Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
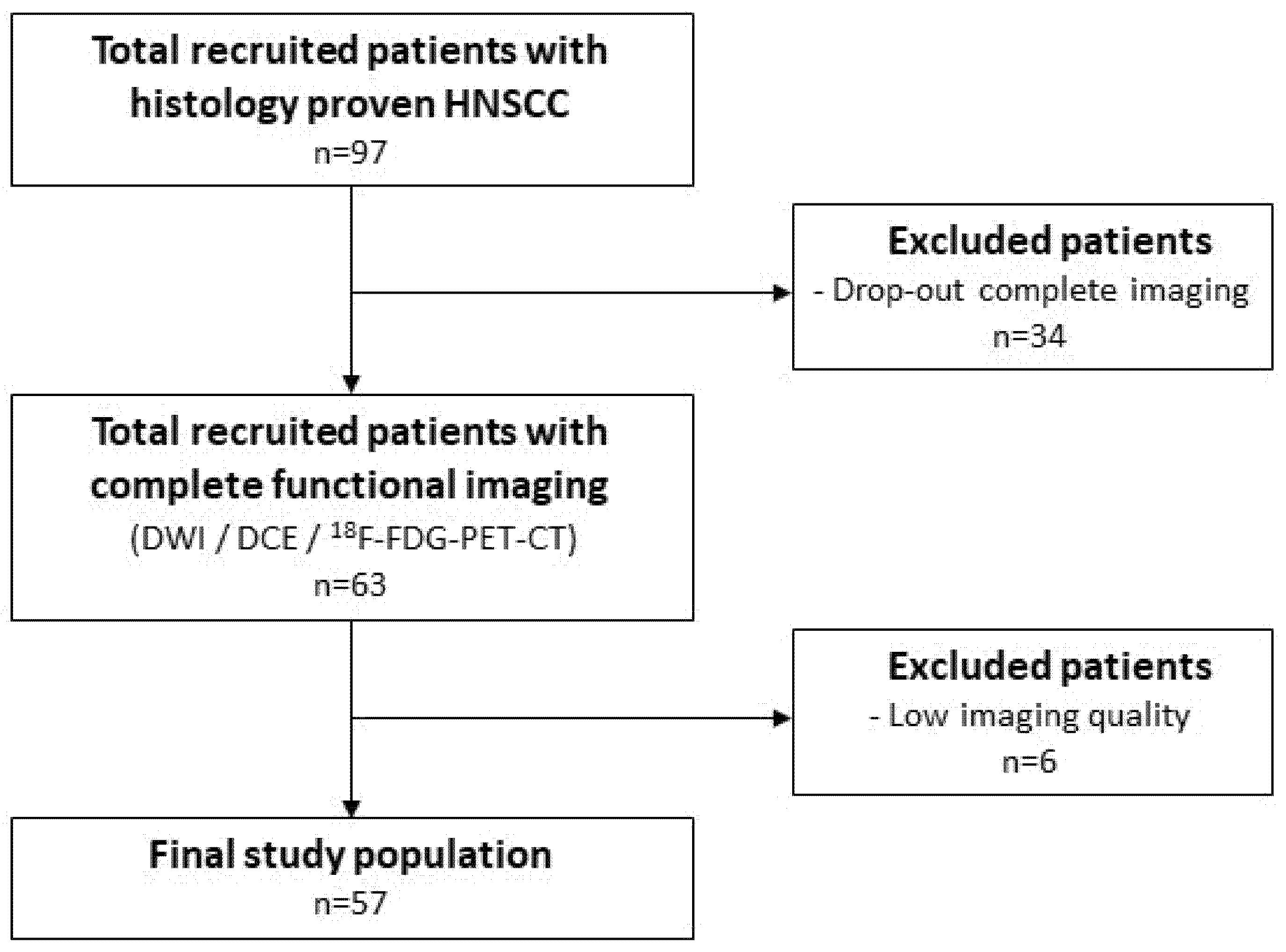
2.1. Patient Selection
2.2. Imaging
2.3. Exclusion Criteria
2.4. Delineation
2.5. Feature Extraction
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Observer Variations
3.3. Parameter Correlations
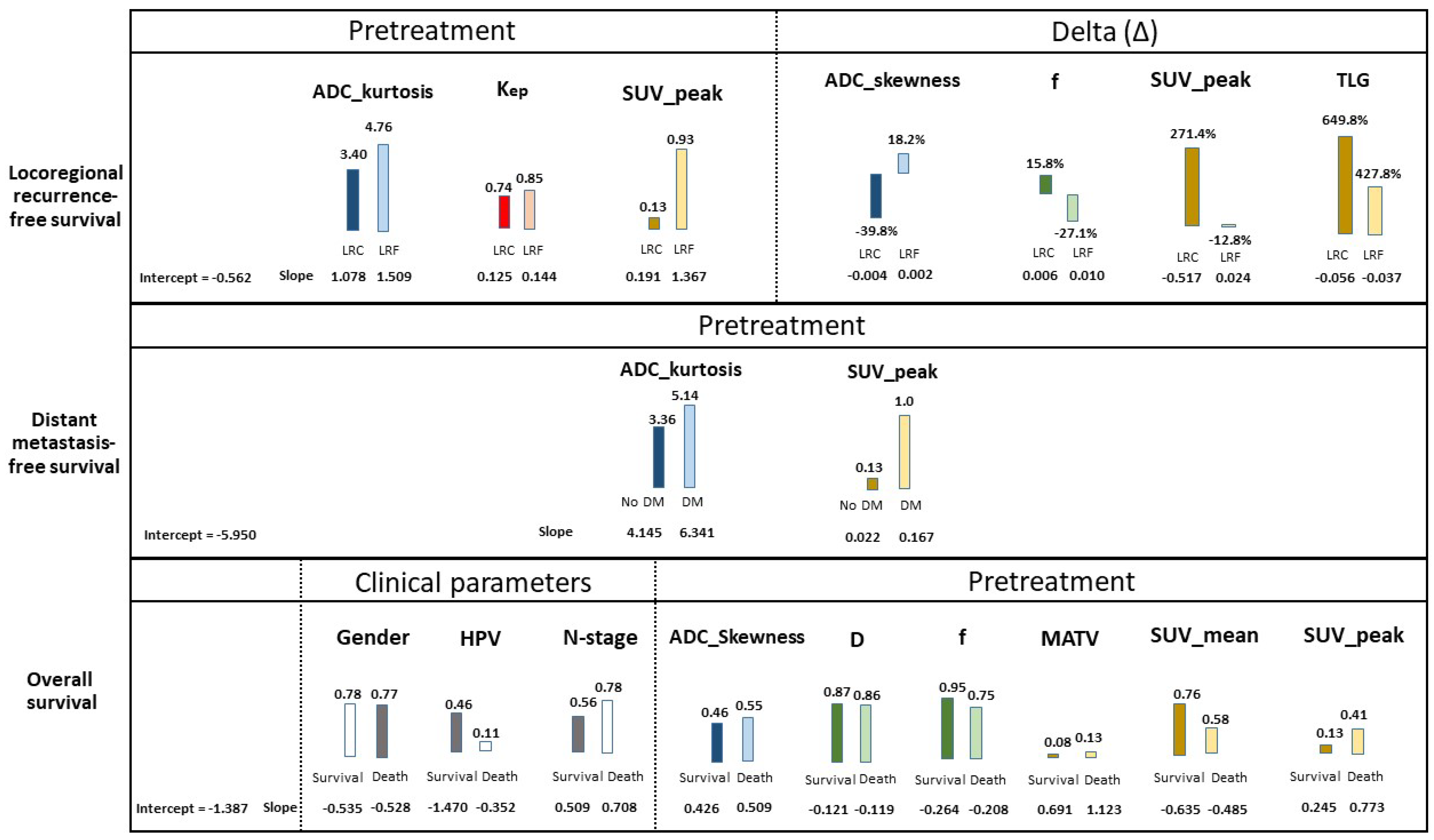
3.4. Prognostic Parameters
3.5. Prognostic Models
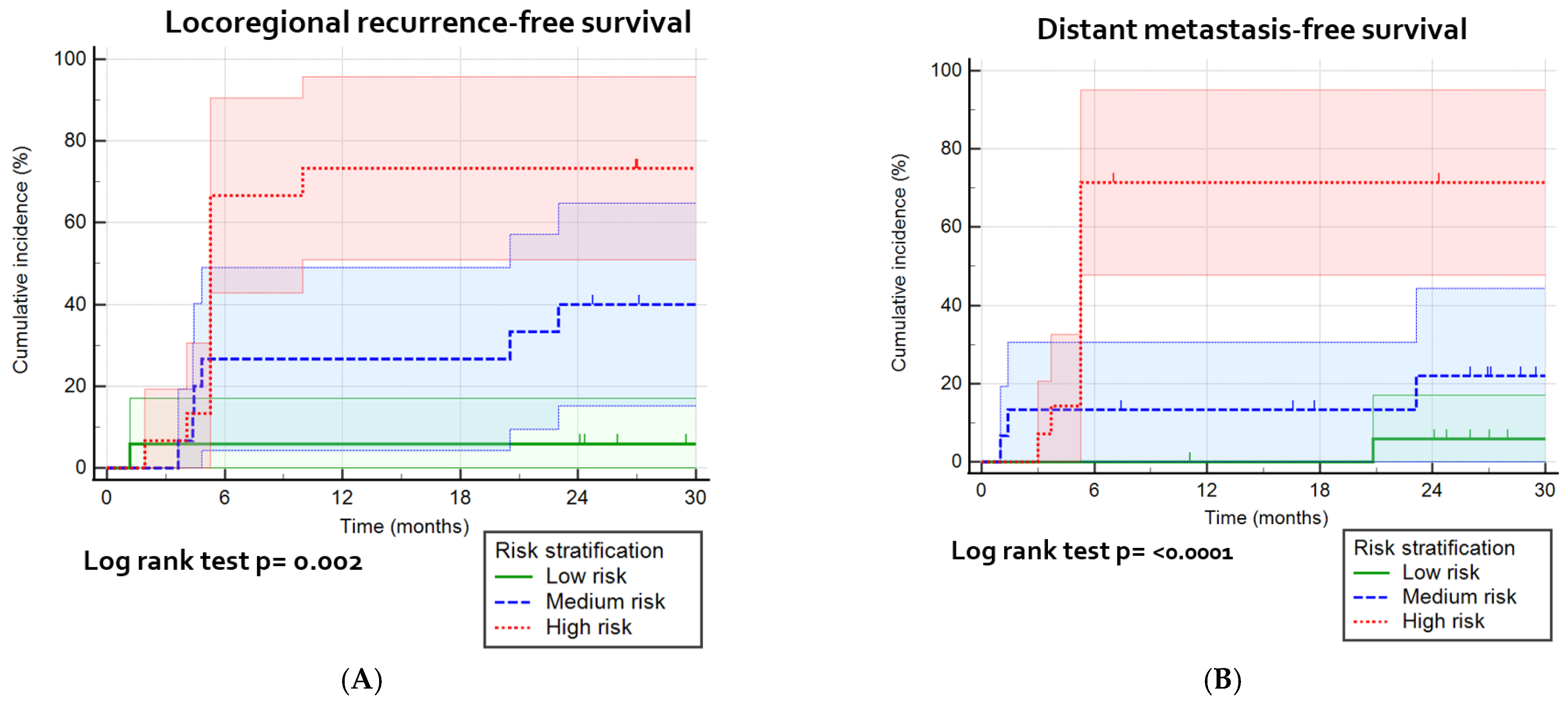
3.6. Risk Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIF | Arterial input function |
| AJCC | American Joint Committee on Cancer |
| D | Pure diffusion coefficient [mm2/s] |
| D* | Pseudo-diffusion coefficient [mm2/s] |
| DCE | Dynamic contrast enhanced |
| DMFS | Distant metastasis-free survival |
| DWI | Diffusion-weighted imaging |
| f | Perfusion fraction |
| GTV | Gross tumor volume |
| HNSCC | Head and neck squamous cell carcinoma |
| IVIM | Intravoxel incoherent motion |
| Kep | The rate constant for transfer of contrast agent from extravascular, extracellular space to the plasma [min−1] |
| Ktrans | The rate constant for transfer of contrast agent from plasma to extravascular, extracellular space [min−1] |
| LRC | Loco-regional control |
| LRF | Loco-regional failure |
| LRFFS | Loco-regional failure-free survival |
| MATV | Mean of the active tumor volume |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| OS | Overall survival |
| SUV_mean | Mean of SUV included in the VOI |
| SUV_peak | Sphere of 8 voxels around the voxel with the highest SUV in the delineated VOI |
| TLG | Total lesion glycolysis (SUVmean × MATV) |
| TLG40% | Total lesion glycolysis, including voxels with SUV higher than 40% of SUV_peak |
