Outcomes after Surgical Treatment of Metastatic Disease in the Adrenal Gland; Valuable for the Patient?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Procedures
3.3. Short-Term and Long-Term Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skandalakis, J.E.; Skandalakis, L.J.; Skandalakis, P.N.; Mirilas, P. The Embryologic and Anatomic Basis of Modern Surgery. Surg. Anat. 2004, 2, 1153–1219. [Google Scholar]
- Bradley, C.T.; Strong, V.E. Surgical management of adrenal metastases. J. Surg. Oncol. 2014, 109, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cedermark, B.J.; Blumenson, L.E.; Pickren, J.W.; Holyoke, D.E.; Elias, E.G. The Significance of Metastases to the Adrenal Glands in Adenocarcinoma of the Colon and Rectum. Surg. Gynecol. Obstet. 1977, 144, 537–546. [Google Scholar]
- Cedermark, B.J.; Blumenson, L.E.; Pickren, J.W.; Elias, E.G. The Significance of Metastases to the Adrenal Gland from Carci-noma of the Stomach and Esophagus. Surg. Gynecol. Obstet. 1977, 145, 41–48. [Google Scholar] [PubMed]
- Seidenwurm, D.J.; Elmer, E.B.; Kaplan, L.M.; Williams, E.K.; Morris, D.G.; Hoffman, A.R. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984, 54, 552–557. [Google Scholar] [CrossRef]
- Mitchell, I.C.; Nwariaku, F.E. Adrenal Masses in the Cancer Patient: Surveillance or Excision. Oncology 2007, 12, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.C.; Shah, M.H.; Liebner, D.A.; Backes, F.J.; Phay, J.; Shirley, L.A. The Adrenal Gland as a Sanctuary Site of Metastases After Pembrolizumab Treatment: A Case Series. J. Natl. Compr. Cancer Netw. 2018, 16, 1279–1283. [Google Scholar] [CrossRef]
- Mercier, O.; Fadel, E.; de Perrot, M.; Mussot, S.; Stella, F.; Chapelier, A.; Dartevelle, P. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2005, 130, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Pfannschmidt, J.; Schlolaut, B.; Muley, T.; Hoffmann, H.; Dienemann, H. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer 2005, 49, 203–207. [Google Scholar] [CrossRef]
- Spartalis, E.; Drikos, I.; Ioannidis, A.; Chrysikos, D.; Athanasiadis, D.I.; Spartalis, M.; Avgerinos, D. Metastatic Carcinomas of the Adrenal Glands: From Diagnosis to Treatment. Anticancer Res. 2019, 39, 2699–2710. [Google Scholar] [CrossRef]
