Assessment of Health-Related Quality of Life in Patients with Advanced Prostate Cancer—Current State and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Importance of HRQOL for Patients with Advanced Prostate Cancer
2.1. Availability of Novel Therapies
2.2. Side Effects of Novel Therapies
2.3. Benefit of Novel Therapies
2.4. Balancing Benefits and Risk of Adverse Events
2.5. Measuring Health-Related Quality of Life (HRQOL)
3. Currently Used Validated Questionnaires
3.1. EQ-5D
3.2. EORTC QLQ-C30
3.3. FACT-P
4. Ease of Use for Clinicians
4.1. Appropriate Use in Current Trials
4.2. Patient Uptake, Potential Strategies for Improvements
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Eisenberger, M.; Hardy-Bessard, A.-C.; Kim, C.S.; Géczi, L.; Ford, D.; Mourey, L.; Carles, J.; Parente, P.; Font, A.; Kacso, G.; et al. Phase III Study Comparing a Reduced Dose of Cabazitaxel (20 mg/m2) and the Currently Approved Dose (25 mg/m2) in Postdocetaxel Patients With Metastatic Castration-Resistant Prostate Cancer—PROSELICA. J. Clin. Oncol. 2017, 35, 3198–3206. [Google Scholar] [CrossRef]
- Cook, A.; Beesley, S.; O’Sullivan, J.M.; Birtle, A.J.; Thalmann, G.; Graham, J.D.; Spears, M.R.; Brock, S.; Srinivasan, R.; Protheroe, A.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- De Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.; Peters, T.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Wille, N.; Badia, X.; Bonsel, G.; Burström, K.; Cavrini, G.; Devlin, N.; Egmar, A.-C.; Greiner, W.; Gusi, N.; Herdman, M.; et al. Development of the EQ-5D-Y: A child-friendly version of the EQ-5D. Qual. Life Res. 2010, 19, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretschmer, A.; Ploussard, G.; Heidegger, I.; Tsaur, I.; Borgmann, H.; Surcel, C.; Mathieu, R.; de Visschere, P.; Valerio, M.; Bergh, R.C.V.D.; et al. Health-related Quality of Life in Patients with Advanced Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2021, 7, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Bullinger, M.; Ahmedzai, S. A Modular Approach to Quality-of-Life Assessment in Cancer Clinical Trials. Recent Res. Cancer Res. 1988, 111, 231–249. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, G.; Bottomley, A.; Fosså, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef]
- Paiva, C.E.; Carneseca, E.C.; Barroso, E.M.; De Camargos, M.G.; Alfano, A.C.C.; Rugno, F.C.; Paiva, B.S.R. Further evaluation of the EORTC QLQ-C30 psychometric properties in a large Brazilian cancer patient cohort as a function of their educational status. Support. Care Cancer 2014, 22, 2151–2160. [Google Scholar] [CrossRef]
