroid Cancer Progression Independent of TERT Promoter Mutations. Front. Oncol. 2021, 11, 649323. [Google Scholar] [CrossRef]
- Minas, T.Z.; Han, J.; Javaheri, T.; Hong, S.H.; Schlederer, M.; Saygideger-Kont, Y.; Celik, H.; Mueller, K.M.; Temel, I.; Ozdemirli, M.; et al. YK-4-279 effectively antagonizes EWS-FLI1 induced leukemia in a transgenic mouse model. Oncotarget 2015, 6, 37678–37694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Bessonova, L.; Taylor, T.H.; Ziogas, A.; Meyskens, F.L., Jr.; Anton-Culver, H. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013, 26, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.H., Jr.; From, L.; Bernardino, E.A.; Mihm, M.C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969, 29, 705–727. [Google Scholar]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; de Vries, E.; Eckel, R.; Nijsten, T.; Eggermont, A.M.; Holzel, D.; Coebergh, J.W.; Engel, J.; Munich Melanoma, G. Gender differences in melanoma survival: Female patients have a decreased risk of metastasis. J. Investig. Dermatol. 2011, 131, 719–726. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zhai, Y.; Fajardo, C.D.; Lang, D. YK-4-279 Attenuates Progression of Pre-Existing Pigmented Lesions to Nodular Melanoma in a Mouse Model. Cancers 2022, 14, 143. https://doi.org/10.3390/cancers14010143
Huang L, Zhai Y, Fajardo CD, Lang D. YK-4-279 Attenuates Progression of Pre-Existing Pigmented Lesions to Nodular Melanoma in a Mouse Model. Cancers. 2022; 14(1):143. https://doi.org/10.3390/cancers14010143
Chicago/Turabian StyleHuang, Lee, Yougang Zhai, Cristian D. Fajardo, and Deborah Lang. 2022. "YK-4-279 Attenuates Progression of Pre-Existing Pigmented Lesions to Nodular Melanoma in a Mouse Model" Cancers 14, no. 1: 143. https://doi.org/10.3390/cancers14010143





