IL-10 Rescues CLL Survival through Repolarization of Inflammatory Nurse-like Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient’s Samples
2.2. CLL Cells Isolation
2.3. Cell Cultures
2.4. Cytokine Treatment
2.4.1. Blocking Antibodies
2.4.2. Co-Culture Experiments
2.5. Flow Cytometry Analysis
2.5.1. CLL Cells Analysis
2.5.2. NLC Analysis
2.6. Fluorescence Microscopy Imaging
2.6.1. Stainings
2.6.2. Image Acquisition
2.6.3. Image Analysis
2.7. Statistical Analysis
3. Results
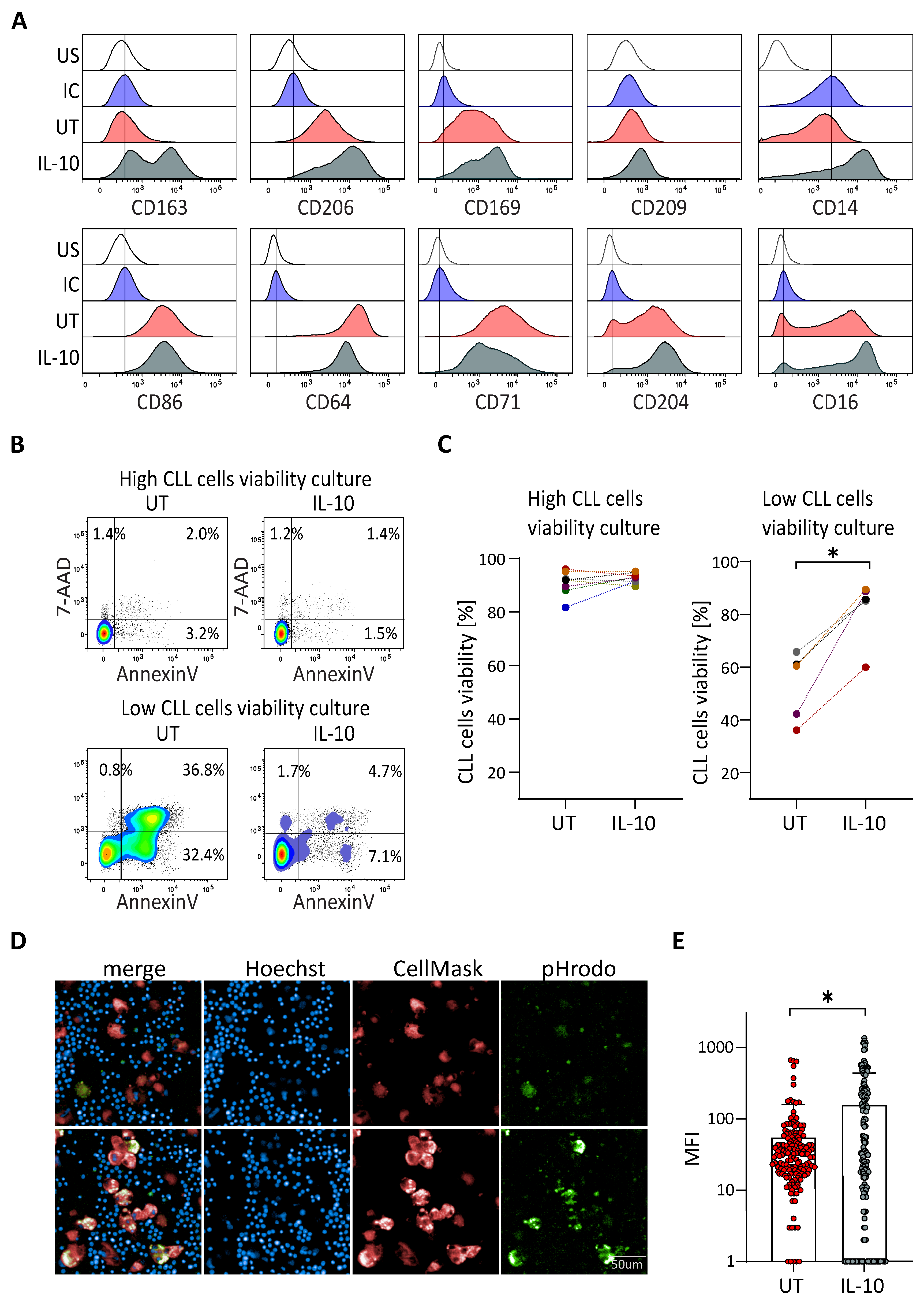
3.1. CLL Cells Viability Depends on M2-like NLCs In Vitro
3.2. IL-10 Rescues the Viability of CLL Cells in Patient Samples without Protective NLC
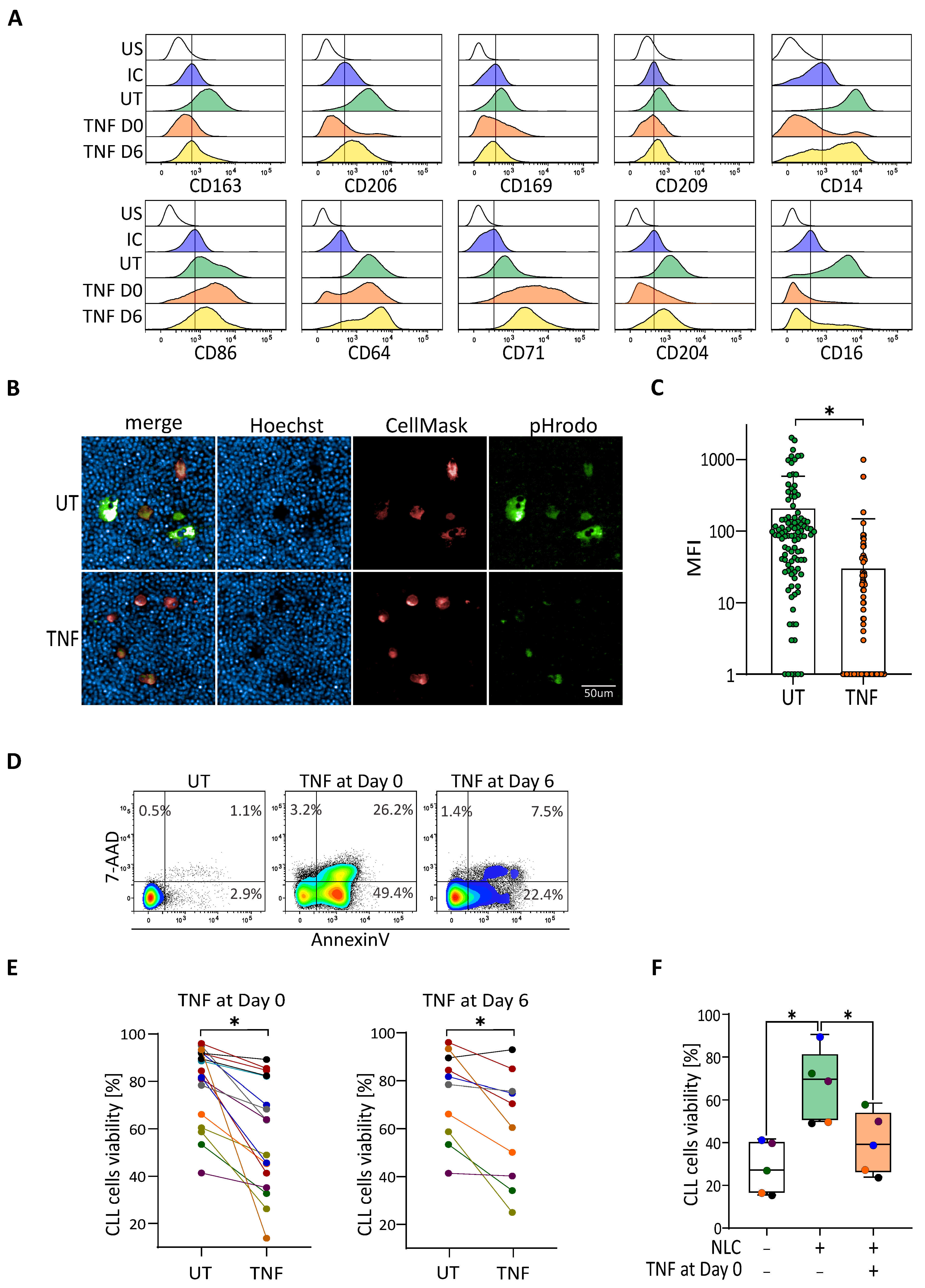
3.3. TNF-Induced Depolarization Abrogates Pro-Survival Functionality of NLC
3.4. IL-10 Repolarizes TNF-Depolarized NLC to Recover the Protective Effect on CLL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boissard, F.; Fournié, J.-J.; Laurent, C.; Poupot, M.; Ysebaert, L. Nurse like cells: Chronic lymphocytic leukemia associated macrophages. Leuk. Lymphoma 2015, 56, 1570–1572. [Google Scholar] [CrossRef]
- Caligaris-Cappio, F.; Gottardi, D.; Alfarano, A.; Stacchini, A.; Gregoretti, M.G.; Ghia, P.; Bertero, M.T.; Novarino, A.; Bergui, L. The nature of the b lymphocyte in b-chronic lymphocytic leukemia. Blood Cells 1993, 19, 601–613. [Google Scholar]
- Lagneaux, L.; Delforge, A.; Bron, D.; De Bruyn, C.; Stryckmans, P. Chronic lymphocytic leukemic b cells but not normal b cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 1998, 91, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Burger, M.; Kipps, T.J. Chronic lymphocytic leukemia b cells express functional cxcr4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood 1999, 94, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Boissard, F.; Fournié, J.-J.; Quillet-Mary, A.; Ysebaert, L.; Poupot, M. Nurse-like cells mediate ibrutinib resistance in chronic lymphocytic leukemia patients. Blood Cancer J. 2015, 5, e355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissard, F.; Laurent, C.; Ramsay, A.G.; Quillet-Mary, A.; Fournié, J.-J.; Poupot, M.; Ysebaert, L. Nurse-like cells impact on disease progression in chronic lymphocytic leukemia. Blood Cancer J. 2016, 6, e381. [Google Scholar] [CrossRef] [Green Version]
