Neuroendocrine Neoplasms of the Breast: The Latest WHO Classification and Review of the Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
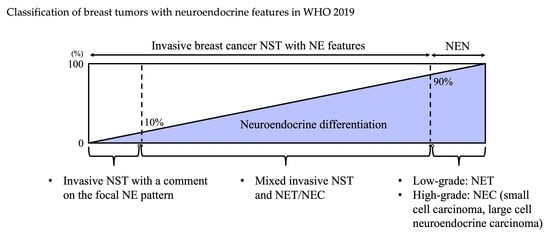
3. WHO 2019 Classification
3.1. WHO Classification: Historical Transition
3.2. Histology: Neuroendocrine Tumors, Neuroendocrine Carcinoma
4. Clinical Features
5. Management
5.1. Surgery
5.2. Radiotherapy
5.3. Chemotherapy
5.4. Hormonal Therapy
5.5. Anti-HER2 Therapy
5.6. Somatostatin Analogue
5.7. Peptide Receptor Radionuclide Therapy (PRRT)
5.8. Other Agents
6. Prognosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NE | neuroendocrine |
| NST | non-special type |
| NET | neuroendocrine tumor |
| NEC | neuroendocrine carcinoma |
| WHO | World Health Organization |
| NEN | neuroendocrine neoplasm |
| HER2 | human epidermal growth factor type 2 |
| HR | hormone receptor |
| CDK4/6 | cyclin-dependent kinase 4/6 |
| IHC | immunohistochemistry |
| IBC-NST | invasive breast cancer of no special type |
| Br-NENs | neuroendocrine neoplasms of the breast |
| CGA | chromogranin A |
| SYN | synaptophysin |
| LCNEC | large cell neuroendocrine carcinoma |
| SCNEC | small cell neuroendocrine carcinoma |
| ER | estrogen receptor |
| PgR | progesterone receptor |
| T-DM1 | trastuzumab-emtansine |
| ADC | antibody-drug conjugate |
| PPRT | Peptide receptor radionuclide therapy |
References
- Hörsch, D.; Schmid, K.W.; Anlauf, M.; Darwiche, K.; Denecke, T.; Baum, R.P.; Spitzweg, C.; Grohe, C.; Presselt, N.; Stremmel, C.; et al. Neuroendocrine Tumors of the Bronchopulmonary System (Typical and Atypical Carcinoid Tumors): Current Strategies in Diagnosis and Treatment. Conclusions of an Expert Meeting February 2011 in Weimar, Germany. Oncol. Res. Treat. 2014, 37, 5. [Google Scholar] [CrossRef] [Green Version]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Marín, J.I.; Lam, A.K.; Ochiai, A.; Odze, R.D.; Washington, K.M.; Fukayama, M.; Rugge, M.; Klimstra, D.S.; Nagtegaal, I.D.; Tan, P.; et al. Gastrointestinal tissue-based molecular biomarkers: A practical categorisation based on the 2019 World Health Organization classification of epithelial digestive tumours. Histopathology 2020, 77, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Marinova, L.; Malinova, D.; Vicheva, S. Primary Neuroendocrine Carcinoma of the Breast: Histopathological Criteria, Prognostic Factors, and Review of the Literature. Case Rep. Pathol. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wei, B.; Albarracin, C.T.; Hu, J.; Abraham, S.C.; Wu, Y. Invasive neuroendocrine carcinoma of the breast: A population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauso, O.; Gustafsson, B.I.; Kidd, M.; Waldum, H.L.; Drozdov, I.; Chan, A.K. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer 2008, 113, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Feyrter, F.; Hartmann, G. On The Carcinoid Growth Form of the Carcinoma Mammae, Especially the Carcinoma Solidum (Gelatinosum) Mammae. Frankf. Z. Pathol. 1963, 73, 24–39. [Google Scholar]
- Cubilla, A.L.M.D.W.; James, M.M.D. Primary carcinoid tumor of the breast. A report of eight patients. Am. J. Surg. Pathol. 1977, 1, 283–292. [Google Scholar] [CrossRef]
- Papotti, M.; Macrí, L.; Bussolati, G.; Reubi, J.C. Correlative study on neuro-endocrine differentiation and presence of somatostatin receptors in breast carcinomas. Int. J. Cancer 1989, 43, 365–369. [Google Scholar] [CrossRef]
