Survival Outcomes after Hyperthermic Intraperitoneal Chemotherapy for a First Ovarian Cancer Relapse: A Systematic Evidence-Based Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Research Strategy
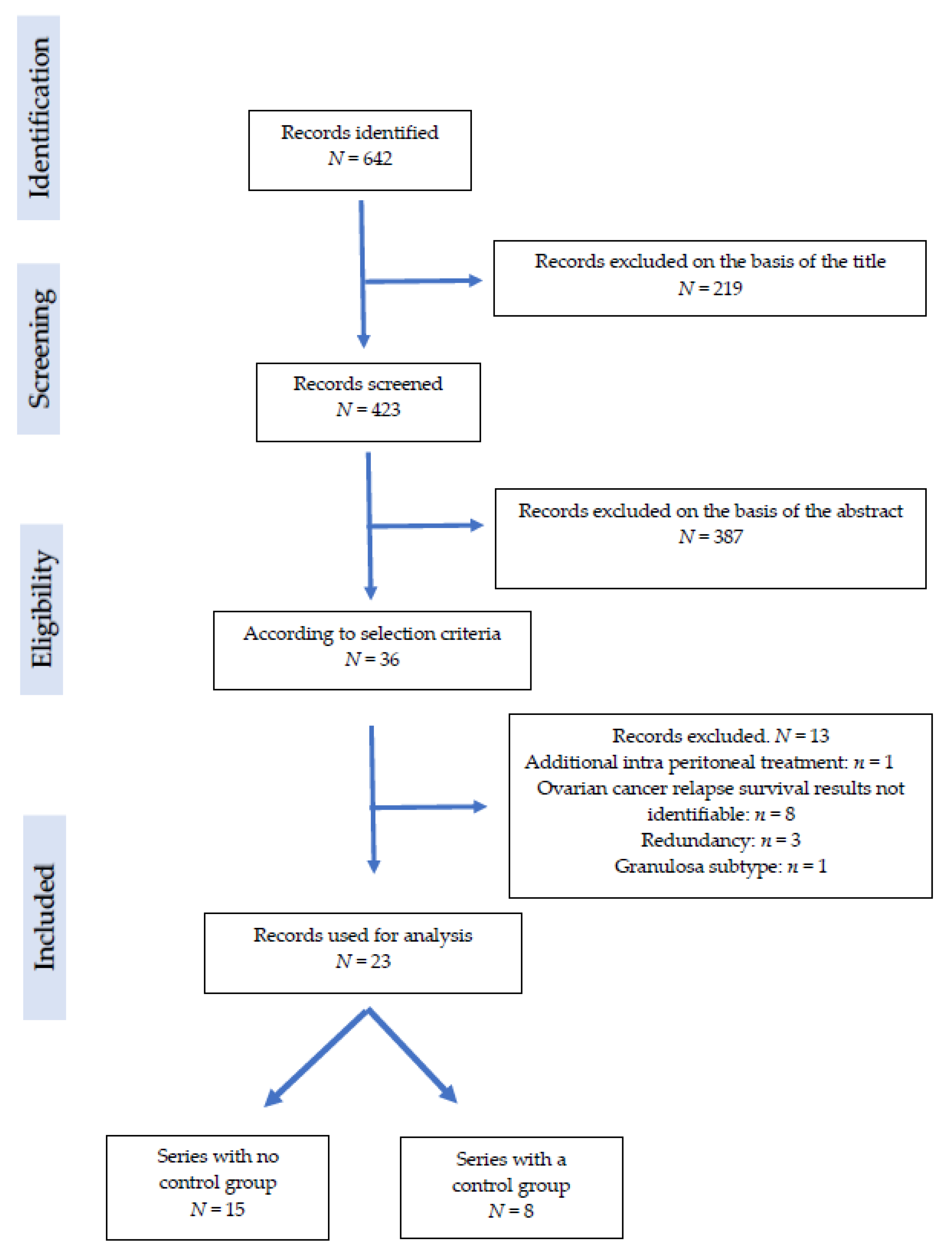
2.2. Study Selection
2.3. Data Extraction and Analysis
2.4. Quality Assessment
3. Results
3.1. Series Characteristics
- Piso 2004 [8]: In this series, 11 patients had additional courses of intraperitoneal chemotherapy after HIPEC.