- Boissard, F.; Tosolini, M.; Ligat, L.; Quillet-Mary, A.; Lopez, F.; Fournié, J.-J.; Ysebaert, L.; Poupot, M. Nurse-like cells promote cll survival through LFA-3/CD2 interactions. Oncotarget 2017, 8, 52225–52236. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Clear, A.; Liu, F.-T.; Matthews, J.; Uddin, N.; McCarthy, A.; Hoxha, E.; Durance, C.; Iqbal, S.; Gribben, J.G. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood 2014, 123, 1709–1719. [Google Scholar] [CrossRef] [Green Version]
- Ysebaert, L.; Fournié, J.-J. Genomic and Phenotypic Characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leuk. Lymphoma 2011, 52, 1404–1406. [Google Scholar] [CrossRef]
- Tsukada, N.; Burger, J.A.; Zvaifler, N.J.; Kipps, T.J. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood 2002, 99, 1030–1037. [Google Scholar] [CrossRef]
- Talbot, H.; Saada, S.; Barthout, E.; Gallet, P.-F.; Gachard, N.; Abraham, J.; Jaccard, A.; Troutaud, D.; Lalloué, F.; Naves, T.; et al. BDNF Belongs to the nurse-like cell secretome and supports survival of b chronic lymphocytic leukemia cells. Sci Rep. 2020, 10, 12572. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Morande, P.E.; Dergan-Dylon, S.; Borge, M.; Toscano, M.A.; Stupirski, J.C.; Bezares, R.F.; Avalos, J.S.; Narbaitz, M.; Gamberale, R.; et al. Nurse-like cells control the activity of chronic lymphocytic leukemia b cells via galectin-1. Leukemia 2013, 27, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Prevosto, C.; Catellani, S.; Rocco, I.; Garuti, A.; Zocchi, M.R. Engagement of CD31 delivers an activating signal that contributes to the survival of chronic lymphocytic leukaemia cells. Br. J. Haematol. 2010, 151, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Tonino, S.H.; Spijker, R.; Luijks, D.M.P.; van Oers, M.H.J.; Kater, A.P. No Convincing evidence for a role of CD31-CD38 interactions in the pathogenesis of chronic lymphocytic leukemia. Blood 2008, 112, 840–843. [Google Scholar] [CrossRef] [Green Version]