| Ve | Fractional volume of the extravascular extracellular space |
References
- Chen, A.M.; Felix, C.; Wang, P.-C.; Hsu, S.; Basehart, V.; Garst, J.; Beron, P.; Wong, D.; Rosove, M.H.; Rao, S.; et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017, 18, 803–811. [Google Scholar] [CrossRef]
- Pigorsch, S.U.; Wilkens, J.J.; Kampfer, S.; Kehl, V.; Hapfelmeier, A.; Schlager, C.; Bier, H.; Schwaiger, M.; Combs, S.E. Do selective radiation dose escalation and tumour hypoxia status impact the loco-regional tumour control after radio-chemotherapy of head & neck tumours? The ESCALOX protocol. Radiat. Oncol. 2017, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, J.; Toustrup, K.; Eriksen, J.G.; Primdahl, H.; Andersen, L.J.; Overgaard, J. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015, 54, 1001–1007. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—A systematic review and meta-analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef]
- Martens, R.; Noij, D.; Ali, M.; Koopman, T.; Marcus, J.T.; Vergeer, M.R.; de Vet, H.; de Jong, M.C.; Leemans, C.R.; Hoekstra, O.S.; et al. Functional imaging early during (chemo)radiotherapy for response prediction in head and neck squamous cell carcinoma; a systematic review. Oral Oncol. 2019, 88, 75–83. [Google Scholar] [CrossRef]
- Martens, R.M.; Koopman, T.; Lavini, C.; Ali, M.; Peeters, C.F.; Noij, D.P.; Zwezerijnen, G.; Marcus, J.T.; Vergeer, M.R.; Leemans, C.R.; et al. Multiparametric functional MRI and (18)F-FDG-PET for survival prediction in patients with head and neck squamous cell carcinoma treated with (chemo)radiation. Eur. Radiol. 