- Higashiyama, M.; Doi, O.; Kodama, K.; Yokouchi, H.; Imaoka, S.; Koyama, H. Surgical treatment of adrenal metastasis following pulmonary resection for lung cancer: Comparison of adrenalectomy with palliative therapy. Int. Surg. 1994, 79, 124–129. [Google Scholar] [PubMed]
- Luketich, J.D.; Burt, M.E. Does Resection of Adrenal Metastases from Non-Small Cell Lung Cancer Improve Survival? Ann. Thorac. Surg. 1996, 62, 1614–1616. [Google Scholar] [CrossRef]
- Sarela, A.I.; Murphy, I.; Coit, D.G. Metastasis to the Adrenal Gland: The Emerging Role of Laparoscopic Surgery. Ann. Surg. Oncol. 2003, 10, 1191–1196. [Google Scholar] [CrossRef]
- Staubitz, J.I.; Clerici, T.; Riss, P.; Watzka, F.; Bergenfelz, A.; Bareck, E.; Fendrich, V.; Goldmann, A.; Grafen, F.; Heintz, A.; et al. EUROCRINE®: Nebennierenoperationen 2015 bis 2019—Überraschende erste Ergebnisse. Chirurg 2021, 92, 448–463. [Google Scholar] [CrossRef]
- Strong, V.E.; D’Angelica, M.; Tang, L.; Prete, F.; Gönen, M.; Coit, D.; Touijer, K.A.; Fong, Y.; Brennan, M.F. Brennan Laparoscopic Adrenalectomy for Isolated Adrenal Metastasis. Ann. Surg. Oncol. 2007, 14, 3392–3400. [Google Scholar] [CrossRef]
- Vrielink, O.M.; Hemmer, P.H.; Kruijff, S. Considerations in Minimally Invasive Adrenal Surgery: The Front door or the Backdoor? Minerva Chir. 2018, 73, 93–99. [Google Scholar]
- Vrielink, O.M.; Wevers, K.P.; Kist, J.W.; Rinkes, I.H.M.B.; Hemmer, P.H.J.; Vriens, M.R.; De Vries, J.; Kruijff, S. Laparoscopic anterior versus endoscopic posterior approach for adrenalectomy: A shift to a new golden standard? Langenbeck’s Arch. Surg. 2017, 402, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Adler, J.T.; Mack, E.; Chen, H. Equal Oncologic Results for Laparoscopic and Open Resection of Adrenal Metastases. J. Surg. Res. 2007, 140, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.R.; Sui, D.; Grubbs, E.G.; Lee, J.E.; Perrier, N.D. Adrenal Metastectomy is Safe in Selected Patients. World J. Surg. 2014, 38, 1336–1342. [Google Scholar] [CrossRef]