- Deaglio, S.; Aydin, S.; Grand, M.M.; Vaisitti, T.; Bergui, L.; D’Arena, G.; Chiorino, G.; Malavasi, F. CD38/CD31 Interactions activate genetic pathways leading to proliferation and migration in chronic lymphocytic leukemia cells. Mol. Med. 2010, 16, 87–91. [Google Scholar] [CrossRef]
- Nishio, M.; Endo, T.; Tsukada, N.; Ohata, J.; Kitada, S.; Reed, J.C.; Zvaifler, N.J.; Kipps, T.J. Nurselike Cells Express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of sdf-1alpha. Blood 2005, 106, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Burger, J.A.; Tsukada, N.; Burger, M.; Zvaifler, N.J.; Dell’Aquila, M.; Kipps, T.J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia b cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000, 96, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Quiroga, M.P.; Hartmann, E.; Bürkle, A.; Wierda, W.G.; Keating, M.J.; Rosenwald, A. high-level expression of the t-cell chemokines ccl3 and ccl4 by chronic lymphocytic leukemia b cells in nurselike cell cocultures and after BCR stimulation. Blood 2009, 113, 3050–3058. [Google Scholar] [CrossRef]
- Polk, A.; Lu, Y.; Wang, T.; Seymour, E.; Bailey, N.G.; Singer, J.W.; Boonstra, P.S.; Lim, M.S.; Malek, S.; Wilcox, R.A. Colony-stimulating factor-1 receptor is required for nurse-like cell survival in chronic lymphocytic leukemia. Clin. Cancer Res. 2016, 22, 6118–6128. [Google Scholar] [CrossRef] [Green Version]
- Kratochvill, F.; Neale, G.; Haverkamp, J.M.; Van de Velde, L.-A.; Smith, A.M.; Kawauchi, D.; McEvoy, J.; Roussel, M.F.; Dyer, M.A.; Qualls, J.E.; et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015, 12, 1902–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesaros, O.; Jimbu, L.; Neaga, A.; Popescu, C.; Berceanu, I.; Tomuleasa, C.; Fetica, B.; Zdrenghea, M. Macrophage polarization in chronic lymphocytic leukemia: Nurse-like cells are the caretakers of leukemic cells. Biomedicines 2020, 8, 516. [Google Scholar] [CrossRef]