- Raspagliesi 2006 [9]: A series of 40 patients treated for advanced ovarian cancer (n = 13) or a relapse (n = 27). Survival results could not be identified separately.
- Cotte 2007 [10]: A series of 81 patients with a mix of first, second or additional relapsed ovarian cancer, and 8 patients with a first relapse not identified separately.
- Di Georgio 2008 [11]: A series of patients possibly merged with Di Georgio 2017
- Pavlov 2009 [12]: A series of 56 patients with a mix of first, second, or additional relapsed ovarian cancer, and 25 patients with a first relapse not identified separately.
- Roviello 2010 [13]: A series of 53 patients treated for advanced ovarian cancer (n = 45) or a relapse (n = 8) not identified separately.
- Carrabin 2010 [14]: A series of 22 patients treated with CRS and HIPEC, 12 for primary ovarian cancer and 10 for relapse, with OS results not identified separately.
- Fagotti 2011 [15]: A series of patients possibly merged with Petrillo 2016.
- Frenel 2011 [16]: A series of patients possibly merged with Classe 2015.
- Ansaloni 2012 [17]: A series of patients with initial treatment or first relapse not identified separately.
- Warschkow 2012 [18]: A series of patients where the survival of patients with a first relapse, with or without HIPEC, was not identified separately.
- Gouy 2013 [19]: A series of patients based on granulosa ovarian tumors.
- Cascales-Campos 2014 [20]: A series of 91 patients treated for advanced ovarian cancer, initially or at the time of the first late relapse, not identified separately.
3.2. Clinical Series without a Group Control
3.3. Clinical Series with Control Groups
4. Discussion
4.1. HIPEC Is Not Standardized
4.2. Weaknesses in the Published Series
4.3. Methodology of the Current Review
4.4. Prospective Randomized Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Helm, C.W. The Role of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer. Oncologist 2009, 14, 683–694. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- de Bree, E.; Helm, C.W. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer: Rationale and Clinical Data. Expert Rev. Anticancer Ther. 2012, 12, 895–911. [Google Scholar] [CrossRef]
- Cianci, S.; Riemma, G.; Ronsini, C.; De Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Ovarian Cancer Recurrence: Systematic Review and Meta-Analysis. Gland Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Hotouras, A.; Desai, D.; Bhan, C.; Murphy, J.; Lampe, B.; Sugarbaker, P.H. Heated IntraPEritoneal Chemotherapy (HIPEC) for Patients With Recurrent Ovarian Cancer: A Systematic Literature Review. Int. J. Gynecol. Cancer 2016, 26, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durieux, N.; Vandenput, S.; Pasleau, F. OCEBM levels of evidence system. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enfermagem. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piso, P.; Dahlke, M.-H.; Loss, M.; Schlitt, H. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Peritoneal Carcinomatosis from Ovarian Cancer. World J. Surg. Onco. 2004, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspagliesi, F.; Kusamura, S.; Campos Torres, J.C.; de Souza, G.A.; Ditto, A.; Zanaboni, F.; Younan, R.; Baratti, D.; Mariani, L.; Laterza, B.; et al. Cytoreduction Combined with Intraperitoneal Hyperthermic Perfusion Chemotherapy in Advanced/Recurrent Ovarian Cancer Patients: The Experience of National Cancer Institute of Milan. Eur. J. Surg. Oncol. 2006, 32, 671–675. [Google Scholar] [CrossRef]