- Bogina, G.; Munari, E.; Brunelli, M.; Bortesi, L.; Marconi, M.; Sommaggio, M.; Lunardi, G.; Gori, S.; Massocco, A.; Pegoraro, M.C.; et al. Neuroendocrine differentiation in breast carcinoma: Clinicopathological features and outcome. Histopathology 2016, 68, 422–432. [Google Scholar] [CrossRef]
- Sapino, A.; Bussolati, G. Is detection of endocrine cells in breast adenocarcinoma of diagnostic and clinical significance? Histopathology 2002, 40, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Board; WCoTE; Breast Tumours. WHO Classification of Tumors, 5nd ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Metovic, J.; Castellano, I.; Marinelli, E.; Osella-Abate, S.; Sapino, A.; Cassoni, P.; Papotti, M. INSM1 Expression in Breast Neoplasms with Neuroedocrine Features. Endocr. Pathol. 2021, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wei, B.; Tian, Z.; Gilcrease, M.Z.; Huo, L.; Albarracin, C.T.; Resetkova, E.; Zhang, H.; Sahin, A.; Chen, J.; et al. Invasive mammary carcinoma with neuroendocrine differentiation: Histological features and diagnostic challenges. Histopathology 2011, 59, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Maluf, H.; Koerner, F. Carcinomas of the breast with endocrine differentiation: A review. Virchows Archiv 1994, 425, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Talu, C.K.; Leblebici, C.; Ozturk, T.K.; Hacihasanoglu, E.; Koca, S.B.; Gucin, Z. Primary breast carcinomas with neuroendocrine features: Clinicopathological features and analysis of tumor growth patterns in 36 cases. Ann. Diagn. Pathol. 2018, 34, 122–130. [Google Scholar] [CrossRef]
- Osamura, R.Y.; Matsui, N.; Okubo, M.; Chen, L.; Field, A.S. Histopathology and Cytopathology of Neuroendocrine Tumors and Carcinomas of the Breast: A Review. Acta Cytol. 2019, 63, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Finzi, G.; Sessa, F.; La Rosa, S. On the Endless Dilemma of Neuroendocrine Neoplasms of the Breast: A Journey Through Concepts and Entities. Endocr. Pathol. 2020, 31, 321–329. [Google Scholar] [CrossRef]
- Shin, S.J.; DeLellis, R.A.; Ying, L.; Rosen, P.P. Small cell carcinoma of the breast: A clinicopathologic and immunohistochemical study of nine patients. Am. J. Surg. Pathol. 2000, 24, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Hare, F.; Giri, S.; Patel, J.K.; Hahn, A.; Martin, M.G. A population-based analysis of outcomes for small cell carcinoma of the breast by tumor stage and the use of radiation therapy. SpringerPlus 2015, 4, 138. [Google Scholar] [CrossRef]
- Wong, Y.N.S.; Jack, R.H.; Mak, V.; Henrik, M.; Davies, E.A. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970–2004. BMC Cancer 2009, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, H.; Nishio, A.; Satake, H.; Naganawa, S.; Imai, T.; Sawaki, M.; Yamamoto, E.; Miyata, T. Neuroendocrine tumor in the breast. Radiat. Med. 2008, 26, 28–32. [Google Scholar] [CrossRef]