- Howell, G.M.; Carty, S.E.; Armstrong, M.J.; Stang, M.T.; McCoy, K.L.; Bartlett, D.L.; Yip, L. Outcome and Prognostic Factors After Adrenalectomy for Patients with Distant Adrenal Metastasis. Ann. Surg. Oncol. 2013, 20, 3491–3496. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, M.A.; Thompson, G.B.; Duh, Q.-Y.; Hamrahian, A.H.; Angelos, P.; Elaraj, D.; Fishman, E.; Kharlip, J.; Garber, J.R.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American Association Of Endocrine Surgeons Medical Guidelines For The Management Of Adrenal Incidentalomas. Endocr. Pr. 2009, 15, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Puccini, M.; Panicucci, E.; Candalise, V.; Ceccarelli, C.; Neri, C.M.; Buccianti, P.; Miccoli, P. The role of laparoscopic resection of metastases to adrenal glands. Gland. Surg. 2017, 6, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsingh, J.; O’Dwyer, P.; Watson, C. Survival outcomes following adrenalectomy for isolated metastases to the adrenal gland. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 631–634. [Google Scholar] [CrossRef]
- Gunjur, A.; Duong, C.; Ball, D.; Siva, S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’—A systematic review. Cancer Treat. Rev. 2014, 40, 838–846. [Google Scholar] [CrossRef]
- Goto, T.; Inoue, T.; Kobayashi, T.; Yamasaki, T.; Ishitoya, S.; Segawa, T.; Ito, N.; Shichiri, Y.; Okumura, K.; Okuno, H.; et al. Feasibility of laparoscopic adrenalectomy for metastatic adrenal tumors in selected patients: A retrospective multicenter study of Japanese populations. Int. J. Clin. Oncol. 2019, 25, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.T.; Beninato, T.; Xiong, M.X.; Shah, N.V.; Kluijfhout, W.P.; Feeney, T.; Suh, I.; Gosnell, J.E.; Shen, W.T.; Duh, Q.-Y. Laparoscopic adrenalectomy for metastatic disease: Retrospective cohort with long-term, comprehensive follow-up. Surgery 2018, 165, 958–964. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Jeffery, M.; Hickey, B.E.; Hider, P.N.; See, A.M. Follow-up Strategies for Patients Treated for Non-Metastatic Colorectal Cancer. Cochrane Database Syst. Rev. 2019, 9, CD002200. [Google Scholar] [CrossRef]
- Lam, K.-Y.; Lo, C.-Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin. Endocrinol. 2002, 56, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gerber, E.; Dinlenc, C.; Wagner, J.R. Laparoscopic Adrenalectomy for Isolated Adrenal Metastasis. JSLS J. Soc. Laparoendosc. Surg. 2004, 8, 314–319. [Google Scholar]