- Cotte, E.; Glehen, O.; Mohamed, F.; Lamy, F.; Falandry, C.; Golfier, F.; Gilly, F.N. Cytoreductive Surgery and Intraperitoneal Chemohyperthermia for Chemoresistant and Recurrent Advanced Epithelial Ovarian Cancer: Prospective Study of 81 Patients. World J. Surg. 2007, 31, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Naticchioni, E.; Biacchi, D.; Sibio, S.; Accarpio, F.; Rocco, M.; Tarquini, S.; Di Seri, M.; Ciardi, A.; Montruccoli, D.; et al. Cytoreductive Surgery (Peritonectomy Procedures) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Diffuse Peritoneal Carcinomatosis from Ovarian Cancer. Cancer 2008, 113, 315–325. [Google Scholar] [CrossRef]
- Pavlov, M.J.; Kovacevic, P.A.; Ceranic, M.S.; Stamenkovic, A.B.; Ivanovic, A.M.; Kecmanovic, D.M. Cytoreductive Surgery and Modified Heated Intraoperative Intraperitoneal Chemotherapy (HIPEC) for Advanced and Recurrent Ovarian Cancer—12-Year Single Center Experience. Eur. J. Surg. Oncol. 2009, 35, 1186–1191. [Google Scholar] [CrossRef]
- Roviello, F.; Pinto, E.; Corso, G.; Pedrazzani, C.; Caruso, S.; Filippeschi, M.; Petrioli, R.; Marsili, S.; Mazzei, M.A.; Marrelli, D. Safety and Potential Benefit of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Peritoneal Carcinomatosis from Primary or Recurrent Ovarian Cancer. J. Surg. Oncol. 2010, 102, 663–670. [Google Scholar] [CrossRef]
- Carrabin, N.; Mithieux, F.; Meeus, P.; Trédan, O.; Guastalla, J.-P.; Bachelot, T.; Labidi, S.I.; Treilleux, I.; Rivoire, M.; Ray-Coquard, I. Hyperthermic intraperitoneal chemotherapy with oxaliplatin and without adjuvant chemotherapy in stage IIIC ovarian cancer. Bull. Cancer 2010, 97, E23–E32. [Google Scholar] [CrossRef] [Green Version]
- Fagotti, A.; Costantini, B.; Vizzielli, G.; Perelli, F.; Ercoli, A.; Gallotta, V.; Scambia, G.; Fanfani, F. HIPEC in Recurrent Ovarian Cancer Patients: Morbidity-Related Treatment and Long-Term Analysis of Clinical Outcome. Gynecol. Oncol. 2011, 122, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.-S.; Leux, C.; Pouplin, L.; Ferron, G.; Berton-Rigaud, D.; Bourbouloux, E.; Dravet, F.; Jaffre, I.; Classe, J.-M. Oxaliplatin-Based Hyperthermic Intraperitoneal Chemotherapy in Primary or Recurrent Epithelial Ovarian Cancer: A Pilot Study of 31 Patients. J. Surg. Oncol. 2011, 103, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ansaloni, L.; Agnoletti, V.; Amadori, A.; Catena, F.; Cavaliere, D.; Coccolini, F.; De Iaco, P.; Di Battista, M.; Framarini, M.; Gazzotti, F.; et al. Evaluation of Extensive Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2012, 22, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Tarantino, I.; Lange, J.; Müller, S.A.; Schmied, B.M.; Zünd, M.; Steffen, T. Does Hyperthermic Intraoperative Chemotherapy Lead to Improved Outcomes in Patients with Ovarian Cancer? A Single Center Cohort Study in 111 Consecutive Patients. Patient Saf. Surg. 2012, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouy, S.; Ferron, G.; Glehen, O.; Bayar, A.; Marchal, F.; Pomel, C.; Quenet, F.; Bereder, J.M.; Le Deley, M.C.; Morice, P. Results of a Multicenter Phase I Dose-Finding Trial of Hyperthermic Intraperitoneal Cisplatin after Neoadjuvant Chemotherapy and Complete Cytoreductive Surgery and Followed by Maintenance Bevacizumab in Initially Unresectable Ovarian Cancer. Gynecol. Oncol. 