- Gallo, M.; Campione, S.; Di Vito, V.; Fortunati, N.; Calzo, F.L.; Messina, E.; Ruggeri, R.M.; Faggiano, A.; Colao, A.A.L. Primary Neuroendocrine Neoplasms of the Breast: Still Open Issues. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Çermik, T.F.; Trabulus, F.D.C.; Talu, E.C.K.; Başaran, Ş. Diagnostic impact of 18F-FDG PET/CT on the management of rare breast carcinomas: Apocrine and neuroendocrine carcinomas. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Foldi, J.; Rozenblit, M.; Park, T.S.; Knowlton, C.A.; Golshan, M.; Moran, M.; Pusztai, L. Optimal Management for Residual Disease Following Neoadjuvant Systemic Therapy. Curr. Treat. Options Oncol. 2021, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Tremelling, A.; Samuel, S.; Murray, M. Primary small cell neuroendocrine carcinoma of the breast—A case report and review of the literature. Int. J. Surg. Case Rep. 2017, 38, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Angarita, F.A.; Rodríguez, J.L.; Meek, E.; Sánchez, J.O.; Tawil, M.; Torregrosa, L. Locally-advanced primary neuroendocrine carcinoma of the breast: Case report and review of the literature. World J. Surg. Oncol. 2013, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutrid, H.; Kassem, M.; Tozbikian, G.; Morgan, E.; White, J.; Shah, M.; VanDeusen, J.; Sardesai, S.; Williams, N.; Stover, D.; et al. TTF-1 Positive Primary Small Cell Carcinoma of the Breast: A Case Report and Review of the Literature. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Uccella, S. The classification of neuroendocrine neoplasms of the breast and its clinical relevance. Virchows Archiv 2021, 1–10. [Google Scholar] [CrossRef]
- Adams, R.; Dyson, P.; Barthelmes, L. Neuroendocrine breast tumours: Breast cancer or neuroendocrine cancer presenting in the breast? Breast 2014, 23, 120–127. [Google Scholar] [CrossRef]
- Richter-Ehrenstein, C.; Arndt, J.; Buckendahl, A.C.; Eucker, J.; Weichert, W.; Kasajima, A. Solid neuroendocrine carcinomas of the breast: Metastases or primary tumors? Breast Cancer Res. Treat. 2010, 124, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheymol, C.; Abramovici, O.; Cao, C.D.; Dumont, A.; Robin, Y.M.; El Hajbi, F. Neuroendocrine tumors of the breast: Myth or reality? A systematic review. Bull. Cancer 2018, 105, 431–439. [Google Scholar] [CrossRef]
- Dalle, I.A.; Abbas, J.; Boulos, F.; Salem, Z.; Assi, H.I. Primary small cell carcinoma of the breast: A case report. J. Med. Case Rep. 2017, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Ding, T.; Xing, Y.; Wei, W.; Tian, Z.; Tang, F. Invasive neuroendocrine carcinoma of the breast: A distinctive subtype of aggressive mammary carcinoma. Cancer 2010, 116, 4463–4473. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Sato, Y.; Hashimoto, S.; Mizuno, K.-I.; Takeuchi, M.; Terai, S. Management of gastric and duodenal neuroendocrine tumors. World J. Gastroenterol. 2016, 22, 6817–6828. [Google Scholar] [CrossRef]
- Trevisi, E.; La Salvia, A.; Daniele, L.; Brizzi, M.P.; De Rosa, G.; Scagliotti, G.V.; Di Maio, M. Neuroendocrine breast carcinoma: A rare but challenging entity. Med. Oncol. 2020, 37, 1–8. [Google Scholar] [CrossRef]
- Inno, A.; Bogina, G.; Turazza, M.; Bortesi, L.; Duranti, S.; Massocco, A.; Zamboni, G.; Carbognin, G.; Alongi, F.; Salgarello, M.; et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist 2016, 21, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Pagano, M.; Asensio, S.; Zanelli, F.; Lococo, F.; Cavazza, A.; Damiani, S.; Rapicetta, C.; Gnoni, R.; Boni, C. Is There a Role for Hormonal Therapy in Neuroendocrine Carcinoma of the Breast? A Paradigmatic Case Report. Clin. Breast Cancer 2014, 14, e99–e101. [Google Scholar] [CrossRef]