2021, 31, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Halfpenny, W.; Hain, S.F.; Biassoni, L.; Maisey, M.N.; Sherman, J.A.; McGurk, M. FDG-PET. A possible prognostic factor in head and neck cancer. Br. J. Cancer 2002, 86, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Noij, D.P.; Martens, R.M.; Marcus, J.T.; de Bree, R.; Leemans, C.R.; Castelijns, J.A.; de Jong, M.C.; de Graaf, P. Intravoxel incoherent motion magnetic resonance imaging in head and neck cancer: A systematic review of the diagnostic and prognostic value. Oral Oncol. 2017, 68, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Gunnar, B.; Buckley, L.D.; Jeffrey, L.E.; Henderson, E.; Knopp, M.V.; Larsson, H.B.W.; Lee, T.-Y.; Mayr, N.A.; Parker, G.J.M.; et al. Estimating kinetic parameters from dynamic contrast-enhancedT1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reason. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Subesinghe, M.; Scarsbrook, A.F.; Sourbron, S.; Wilson, D.J.; McDermott, G.; Speight, R.; Roberts, N.; Carey, B.; Forrester, R.; Gopal, S.V.; et al. Alterations in anatomic and functional imaging parameters with repeated FDG PET-CT and MRI during radiotherapy for head and neck cancer: A pilot study. BMC Cancer 2015, 15, 137. [Google Scholar] [CrossRef]
- Wong, K.H.; Panek, R.; Dunlop, A.; McQuaid, D.; Riddell, A.; Welsh, L.C.; Murray, I.; Koh, D.M.; Leach, M.O.; Bhide, S.A.; et al. Changes in multimodality functional imaging parameters early during chemoradiation predict treatment response in patients with locally advanced head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 45, 759–767. [Google Scholar] [CrossRef]