2016, 142, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Cascales-Campos, P.; Gil, J.; Gil, E.; Feliciangeli, E.; López, V.; Gonzalez, A.G.; Ruiz-Pardo, J.; Nieto, A.; Parrilla, P. Cytoreduction and HIPEC after Neoadjuvant Chemotherapy in Stage IIIC–IV Ovarian Cancer. Critical Analysis in Elderly Patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Massari, R.; Barone, M.; Basilico, R.; Carella, C.; Colasante, A.; De Tursi, M.; Filippone, A.; Guetti, L.; Mani, A. Peritonectomy and Hyperthermic Chemotherapy in Patients with Advanced or Recurrent Ephitelial Ovarian Cancer: A Single Center Cohort Study. Minerva Chir. 2014, 69, 17–26. [Google Scholar]
- Königsrainer, I.; Beckert, S.; Becker, S.; Zieker, D.; Fehm, T.; Grischke, E.-M.; Lauk, O.; Glatzle, J.; Brücher, B.; Wallwiener, D.; et al. Cytoreductive Surgery and HIPEC in Peritoneal Recurrent Ovarian Cancer: Experience and Lessons Learned. Langenbecks Arch. Surg. 2011, 396, 1077–1081. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; Marsanic, P.; Mellano, A.; Borsano, A.; Cinquegrana, A.; Sottile, A.; De Simone, M. Treatment of Peritoneal Carcinomatosis from Ovarian Cancer by Surgical Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Minerva Chir. 2014, 69, 27–35. [Google Scholar]
- Di Giorgio, A.; De Iaco, P.; De Simone, M.; Garofalo, A.; Scambia, G.; Pinna, A.D.; Verdecchia, G.M.; Ansaloni, L.; Macrì, A.; Cappellini, P.; et al. Cytoreduction (Peritonectomy Procedures) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Retrospective Italian Multicenter Observational Study of 511 Cases. Ann. Surg. Oncol. 2017, 24, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Zanon, C.; Clara, R.; Chiappino, I.; Bortolini, M.; Cornaglia, S.; Simone, P.; Bruno, F.; De Riu, L.; Airoldi, M.; Pedani, F. Cytoreductive Surgery and Intraperitoneal Chemohyperthermia for Recurrent Peritoneal Carcinomatosis from Ovarian Cancer. World J. Surg. 2004, 28, 1040–1045. [Google Scholar] [CrossRef]
- Rufián, S.; Muñoz-Casares, F.C.; Briceño, J.; Díaz, C.J.; Rubio, M.J.; Ortega, R.; Ciria, R.; Morillo, M.; Aranda, E.; Muntané, J.; et al. Radical Surgery-Peritonectomy and Intraoperative Intraperitoneal Chemotherapy for the Treatment of Peritoneal Carcinomatosis in Recurrent or Primary Ovarian Cancer. J. Surg. Oncol. 2006, 94, 316–324. [Google Scholar] [CrossRef]
- Ceelen, W.P.; Van Nieuwenhove, Y.; Van Belle, S.; Denys, H.; Pattyn, P. Cytoreduction and Hyperthermic Intraperitoneal Chemoperfusion in Women with Heavily Pretreated Recurrent Ovarian Cancer. Ann. Surg. Oncol. 2012, 19, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Bayon, L.; Steiner, M.A.; Vasquez Jimenez, W.; Asencio, J.M.; Alvarez de Sierra, P.; Atahualpa Arenas, F.; Rodriguez del Campo, J.; Garcia Sabrido, J.L. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Advanced Epithelial Ovarian Carcinoma: Upfront Therapy, at First Recurrence, or Later? Eur. J. Surg. Oncol. 2013, 39, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; De Iaco, P.; Cianci, S.; Perrone, M.; Costantini, B.; Ronsini, C.; Scambia, G.; Fagotti, A. Long-Term Survival for Platinum-Sensitive Recurrent Ovarian Cancer Patients Treated with Secondary Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2016, 23, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.-M.; Glehen, O.; Decullier, E.; Bereder, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for First Relapse of Ovarian Cancer. Anticancer Res. 2015, 35, 4997–5005. [Google Scholar]