- Buttar, A.; Mittal, K.; Khan, A.; Bathini, V. Effective Role of Hormonal Therapy in Metastatic Primary Neuroendocrine Breast Carcinoma. Clin. Breast Cancer 2011, 11, 342–345. [Google Scholar] [CrossRef]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; Andre, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Shanks, A.; Choi, J.; Karur, V. Dramatic response to cyclin D-dependent kinase 4/6 inhibitor in refractory poorly differentiated neuroendocrine carcinoma of the breast. Proc. Bayl. Univ. Med. Cent. 2018, 31, 352–354. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.M.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef]
- Lavigne, M.; Menet, E.; Tille, J.-C.; Lae, M.; Fuhrmann, L.; Bonneau, C.; Deniziaut, G.; Melaabi, S.; Ng, C.C.K.; Marchiò, C.; et al. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod. Pathol. 2018, 31, 68–82. [Google Scholar] [CrossRef]
- Marchiò, C.; Geyer, F.C.; Ng, C.K.Y.; Piscuoglio, S.; De Filippo, M.R.; Cupo, M.; Schultheis, A.M.; Lim, R.S.; Burke, K.A.; Rocco, E.G.; et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J. Pathol. 2017, 241, 405–419. [Google Scholar] [CrossRef]
- McCullar, B.; Pandey, M.; Yaghmour, G.; Hare, F.; Patel, K.; Stein, M.; Feldman, R.; Chandler, J.C.; Martin, M.G. Genomic landscape of small cell carcinoma of the breast contrasted to small cell carcinoma of the lung. Breast Cancer Res. Treat. 2016, 158, 195–202. [Google Scholar] [CrossRef]
- Ang, D.; Ballard, M.; Beadling, C.; Warrick, A.; Schilling, A.; O’Gara, R. Novel mutations in neuroendocrine carcinoma of the breast: Possible therapeutic targets. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 97–103. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; De Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ke, X.; Yu, J.; Jing, Q.; Bu, H.; Zeng, X.; Wei, B. Clinical and genomic analyses of neuroendocrine neoplasms of the breast. Mod. Pathol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, A.; Bregni, G.; Galli, G.; Zanardi, E.; de Braud, F.G.M.; Di Cosimo, S. HER2-Positive Neuroendocrine Breast Cancer: Case Report and Review of Literature. Breast Care 2016, 11, 424–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marijanovic, I.; Kraljevic, M.; Buhovac, T.; Krizanac, D.K. Rare Human Epidermal Growth Factor Receptor 2 (HER-2)-Positive Neuroendocrine Carcinoma of the Breast: A Case Report with 9-Year Follow-up. Am. J. Case Rep. 2020, 21, e925895. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Ryan, Q.; Ibrahim, A.; Cohen, M.H.; Johnson, J.; Ko, C.; Sridhara, R.; Justice, R.; Pazdur, R. FDA Drug Approval Summary: Lapatinib in Combination with Capecitabine for Previously Treated Metastatic Breast Cancer That Overexpresses HER-2. Oncology 2008, 13, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With >/= 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Thodou, E.; Choreftaki, T. Investigation of somatostatin receptor profile of neuroendocrine carcinomas of the breast. Pathol. Res. Pract. 2020, 216, 153066. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas, J.J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, J.; Kulkarni, H.R.; Baum, R.P. 177Lu-DOTATOC Peptide Receptor Radionuclide Therapy in a Patient with Neuroendocrine Breast Carcinoma and Breast Invasive Ductal Carcinoma. Clin. Nucl. Med. 2020, 45, e232–e235. [Google Scholar] [CrossRef] [PubMed]