- Dürr, C.; Hanna, B.S.; Schulz, A.; Lucas, F.; Zucknick, M.; Benner, A.; Clear, A.; Ohl, S.; Öztürk, S.; Zenz, T.; et al. tumor necrosis factor receptor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid wogonin. Haematologica 2018, 103, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lech-Maranda, E.; Grzybowska-Izydorczyk, O.; Wyka, K.; Mlynarski, W.; Borowiec, M.; Antosik, K.; Cebula-Obrzut, B.; Makuch-Lasica, H.; Nowak, G.; Klimkiewicz-Wojciechowska, G.; et al. Serum tumor necrosis factor-α and interleukin-10 levels as markers to predict outcome of patients with chronic lymphocytic leukemia in different risk groups defined by the IGHV mutation status. Arch. Immunol. Exp. 2012, 60, 477–486. [Google Scholar] [CrossRef]
- Mainou-Fowler, T.; Miller, S.; Proctor, S.J.; Dickinson, A.M. The levels of TNF alpha, IL4 and IL10 production by T-cells in B-cell chronic lymphocytic leukaemia (B-CLL). Leuk. Res. 2001, 25, 157–163. [Google Scholar] [CrossRef]
- Bojarska-Junak, A.; Hus, I.; Szczepanek, E.W.; Dmoszyńska, A.; Roliński, J. Peripheral blood and bone marrow TNF and TNF receptors in early and advanced stages of B-CLL in correlation with ZAP-70 protein and CD38 antigen. Leuk. Res. 2008, 32, 225–233. [Google Scholar] [CrossRef]
- Rivas, J.R.; Liu, Y.; Alhakeem, S.S.; Eckenrode, J.M.; Marti, F.; Collard, J.P.; Zhang, Y.; Shaaban, K.A.; Muthusamy, N.; Hildebrandt, G.C.; et al. Interleukin-10 suppression enhances t-cell antitumor immunity and responses to checkpoint blockade in chronic lymphocytic leukemia. Leukemia 2021. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Weinberg, J.B.; Yoshizaki, A.; Horikawa, M.; Bryant, J.M.; Iwata, Y.; Matsushita, T.; Matta, K.M.; Chen, Y.; Venturi, G.M.; et al. Chronic lymphocytic leukemia and regulatory b cells share il-10 competence and immunosuppressive function. Leukemia 2013, 27, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, A.; Dürr, C.; Zenz, T.; Döhner, H.; Stilgenbauer, S.; Lichter, P.; Seiffert, M. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood 2013, 121, 2503–2511. [Google Scholar] [CrossRef]