- Deraco, M.; Virzì, S.; Iusco, D.; Puccio, F.; Macrì, A.; Famulari, C.; Solazzo, M.; Bonomi, S.; Grassi, A.; Baratti, D.; et al. Secondary Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Recurrent Epithelial Ovarian Cancer: A Multi-Institutional Study: Cytoreductive Surgery and HIPEC in Recurrent Ovarian Cancer. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 800–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helm, C.W.; Richard, S.D.; Pan, J.; Bartlett, D.; Goodman, M.D.; Hoefer, R.; Lentz, S.S.; Levine, E.A.; Loggie, B.W.; Metzinger, D.S.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer: First Report of the HYPER-O Registry. Int. J. Gynecol. Cancer 2010, 20, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, O.; Abramian, A.; Kullmann, M.; Fuhrmann, C.; Coch, C.; Hoeller, T.; Ruehs, H.; Keyver-Paik, M.D.; Rudlowski, C.; Weber, S.; et al. HIPEC ROC I: A Phase i Study of Cisplatin Administered as Hyperthermic Intraoperative Intraperitoneal Chemoperfusion Followed by Postoperative Intravenous Platinum-Based Chemotherapy in Patients with Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: HIPEC in Recurrent Ovarian Cancer. Int. J. Cancer 2015, 136, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Delotte, J.; Arias, T.; Guerin, O.; Boulahssass, R.; Bereder, I.; Bongain, A.; Benchimol, D.; Bereder, J.M. Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Recurrent Ovarian Cancer in Elderly Women. Acta Obstet. Gynecol. Scand. 2015, 94, 435–439. [Google Scholar] [CrossRef]
- Cascales-Campos, P.A.; Gil, J.; Feliciangeli, E.; Gil, E.; González-Gil, A.; López, V.; Ruiz-Pardo, J.; Nieto, A.; Parrilla, J.J.; Parrilla, P. The Role of Hyperthermic Intraperitoneal Chemotherapy Using Paclitaxel in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer Patients with Microscopic Residual Disease after Cytoreduction. Ann. Surg. Oncol. 2015, 22, 987–993. [Google Scholar] [CrossRef]
- Muñoz-Casares, F.C.; Rufián, S.; Rubio, M.J.; Díaz, C.J.; Díaz, R.; Casado, A.; Arjona, A.; Muñoz-Villanueva, M.C.; Muntané, J. The Role of Hyperthermic Intraoperative Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Peritoneal Carcinomatosis in Recurrent Ovarian Cancer. Clin. Transl. Oncol. 2009, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Costantini, B.; Petrillo, M.; Vizzielli, G.; Fanfani, F.; Margariti, P.A.; Turco, L.C.; Piovano, E.; Scambia, G. Cytoreductive Surgery plus HIPEC in Platinum-Sensitive Recurrent Ovarian Cancer Patients: A Case-Control Study on Survival in Patients with Two Year Follow-Up. Gynecol. Oncol. 2012, 127, 502–505. [Google Scholar] [CrossRef]