- Savelli, G.; Zaniboni, A.; Bertagna, F.; Bosio, G.; Nisa, L.; Rodella, C.; Biasiotto, G.; Bettinsoli, G.; Migliorati, E.; Peli, A.; et al. Peptide Receptor Radionuclide Therapy (PRRT) in a Patient Affected by Metastatic Breast Cancer with Neuroendocrine Differentiation. Breast Care 2012, 7, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Ferrata, M.; Schad, A.; Zimmer, S.; Musholt, T.J.; Bahr, K.; Kuenzel, J.; Becker, S.; Springer, E.; Roth, W.; Weber, M.M.; et al. PD-L1 Expression and Immune Cell Infiltration in Gastroenteropancreatic (GEP) and Non-GEP Neuroendocrine Neoplasms with High Proliferative Activity. Front. Oncol. 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Maiorano, B.; Azzali, I.; Liverani, C.; Bocchini, M.; Fausti, V.; Di Menna, G.; Grassi, I.; Sansovini, M.; Riva, N.; et al. Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Sapino, A.; Righi, L.; Cassoni, P.; Papotti, M.; Gugliotta, P.; Bussolati, G. Expression of Apocrine Differentiation Markers in Neuroendocrine Breast Carcinomas of Aged Women. Mod. Pathol. 2001, 14, 768–776. [Google Scholar] [CrossRef]
- Yang, L.; Roy, M.; Lin, H.; Shen, Y.; Albarracin, C.; Huo, L.; Chen, H.; Wei, B.; Bedrosian, I.; Bu, H.; et al. Validation of prognostic significance of the proposed uniform classification framework in neuroendocrine neoplasms of the breast. Breast Cancer Res. Treat. 2021, 186, 403–415. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Yang, R.L.; Allison, K.H.; Norton, J.A.; Hernandez-Boussard, T.; Wapnir, I.L. Impact of histological subtype on long-term outcomes of neuroendocrine carcinoma of the breast. Breast Cancer Res. Treat. 2014, 148, 637–644. [Google Scholar] [CrossRef]
- Lai, B.S.; Tsang, J.Y.; Poon, I.K.; Shao, Y.; Chan, S.; Tam, F.K.; Cheung, S.; Shea, K.; Tse, G.M. The Clinical Significance of Neuroendocrine Features in Invasive Breast Carcinomas. Oncologist 2020, 25, e1318–e1329. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wei, B.; Tang, F.; Wei, W.; Gilcrease, M.Z.; Huo, L.; Albarracin, C.T.; Resetkova, E.; Middleton, L.; Sahin, A.; et al. Prognostic significance of tumor grading and staging in mammary carcinomas with neuroendocrine differentiation. Hum. Pathol. 2011, 42, 1169–1177. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaki, Y.; Miura, S.; Oki, R.; Morikawa, T.; Uchino, K. Neuroendocrine Neoplasms of the Breast: The Latest WHO Classification and Review of the Literature. Cancers 2022, 14, 196. https://doi.org/10.3390/cancers14010196
Ozaki Y, Miura S, Oki R, Morikawa T, Uchino K. Neuroendocrine Neoplasms of the Breast: The Latest WHO Classification and Review of the Literature. Cancers. 2022; 14(1):196. https://doi.org/10.3390/cancers14010196
Chicago/Turabian StyleOzaki, Yukinori, Sakiko Miura, Ryosuke Oki, Teppei Morikawa, and Keita Uchino. 2022. "Neuroendocrine Neoplasms of the Breast: The Latest WHO Classification and Review of the Literature" Cancers 14, no. 1: 196. https://doi.org/10.3390/cancers14010196
APA StyleOzaki, Y., Miura, S., Oki, R., Morikawa, T., & Uchino, K. (2022). Neuroendocrine Neoplasms of the Breast: The Latest WHO Classification and Review of the Literature. Cancers, 14(1), 196. https://doi.org/10.3390/cancers14010196