- Groenvold, M.; Klee, M.C.; Sprangers, M.A.; Aaronson, N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997, 50, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Cocks, K.; King, M.; Velikova, G.; de Castro, G.; St-James, M.M.; Fayers, P.; Brown, J. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur. J. Cancer 2012, 48, 1713–1721. [Google Scholar] [CrossRef]
- Fayers, P.M. Interpreting quality of life data: Population-based reference data for the EORTC QLQ-C30. Eur. J. Cancer 2001, 37, 1331–1334. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Sparano, F.; Moris, L.; Beyer, K.; Cottone, F.; Sprangers, M.; Efficace, F. Harnessing the patient voice in prostate cancer research: Systematic review on the use of patient-reported outcomes in randomized controlled trials to support clinical decision-making. Cancer Med. 2020, 9, 4039–4058. [Google Scholar] [CrossRef] [PubMed]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Chi, K.N.; Protheroe, A.; Rodríguez-Antolín, A.; Facchini, G.; Suttman, H.; Matsubara, N.; Ye, Z.; Keam, B.; Damião, R.; Li, T.; et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206. [Google Scholar] [CrossRef]
- Morgans, A.K.; Chen, Y.-H.; Sweeney, C.J.; Jarrard, D.F.; Plimack, E.R.; Gartrell, B.A.; Carducci, M.A.; Hussain, M.; Garcia, J.A.; Cella, D.; et al. Quality of Life During Treatment With Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer. J. Clin. Oncol. 2018, 36, 1088–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, N.; McQuarrie, K.; Bjartell, A.; Chowdhury, S.; Gomes, A.J.P.D.S.; Chung, B.H.; Özgüroglu, M.; Soto, Á.J.; Merseburger, A.S.; Uemura, H.; et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): A randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019, 20, 1518–1530. [Google Scholar] [CrossRef]
- Stenzl, A.; Dunshee, C.; De Giorgi, U.; Alekseev, B.; Iguchi, T.; Szmulewitz, R.Z.; Flaig, T.W.; Tombal, B.; Morlock, R.; Ivanescu, C.; et al. Effect of Enzalutamide plus Androgen Deprivation Therapy on Health-related Quality of Life in Patients with Metastatic Hormone-sensitive Prostate Cancer: An Analysis of the ARCHES Randomised, Placebo-controlled, Phase 3 Study. Eur. Urol. 2020, 78, 603–614. [Google Scholar] [CrossRef]
- Tombal, B.; Saad, F.; Penson, D.; Hussain, M.; Sternberg, C.N.; Morlock, R.; Ramaswamy, K.; Ivanescu, C.; Attard, G. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 556–569. [Google Scholar] [CrossRef]
- Smith, M.R.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the phase III ARAMIS trial. Eur. J. Cancer 2021, 154, 138–146. [Google Scholar] [CrossRef]