- Baiocchi, G.; Ferreira, F.O.; Mantoan, H.; da Costa, A.A.B.A.; Faloppa, C.C.; Kumagai, L.Y.; de Mello, C.A.L.; Takahashi, R.M.; Nakagawa, W.T.; Aguiar, S.; et al. Hyperthermic Intraperitoneal Chemotherapy after Secondary Cytoreduction in Epithelial Ovarian Cancer: A Single-Center Comparative Analysis. Ann. Surg. Oncol. 2016, 23, 1294–1301. [Google Scholar] [CrossRef]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive Surgery and HIPEC in Recurrent Epithelial Ovarian Cancer: A Prospective Randomized Phase III Study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef]
- Le Brun, J.-F.; Campion, L.; Berton-Rigaud, D.; Lorimier, G.; Marchal, F.; Ferron, G.; Oger, A.S.; Dravet, F.; Jaffre, I.; Classe, J.-M. Survival Benefit of Hyperthermic Intraperitoneal Chemotherapy for Recurrent Ovarian Cancer: A Multi-Institutional Case Control Study. Ann. Surg. Oncol. 2014, 21, 3621–3627. [Google Scholar] [CrossRef]
- Marocco, F.; Vaira, M.; Milani, A.; Genta, S.; Maggiorotto, F.; Magistris, A.; Cinquegrana, A.; Robella, M.; De Simone, M.; Aglietta, M.; et al. Secondary Cytoreductive Surgery, Hyperthermic Intraperitoneal Intraoperative Chemotherapy, and Chemotherapy Alone: A Retrospective Comparison of Alternative Approaches in Relapsed Platinum Sensitive Ovarian Cancer. Eur. J. Gynaecol. Oncol. 2016, 37, 638–643. [Google Scholar] [PubMed]
- Safra, T.; Grisaru, D.; Inbar, M.; Abu-Abeid, S.; Dayan, D.; Matceyevsky, D.; Weizman, A.; Klausner, J.M. Cytoreduction Surgery with Hyperthermic Intraperitoneal Chemotherapy in Recurrent Ovarian Cancer Improves Progression-Free Survival, Especially in BRCA-Positive Patients-A Case-Control Study: Cytoreduction & IP Chemotherapy in REOC. J. Surg. Oncol. 2014, 110, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, X.; Lewsley, L.-A.; Daniele, G.; Cook, A.; Yanaihara, N.; Tinker, A.; Kristensen, G.; Ottevanger, P.B.; Aravantinos, G.; Miller, A.; et al. Assessment of Progression-Free Survival as a Surrogate End Point of Overall Survival in First-Line Treatment of Ovarian Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e1918939. [Google Scholar] [CrossRef]
- Shimokawa, M.; Kogawa, T.; Shimada, T.; Saito, T.; Kumagai, H.; Ohki, M.; Kaku, T. Overall Survival and Post-Progression Survival Are Potent Endpoint in Phase III Trials of Second/Third-Line Chemotherapy for Advanced or Recurrent Epithelial Ovarian Cancer. J. Cancer 2018, 9, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metelli, S.; Chaimani, A. Challenges in Meta-Analyses with Observational Studies. Evid. Based Ment. Health 2020, 23, 83–87. [Google Scholar] [CrossRef] [Green Version]

| Author (Year) (Country) | n | OQL | Pro/Retro (Period) M | Systemic Chemo before HIPEC | HIPEC Technique | Drug IP (Poso) | Temp. | Dur. (min) |
|---|---|---|---|---|---|---|---|---|
| Zanon (2004) [25] (Italy) | 30 | 4 | Pro (January 1998–September 2003) | No | open | CDDP (100 mg/m2) (150 mg/m2 + Thiosulfate) | 41.5 °C | 60 |
| Piso (2004) [8] (Germany) | 11 | 4 | Retro | Mixed | open | CDDP (75 mg/m2) or Mitomycin (15 mg/m2) | 41.5 °C | 90 |
| Rufian (2006) [26] (Spain) | 14 | 4 | Retro (January 1997–December 2004) | No | open | Paclitaxel (60 mg/m2) | 41–43 °C | 60 |
| Konigsrainer (2011)12/20/2021 3:31:00 PM (Germany) | 31 | 4 | Retro (February 2007–February 2010) | No | open | CDDP (50 mg/m2) | 42 °C | 90 |
| Helm (2010) [32] (Germany) | 83 | 4 | (September 2005–June 2008) M | Mixed | Open closed | Platinum Platinum Mito combinationPlatinum Doxo combination(Posology UK) | NP | From 60 to 120 |