- Staubitz, J.; Hoppe-Lotichius, M.; Baumgart, J.; Mittler, J.; Lang, H.; Musholt, T. Survival After Adrenalectomy for Metastatic Hepatocellular Carcinoma: A 25-year Institutional Experience. World J. Surg. 2021, 45, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Martin, A.C.R.; A Wicherts, D.; Azoulay, D.; Castaing, D.; Adam, R. Long-term outcome in patients with adrenal metastases following resection of colorectal liver metastases. BJS 2009, 96, 935–940. [Google Scholar] [CrossRef]
- Kocijancic, I.; Vidmar, K.; Zwitter, M.; Snoj, M. The significance of adrenal metastases from lung carcinoma. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 87–88. [Google Scholar] [CrossRef]
- Balla, A.; Palmieri, L.; Meoli, F.; Corallino, D.; Ortenzi, M.; Ursi, P.; Guerrieri, M.; Quaresima, S.; Paganini, A.M. Are Adrenal Lesions of 6 cm or More in Diameter a Contraindication to Laparoscopic Adrenalectomy? A Case–Control Study. World J. Surg. 2019, 44, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.C.; Gunseren, K.O.; Senol, K.; Vuruskan, H.; Yavascaoglu, I. Is 6 cm Diameter an Upper Limit for Adrenal Tumors to Perform Laparoscopic Adrenalectomy? J. Laparoendosc. Adv. Surg. Tech. 2021, 31, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Di Buono, G.; Buscemi, S.; Monte, A.I.L.; Geraci, G.; Sorce, V.; Citarrella, R.; Gulotta, E.; Palumbo, V.D.; Fazzotta, S.; Gulotta, L.; et al. Laparoscopic adrenalectomy: Preoperative data, surgical technique and clinical outcomes. BMC Surg. 2019, 18, 128. [Google Scholar] [CrossRef] [Green Version]
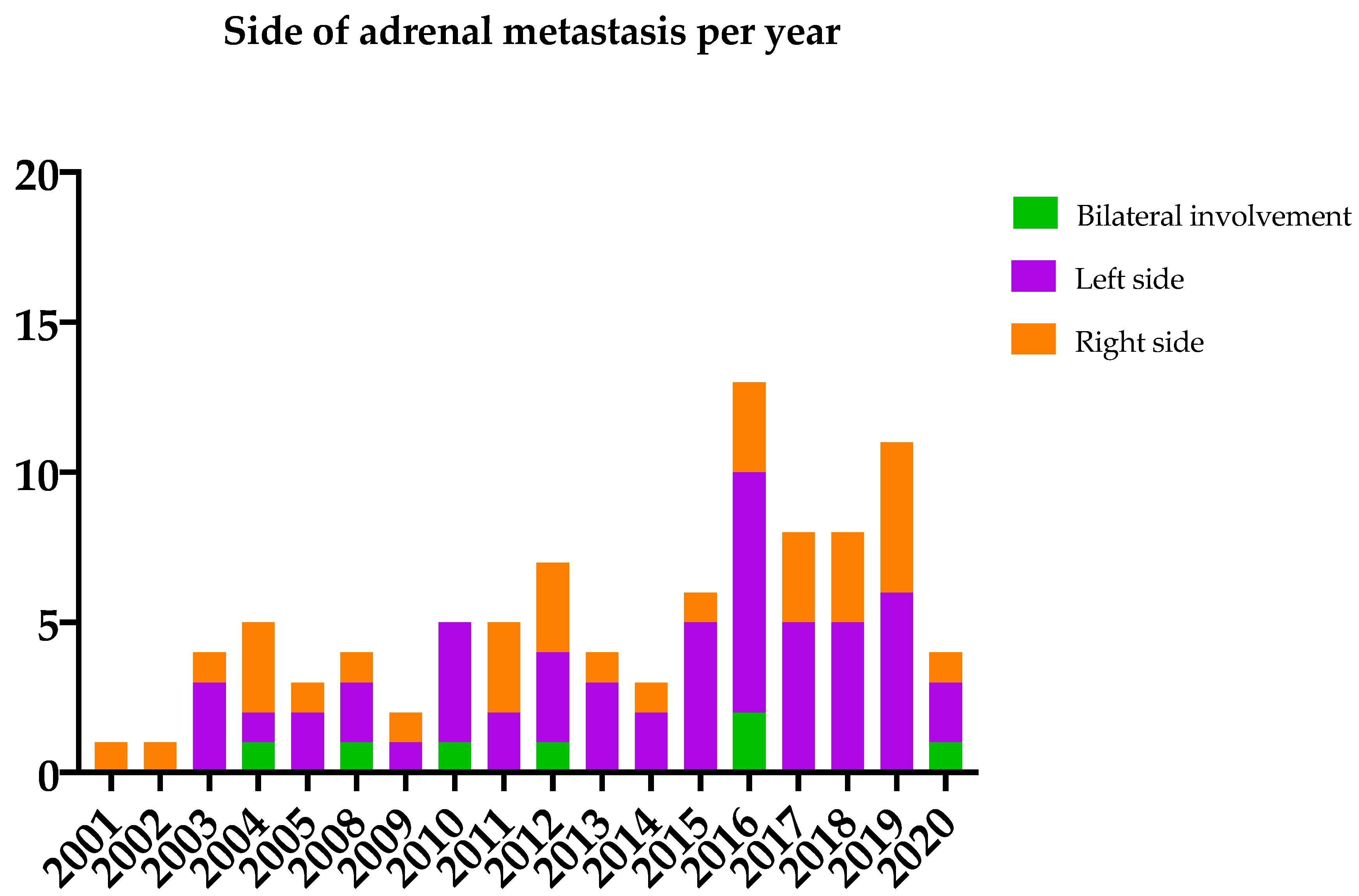
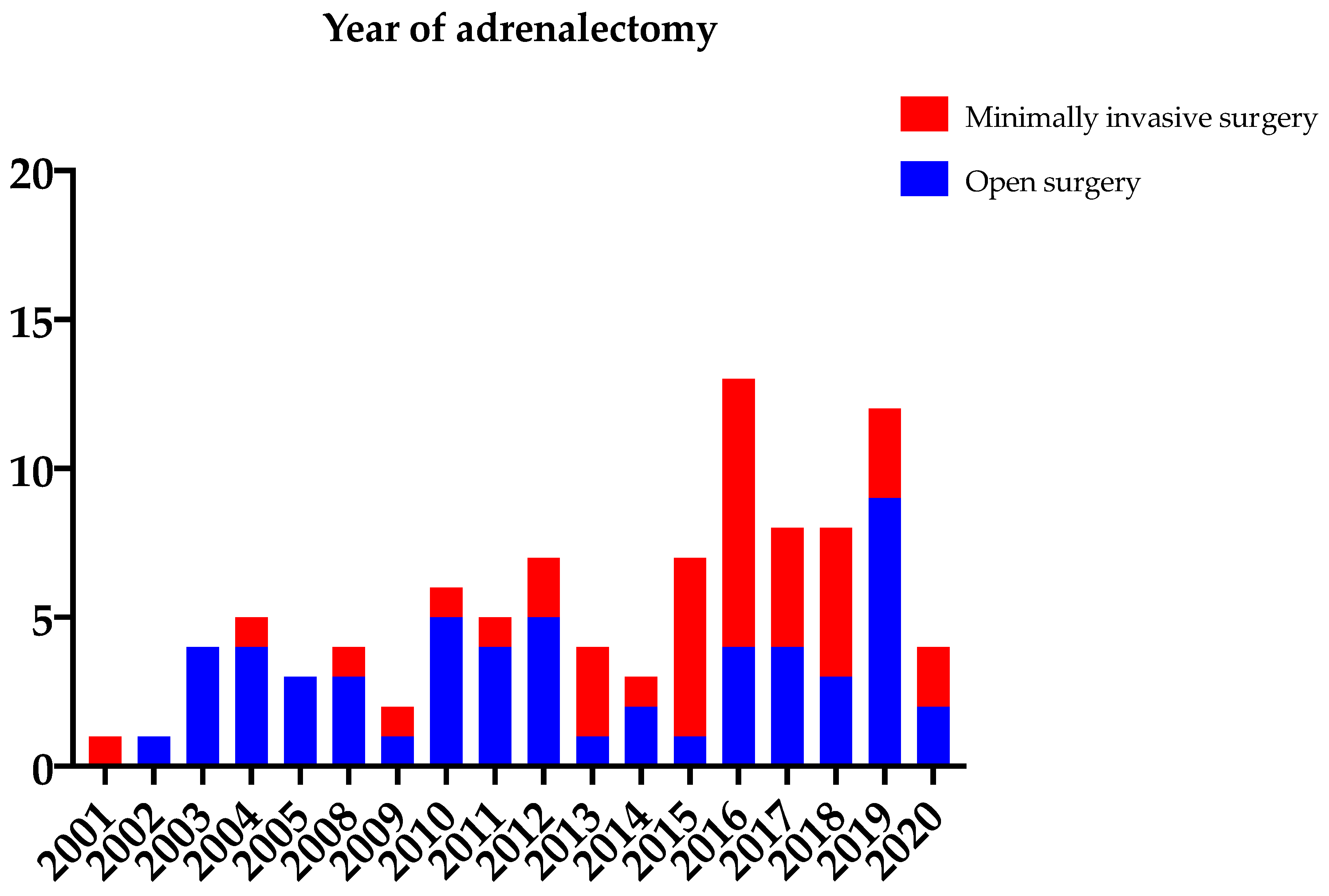
| Primary Tumor | Patients n (%) | Type of Surgery Open versus Minimally Invasive n (%) versus n (%) | Onset of Adrenal Metastasis Synchronous versus Metachronous n (%) versus n (%) | Concomitant Metastases n (n Synchronous versus n Metachronous Disease) | Resection Margin Radical versus Non-Radical n (%) versus n (%) |
|---|---|---|---|---|---|
| All adrenal metastasis | 95 (100) | 42 (44.2) versus 53 (55.8) | 20 (21.1) versus 75 (78.9) | 15 (8 versus 7) | 51 (70.8) versus 21 (29.2) 1 |
| Colorectal cancer | 25 (27) | 14 (56.0) versus 11 (44.0) | 2 (8.0) versus 23 (92) | 3 (0 versus 3) | 12 (63.2) versus 7 (36.8) |