- Saad, F.; Cella, D.; Basch, E.; Hadaschik, B.; Mainwaring, P.N.; Oudard, S.; Graff, J.N.; McQuarrie, K.; Li, S.; Hudgens, S.; et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1404–1416. [Google Scholar] [CrossRef]
- Devlin, N.; Herdman, M.; Pavesi, M.; Phung, D.; Naidoo, S.; Beer, T.M.; Tombal, B.; Loriot, Y.; Ivanescu, C.; Parli, T.; et al. Health-related quality of life effects of enzalutamide in patients with metastatic castration-resistant prostate cancer: An in-depth post hoc analysis of EQ-5D data from the PREVAIL trial. Health Qual. Life Outcomes 2017, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Cella, D.; Ivanescu, C.; Holmstrom, S.; Bui, C.; Spalding, J.; Fizazi, K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: Additional analyses from the AFFIRM randomized clinical trial. Ann. Oncol. 2015, 26, 179–185. [Google Scholar] [CrossRef]
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial—FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef]
- Harland, S.; Staffurth, J.; Molina, A.; Hao, Y.; Gagnon, D.D.; Sternberg, C.N.; Cella, D.; Fizazi, K.; Logothetis, C.J.; Kheoh, T.; et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur. J. Cancer 2013, 49, 3648–3657. [Google Scholar] [CrossRef]
- Basch, E.; Autio, K.; Ryan, C.J.; Mulders, P.; Shore, N.; Kheoh, T.; Fizazi, K.; Logothetis, C.J.; Rathkopf, D.; Smith, M.R.; et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013, 14, 1193–1199. [Google Scholar] [CrossRef]
- Pickard, A.S.; Ray, S.; Ganguli, A.; Cella, D. Comparison of FACT- and EQ-5D–Based Utility Scores in Cancer. Value Health 2012, 15, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaltsa, K.; Longworth, L.; Ivanescu, C.; Phung, D.; Holmstrom, S. Mapping the FACT-P to the Preference-Based EQ-5D Questionnaire in Metastatic Castration-Resistant Prostate Cancer. Value Health 2014, 17, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Diels, J.; Hamberg, P.; Ford, D.; Price, P.W.; Spencer, M.; Dass, R.N. Mapping FACT-P to EQ-5D in a large cross-sectional study of metastatic castration-resistant prostate cancer patients. Qual. Life Res. 2015, 24, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Snyder, C.F.; Blackford, A.L.; Okuyama, T.; Akechi, T.; Yamashita, H.; Toyama, T.; Carducci, M.A.; Wu, A.W. Using the EORTC-QLQ-C30 in clinical practice for patient management: Identifying scores requiring a clinician’s attention. Qual. Life Res. 2013, 22, 2685–2691. [Google Scholar] [CrossRef] [Green Version]
- A Kessel, K.; Vogel, M.M.; Alles, A.; Dobiasch, S.; Fischer, H.; E Combs, S. Mobile App Delivery of the EORTC QLQ-C30 Questionnaire to Assess Health-Related Quality of Life in Oncological Patients: Usability Study. JMIR mHealth uHealth 2018, 6, e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fankhauser, C.D.; Wettstein, M.S.; Pedregal, M.; Clarke, N.W.; Sweeney, C.J. A Call for Standardized Reporting of Adverse Events. Eur. Urol. 2020, 78, 481–482. [Google Scholar] [CrossRef]