- Filip, A.A.; Ciseł, B.; Wąsik-Szczepanek, E. Guilty bystanders: Nurse-like cells as a model of microenvironmental support for leukemic lymphocytes. Clin. Exp. Med. 2015, 15, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Fluckiger, A.C.; Durand, I.; Banchereau, J. Interleukin 10 induces apoptotic cell death of b-chronic lymphocytic leukemia cells. J. Exp. Med. 1994, 179, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Moseman, E.A.; Farrar, M.A.; Bachanova, V.; Weisdorf, D.J.; Blazar, B.R.; Chen, W. Toll-like Receptor 9 Signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia b cells. Blood 2010, 115, 5041–5052. [Google Scholar] [CrossRef] [Green Version]
- Kitabayashi, A.; Hirokawa, M.; Miura, A.B. The Role of Interleukin-10 (IL-10) in Chronic B-lymphocytic leukemia: IL-10 prevents leukemic cells from apoptotic cell death. Int. J. Hematol. 1995, 62, 99–106. [Google Scholar] [CrossRef]
- Morabito, F.; Filangeri, M.; Sculli, G.; Oliva, B. In vitro modulation of bcl-2 protein expression, drug-induced apoptosis and cytotoxicity by interleukin-10 in chronic lymphocytic leukemia. Haematologica 1998, 83, 1046–1048. [Google Scholar] [PubMed]
- Fiorcari, S.; Maffei, R.; Audrito, V.; Martinelli, S.; Ten Hacken, E.; Zucchini, P.; Grisendi, G.; Potenza, L.; Luppi, M.; Burger, J.A.; et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016, 7, 65968–65981. [Google Scholar] [CrossRef] [PubMed]
- Manso, B.A.; Zhang, H.; Mikkelson, M.G.; Gwin, K.A.; Secreto, C.R.; Ding, W.; Parikh, S.A.; Kay, N.E.; Medina, K.L. Bone Marrow hematopoietic dysfunction in untreated chronic lymphocytic leukemia patients. Leukemia 2019, 33, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Morva, A.; Lemoine, S.; Hillion, S.; Bordron, A.; Pers, J.-O.; Berthou, C.; Mageed, R.A.; Renaudineau, Y.; Youinou, P. CD5 promotes IL-10 production in chronic lymphocytic leukemia b cells through STAT3 and NFAT2 activation. J. Immunol. 2011, 186, 4835–4844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, N.; Zhang, R.; Li, J.; Zhang, Z.; Yuan, H.; Chen, G.; Zhao, F.; Wang, L.; Cao, H.; Qu, J.; et al. Increased IL-10/IL-17 Ratio is aggravated along with the prognosis of patients with chronic lymphocytic leukemia. Int. Immunopharmacol. 2016, 40, 57–64. [Google Scholar] [CrossRef]