| Lung cancer | 25 (27) | 6 (24.0) versus 19 (76.0) | 11 (44.0) versus 14 (56.0) | 4 (4 versus 0) | 15 (65.2) versus 8 (34.8) |
| Melanoma | 16 (17) | 8 (50.0) versus 8 (50.0) | 2 (12.5) versus 14 (87.5) | 4 (1 versus 3) | 9 (75.0) versus 3 (25.0) |
| Renal cell cancer | 7 (7) | 4 (57.1) versus 3 (42.9) | 0 (0) versus 8 (100) | N/A | 6 (100.0) versus 0 (0.0) |
| Breast cancer | 4 (4) | 0 (0.0) versus 4 (100.0) | 1 (25.0) versus 3 (75.0) | 1 (1 versus 0) | 2 (100.0) versus 0 (0.0) |
| Other cancer types | 18 (19) | 10 (55.5) versus 8 (44.5) | 5 (27.8) versus 13 (72.2) | 3 (2 versus 0) | 6 (75.0) versus 2 (25.0) |
| Radiological | Histological | |||
|---|---|---|---|---|
| Primary Tumor | Adrenal Metastasis (n) | Maximum Tumor Diameter (mm) Median (IQR) | Adrenal Metastasis (n) | Maximum Histological Tumor Diameter (mm) Median (IQR) |
| All adrenal metastasis | 77 | 33 (21) | 83 | 35 (36) |
| Colorectal cancer | 21 | 32 (17) | 25 | 39 (38) |
| Lung cancer | 18 | 40 (18.25) | 19 | 40 (37) |
| Melanoma | 13 | 36 (27) | 12 | 38.5 (41) |
| Renal cell cancer | 8 | 34 (14.75) | 7 | 28 (15) |
| Breast cancer | 3 | 28 (34) | 3 | 28 (28) |
| Other cancer types | 14 | 22 (8.25) | 17 | 35 (36) |
| Primary Tumor | Patients n (%) | Complications n (% per Tumor Type) |
|---|---|---|
| All adrenal metastasis | 95 (100) | 36 (100) |
| Colorectal cancer | 25 (27) | 10 (40) |
| Lung cancer | 25 (27) | 8 (32) |
| Melanoma | 16 (17) | 5 (31.25) |
| Renal cell cancer | 7 (7) | 4 (57.14) |
| Breast cancer | 4 (4) | 2 (50) |
| Other cancer types | 18 (19) | 7 (38.89) |
| Complication | Patients n (%) |
|---|---|
| All complications | 54 (100) |
| Ileus/gastroparesis | 7 (13.0) |
| Wound problems | 6 (11.1) |
| Pneumonia | 5 (9.3) |
| Heart arrhythmias | 5 (9.3) |
| Delirium | 4 (7.4) |
| Fluid overload/edema | 3 (5.6) |
| Electrolyte imbalance | 3 (5.6) |
| Urinary tract infection | 2 (3.7) |
| Bladder retention | 2 (3.7) |
| Anemia | 2 (3.7) |
| Bleeding | 2 (3.7) |
| Postoperative pain | 2 (3.7) |
| Septic shock | 1 (1.8) |
| Bowel perforation | 1 (1.8) |
| Bile leakage | 1 (1.8) |
| Pneumatic embolism | 1 (1.8) |
| Pneumothorax | 1 (1.8) |
| Decubitus | 1 (1.8) |
| Abscess | 1 (1.8) |