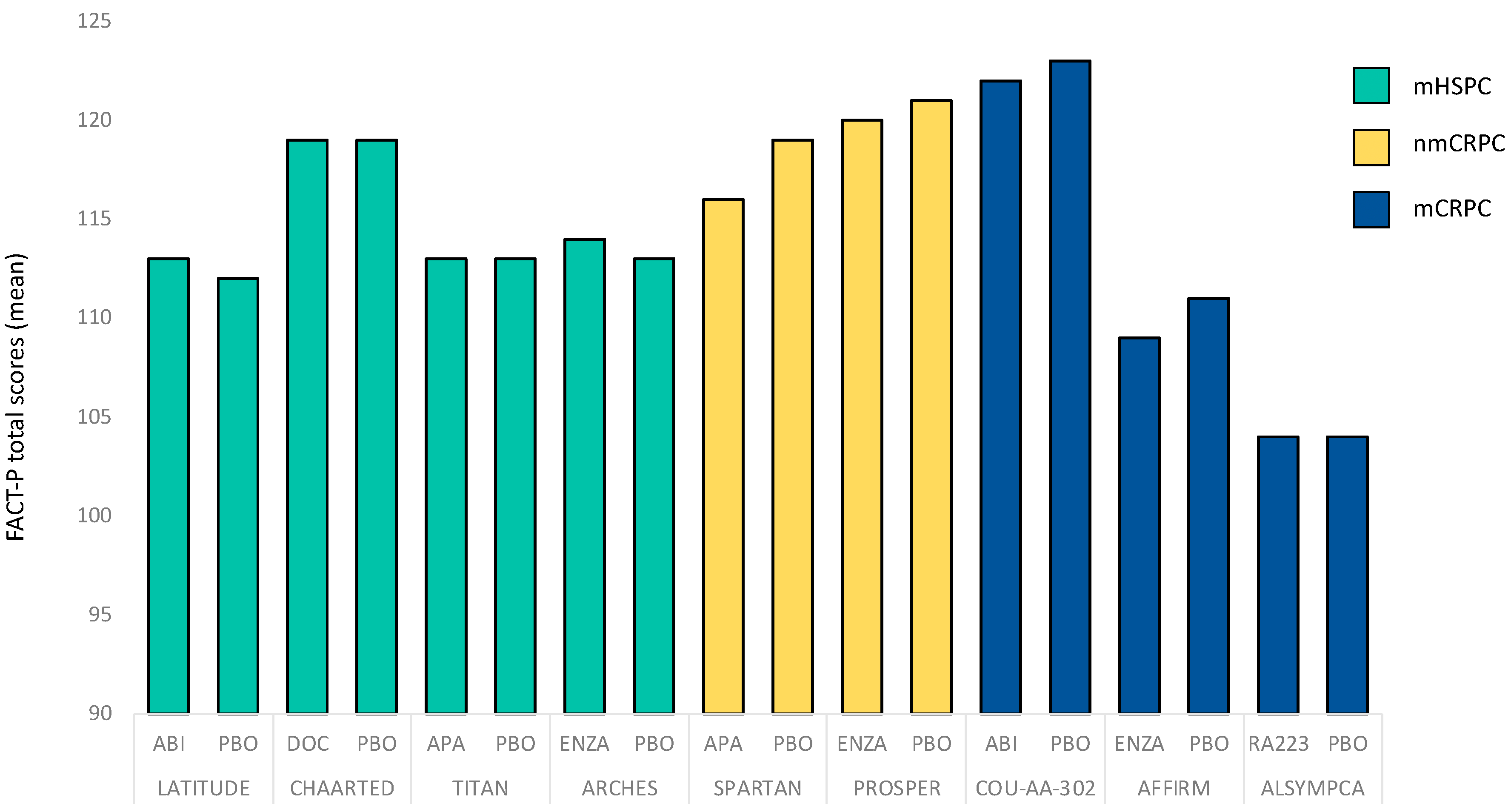
| Study | Year | Clinical Stage | Intervention | HRQOL Primary Endpoint | HRQOL Assessment Tool | HRQOL Baseline Values Reported |
|---|---|---|---|---|---|---|
| LATITUDE [29] | 2018 | mHSPC | ABI vs. PBO | no | FACT-P EQ-5D (-5L) | yes |
| E3805 CHAARTED [30] | 2018 | mHSPC | DOC + ADT vs. ADT | no | FACT-P (FACT-Taxane) | yes |
| TITAN [31] | 2019 | mHSPC | APA vs. PBO | no | FACT-P EQ-5D (-5L) | yes |
| ARCHES [32] | 2020 | mHSPC | ENZA vs. PBO | no | FACT-P EQ-5D (-5L) QLQ-PR25 | yes |
| PROSPER [33] | 2019 | nmCRPC | ENZA vs. PBO | no | FACT-P QLQ-PR25 EQ-5D (-5L) | yes |
| ARAMIS [34] | 2021 | nmCRPC | DARO vs. PBO | no | FACT-P QLQ-PR25 | |
| SPARTAN [35] | 2018 | nmCRPC | APA vs. PBO | no | FACT-P 5Q-5D (-3L) | yes |
| PREVAIL [36] | 2017 | mCRPC | ENZA vs. PBO | no | EQ-5D (-3L) | yes |
| AFFIRM [37] | 2014 | mCRPC | ENZA vs. PBO | no | FACT-P | yes |
| ALSYMPCA [7] | 2016 | mCRPC | RA223 vs. PBO | no | FACT-P EQ-5D (-5L) | yes |
| PROSELICA [5] | 2017 | mCRPC | CAB 20 vs. CAB 25 | no | FACT-P | no |
| FIRSTANA [38] | 2017 | mCRPC | CAB 20 vs. CAB 25 vs. DOC | no | FACT-P | no |
| COU-AA-301 [39] | 2011 | mCRPC | ABI + ADT vs. PBO + ADT | no | FACT-P | no |
| COU-AA-302 [40] | 2013 | mCRPC | ABI + ADT vs. PBO + ADT | no | FACT-P | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kretschmer, A.; van den Bergh, R.C.N.; Martini, A.; Marra, G.; Valerio, M.; Tsaur, I.; Heidegger, I.; Kasivisvanathan, V.; Kesch, C.; Preisser, F.; et al. Assessment of Health-Related Quality of Life in Patients with Advanced Prostate Cancer—Current State and Future Perspectives. Cancers 2022, 14, 147. https://doi.org/10.3390/cancers14010147
Kretschmer A, van den Bergh RCN, Martini A, Marra G, Valerio M, Tsaur I, Heidegger I, Kasivisvanathan V, Kesch C, Preisser F, et al. Assessment of Health-Related Quality of Life in Patients with Advanced Prostate Cancer—Current State and Future Perspectives. Cancers. 2022; 14(1):147. https://doi.org/10.3390/cancers14010147
Chicago/Turabian StyleKretschmer, Alexander, Roderick C. N. van den Bergh, Alberto Martini, Giancarlo Marra, Massimo Valerio, Igor Tsaur, Isabel Heidegger, Veeru Kasivisvanathan, Claudia Kesch, Felix Preisser, and et al. 2022. "Assessment of Health-Related Quality of Life in Patients with Advanced Prostate Cancer—Current State and Future Perspectives" Cancers 14, no. 1: 147. https://doi.org/10.3390/cancers14010147