- Kamper, E.F.; Papaphilis, A.D.; Angelopoulou, M.K.; Kopeikina, L.T.; Siakantaris, M.P.; Pangalis, G.A.; Stavridis, J.C. Serum levels of tetranectin, intercellular adhesion molecule-1 and interleukin-10 in b-chronic lymphocytic leukemia. Clin. Biochem. 1999, 32, 639–645. [Google Scholar] [CrossRef]
- Jablonska, E.; Kiersnowska-Rogowska, B.; Rogowski, F.; Parfienczyk, A.; Puzewska, W.; Bukin, M. TNF family molecules in the serum of patients with b-cell chronic lymphocytic leukemia (B-CLL). Leuk. Lymphoma 2005, 46, 1307–1312. [Google Scholar] [CrossRef]
- El Hajj, H.; Hleihel, R.; El Sabban, M.; Bruneau, J.; Zaatari, G.; Cheminant, M.; Marçais, A.; Akkouche, A.; Hasegawa, H.; Hall, W.; et al. Loss of interleukin-10 activates innate immunity to eradicate adult t-cell leukemia-initiating cells. Haematologica 2021, 106, 1443–1456. [Google Scholar] [CrossRef]
- Gordy, J.T.; Luo, K.; Francica, B.; Drake, C.; Markham, R.B. Anti-IL-10-mediated enhancement of antitumor efficacy of a dendritic cell-targeting mip3α-gp100 vaccine in the b16f10 mouse melanoma model is dependent on type i interferons. J. Immunother. 2018, 41, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Spaner, D.E.; Venema, R.; Huang, J.; Norris, P.; Lazarus, A.; Wang, G.; Shi, Y. Association of blood igg with tumor necrosis factor-alpha and clinical course of chronic lymphocytic leukemia. EBioMedicine 2018, 35, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domagala, M.; Ysebaert, L.; Ligat, L.; Lopez, F.; Fournié, J.-J.; Laurent, C.; Poupot, M. IL-10 Rescues CLL Survival through Repolarization of Inflammatory Nurse-like Cells. Cancers 2022, 14, 16. https://doi.org/10.3390/cancers14010016
Domagala M, Ysebaert L, Ligat L, Lopez F, Fournié J-J, Laurent C, Poupot M. IL-10 Rescues CLL Survival through Repolarization of Inflammatory Nurse-like Cells. Cancers. 2022; 14(1):16. https://doi.org/10.3390/cancers14010016
Chicago/Turabian StyleDomagala, Marcin, Loïc Ysebaert, Laetitia Ligat, Frederic Lopez, Jean-Jacques Fournié, Camille Laurent, and Mary Poupot. 2022. "IL-10 Rescues CLL Survival through Repolarization of Inflammatory Nurse-like Cells" Cancers 14, no. 1: 16. https://doi.org/10.3390/cancers14010016