| Hypertension | 1 (1.8) |
| Diabetes de novo | 1 (1.8) |
| Constipation | 1 (1.8) |
| Fever | 1 (1.8) |
| Primary Tumor | Patients n (%) | Deceased Patients n (%) | Onset of Adrenal Metastasis Synchronous vs. Metachronous n versus n | Median Time to Death after MA Months (IQR) |
|---|---|---|---|---|
| All adrenal metastasis | 95 (100) | 53 (100) | 16 versus 37 | 20.2 (24.9) 1 |
| Colorectal cancer | 25 (27) | 17 (68) | 2 versus 15 | 29.97 (25.17) |
| Lung cancer | 25 (27) | 18 (72) | 10 versus 8 | 8.49 (15.88) 2 |
| Melanoma | 16 (17) | 3 (18.75) | 1 versus 2 | 7.96 (10.06) |
| Renal cell cancer | 7 (7) | 3 (42.86) | 0 versus 3 | 40.37 (71.29) |
| Breast cancer | 4 (4) | 1 (25) | 0 versus 1 | 46.82 (N/A) |
| Other cancer types | 18 (19) | 11 (57.89) | 3 versus 8 | 22.08 (24.57) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metman, M.J.H.; Viëtor, C.L.; Seinen, A.J.; Berends, A.M.A.; Hemmer, P.H.J.; Kerstens, M.N.; Feelders, R.A.; Franssen, G.J.H.; van Ginhoven, T.M.; Kruijff, S. Outcomes after Surgical Treatment of Metastatic Disease in the Adrenal Gland; Valuable for the Patient? Cancers 2022, 14, 156. https://doi.org/10.3390/cancers14010156
Metman MJH, Viëtor CL, Seinen AJ, Berends AMA, Hemmer PHJ, Kerstens MN, Feelders RA, Franssen GJH, van Ginhoven TM, Kruijff S. Outcomes after Surgical Treatment of Metastatic Disease in the Adrenal Gland; Valuable for the Patient? Cancers. 2022; 14(1):156. https://doi.org/10.3390/cancers14010156
Chicago/Turabian StyleMetman, Madelon J. H., Charlotte L. Viëtor, Auke J. Seinen, Annika M. A. Berends, Patrick H. J. Hemmer, Michiel N. Kerstens, Richard A. Feelders, Gaston J. H. Franssen, Tessa M. van Ginhoven, and Schelto Kruijff. 2022. "Outcomes after Surgical Treatment of Metastatic Disease in the Adrenal Gland; Valuable for the Patient?" Cancers 14, no. 1: 156. https://doi.org/10.3390/cancers14010156
APA StyleMetman, M. J. H., Viëtor, C. L., Seinen, A. J., Berends, A. M. A., Hemmer, P. H. J., Kerstens, M. N., Feelders, R. A., Franssen, G. J. H., van Ginhoven, T. M., & Kruijff, S. (2022). Outcomes after Surgical Treatment of Metastatic Disease in the Adrenal Gland; Valuable for the Patient? Cancers, 14(1), 156. https://doi.org/10.3390/cancers14010156