| Ceelen (2012) [27] (Belgium) | 42 | 4 | Pro (October 2002–January 2009) | Mixed | Open (if Oxali) Closed (if CDDP) | Oxali (460 mg/m2) CDDP (100 to 250 mg/m2) | 41 °C | 30 (if Oxali) 90 (if CDDP) |
| Deraco (2012) [31] (Italy) | 56 | 4 | Retro (April 1995–May 2010) M | Mixed | Closed | CDDP + Doxo CDDP + Mito | 42.5 °C | 90 |
| Gonzalez Bayon (2013) [28] (Spain) | 19 | 4 | Retro (June 2002–October 2011) | No | Open | CDDP (100 mg/m2) + Doxo (30 mg/m2) | 42 °C | 90 |
| Robella (2014) [23] (Italy) | 25 | 4 | Retro (October 1995–December 2011) | No | Closed | CDDP (100 mg/m2) + Doxo (15.2 mg/m2) | 41.5 °C | 60 |
| Massari (2014) [21] (Italy) | 11 | 4 | Retro (October 2006–December 2009) | Mixed | Closed | CDDP + Caelix Docetaxel + Caelix | 42.5 °C | 60 |
| Zivanovic (2014) [33] (Germany) | 12 | 4 | Pro (October 2011–January 2013) | No | Closed | CDDP (60 mg/m2 or 80 mg/m2 or 100 mg/m2 | 41–43 °C | 90 |
| Classe (2015) [30](France) | 314 | 4 | Retro (January 2001–December 2010) M | Mixed | Closed (25%) Open (75%) | NP | 42 °C (median) | 90 (median) |
| Delotte (2015) [34] (France) | 15 | 4 | Retro (January 2012–January 2014) | No | Open | CDDP (50 mg/m2) Doxo (15 mg/m2) | 43 °C | 60 |
| Petrillo (2016) [29](Italy) | 70 | 4 | Retro (December 2004–June 2015) | No | NP | CDDP (75 mg/m2) Oxali (460 mg/m2) | 41.5 °C | 60 (CDDP) 30 (Oxali) |
| Di Georgio (2017) [24] (Italy) | 179 | 4 | Retro (December 1998–December 2014) M | Mixed | Closed Open Semi closed | CDDP alone (75 mg/m2) CDDP mixed with Doxo, Pacli, Mito Oxali (460 mg/m2) | UK | 60 (CDDP) 30 (Oxali) |
| Author (Year) | n | Residual | DFI (Median) (mos) | OS (Median) (mos) | PFS (mos) |
|---|---|---|---|---|---|
| Zanon (2004) [25] | 30 | CC0-CC1 77% | UK | 28.1 | 17.1 |
| Piso (2004) [8] | 11 | UK | 18 | 30 | UK |
| Rufian (2006) [26] | 14 | UK | UK | 57 | UK |
| Konigsrainer (2011)12/20/2021 3:31:00 PM | 31 | CC0 65%–CC1 25% | 24 | 24 | 12 |
| Helm (2010) [32] | 83 | UK | UK | 23.5 | 13.7 |
| Ceelen(2012) [27] | 42 | No residual 50% | 3(median) | 37 | 13 |
| Deraco (2012) [31] | 56 | CC0 82%–CC1 12% | <6 (23%) >6 (58%) | 25.7(whole population) | 10.8 (whole population) |
| Gonzalez Bayon (2013) [28] | 19 | CC0 73%–CC1 26% | UK | 62.8 | 18.1 |
| Robella (2014) [23] | 25 | CC0 78.6%–CC1 12.8% | UK | 28 | NP |
| Massari (2014) [21] | 11 | UK | UK | 27 | 11.9 |
| Zivanovic (2014) [33] | 12 | CC0 58%–CC18% | >6 | At 20 mos66.6% | 13.6 |
| Classe (2015) [30] | 314 | CC0 79%–CC1 19% | <6 (53%) >6 (47%) | 5142 | 1413 |
| Delotte (2015) [34] | 15 | CC0 60%–CC1 40% | UK | 35 | 15.6 |
| Petrillo (2016) [29] | 70 | CC0 88.6%–CC1 11.4% | 19 | 63 | 27 |
| Di Georgio (2017) [24] | 179 | CC0 79.9% | <12 (20%) >12 (80%) | 52.4 | 16.6 |
| Author (Year) (Country) | n (H/no H) | OQL | Pro/Retro (Period)M | Systemic Chemo before HIPEC | HIPEC Technique | Chemo IP (poso) | Temp. | Dur. min |
|---|---|---|---|---|---|---|---|---|
| Non randomized | ||||||||
| Munoz Casares (2009) [36] (Spain) | 14/12 | 4 | Retro (January 1997–December 2004) | No | Open | Pacli (60 mg/m2) | 41 °C/43 °C | 60 |
| Fagotti (2012) [37] (Italy) | 30/37 | 4 | Retro (May 2005–October 2009) | No | Closed | Oxali (460 mg/m2) | 41.5 °C | 30 |
| Safra (2014) [42] (Israel) | 27/84 | 4 | Retro (UK) | UK | Closed | CDDP (50 mg/m2) + Doxo (15 mg/m2); Pacli (60 mg/m2) + Carboplatinum (AUC4) | 42.5 °C | 60 |
| Lebrun (2014) [40] (France) | 23/19 | 4 | Retro (June 1997–July 2011) | Yes | Open | CDDP Oxaliplatinum | 42 °C | 6030 |
| Baiocchi (2016) [38] (Brazil) | 29/50 | 4 | Retro (May 2000–January 2014) | Mixed | Closed | CDDP (50 mg/m2) + Mito (10 mg/m2); CDDP (50 mg/m2) + Doxo; CDDP alone; Oxali alone | 41–42 °C | 90 |
| Cascales Campos (2015) [35] (Spain) | 32/22 | 4 | Retro (January 2001–July 2012) | Mixed | Open | Pacli (60 mg/m2) | 42 °C | - |
| Marocco (2016) [41] (Italy) | 19/11 | 4 | Retro (1995–2012) | No | Semi-closed | CDDP (100 mg/m2) + Doxo (15 mg/L) | 41.5 °C | 60 |
| Randomized | ||||||||
| Spilliotis (2014) [39] (Greece) | 60/60 | 2 | Pro randomized (2006/2013) | No | Open (n = 40) Closed (n = 20) | CDDP (100 mg/m2) + Pacli (175 mg/m2); Doxo (35 mg/m2) + Pacli (175 mg/m2); Mito (15 mg/m2) + Pacli (175 mg/m2) | 42.5 °C | 60 |
| Author (Year) | n (H/no H) | Residual | DFI (mos) | OS | PFS (mos) |
|---|---|---|---|---|---|
| Not randomized | |||||
| Munoz Casares (2009) [36] | 14/12 | CC0: (H) 64%/(No H) 58% | UK | 5 years 58%/17% (p = 0.046) | NP |
| Fagotti (2012) [37] | 30/37 | CC0: (H) 96.7%/(No H) 100%) | 20/22 | 5 years 68%/42% (p = 0.017) | 26/15 (p = 0.004) |
| Safra (2014) [42] | 27/84 | UK | 24/21 | 5 years 79%/45% (p = 0.016) | 15/6 (p = 0.001) |
| Lebrun (2014) [40] | 23/19 | CC0: (H) 65%/(No H) 42% | >12 (19/18) | 4 years 75.6%/19.4% (p = 0.013) | NP |
| Baiocchi (2016) [38] | 29/50 | CC0: (H) 79% (No H) 74% | 28 | 58 mos/59 mos (p = 0.95) | 15.8/18.6 (p = 0.82) |
| Cascales Campos (2015) [35] | 32/22 | UK | 22 | NP | 3 years 45%23% (NS) (p = 0.078) |
| Marocco (2016) [41] | 19/11 | CC0: 100% | 22/26.9 | 51.5/NP | 19.9/23 |
| Randomized | |||||
| Spilliotis (2014) [39] | 60/60 | CC0: (H) 65%/(No H) 55% | UK | 26.7 mos/13.4 mos, (p = 0.006) | NP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Classe, J.-M.; Asselain, B.; Campion, L.; Berton, D.; Frenel, J.-S.; Lécuru, F.; Ferron, G.; Gladieff, L.; Bourgin, C.; Loaec, C. Survival Outcomes after Hyperthermic Intraperitoneal Chemotherapy for a First Ovarian Cancer Relapse: A Systematic Evidence-Based Review. Cancers 2022, 14, 172. https://doi.org/10.3390/cancers14010172
Classe J-M, Asselain B, Campion L, Berton D, Frenel J-S, Lécuru F, Ferron G, Gladieff L, Bourgin C, Loaec C. Survival Outcomes after Hyperthermic Intraperitoneal Chemotherapy for a First Ovarian Cancer Relapse: A Systematic Evidence-Based Review. Cancers. 2022; 14(1):172. https://doi.org/10.3390/cancers14010172
Chicago/Turabian StyleClasse, Jean-Marc, Bernard Asselain, Loic Campion, Dominique Berton, Jean-Sébastien Frenel, Fabrice Lécuru, Gwenael Ferron, Laurence Gladieff, Charlotte Bourgin, and Cecile Loaec. 2022. "Survival Outcomes after Hyperthermic Intraperitoneal Chemotherapy for a First Ovarian Cancer Relapse: A Systematic Evidence-Based Review" Cancers 14, no. 1: 172. https://doi.org/10.3390/cancers14010172
APA StyleClasse, J.-M., Asselain, B., Campion, L., Berton, D., Frenel, J.-S., Lécuru, F., Ferron, G., Gladieff, L., Bourgin, C., & Loaec, C. (2022). Survival Outcomes after Hyperthermic Intraperitoneal Chemotherapy for a First Ovarian Cancer Relapse: A Systematic Evidence-Based Review. Cancers, 14(1), 172. https://doi.org/10.3390/cancers14010172