- Martens, R.M.; Koopman, T.; Noij, D.P.; de Bree, R.; Vergeer, M.R.; Zwezerijnen, G.; Leemans, C.R.; de Graaf, P.; Boellaard, R.; Castelijns, J.A. Adherence to pretreatment and intratreatment imaging of head and neck squamous cell carcinoma patients undergoing (chemo) radiotherapy in a research setting. Clin. Imaging 2021, 69, 82–90. [Google Scholar] [CrossRef]
- Lavini, C.; Kramer, G.; Pieters-den Bos, I.; Hoekstra, O.; Marcus, J.T. MRI protocol optimization for quantitative DCE-MRI of the spine. Magn. Reason. Imaging 2017, 44, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Frings, V.; de Langen, A.J.; Smit, E.F.; van Velden, F.H.; Hoekstra, O.S.; van Tinteren, H.; Boellaard, R. Repeatability of metabolically active volume measurements with 18F-FDG and 18F-FLT PET in non-small cell lung cancer. J. Nucl. Med. 2010, 51, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Koopman, T.; Martens, R.; Gurney-Champion, O.J.; Yaqub, M.; Lavini, C.; de Graaf, P.; Castelijns, J.; Boellaard, R.; Marcus, J.T. Repeatability of IVIM biomarkers from diffusion-weighted MRI in head and neck: Bayesian probability versus neural network. Magn. Reason. Med. 2021, 85, 3394–3402. [Google Scholar] [CrossRef]
- Parker, G.J.; Roberts, C.; Macdonald, A.; Buonaccorsi, G.A.; Cheung, S.; Buckley, D.L.; Jackson, A.; Watson, Y.; Davies, K.; Jayson, G.C. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn. Reason. Med. 2006, 56, 993–1000. [Google Scholar] [CrossRef]
- King, A.D.; Chow, K.K.; Yu, K.H.; Mo, F.K.; Yeung, D.K.; Yuan, J.; Bhatia, K.S.; Vlantis, A.C.; Ahuja, A.T. Head and neck squamous cell carcinoma: Diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology 2013, 266, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Riaz, N.; Hunt, M.; Wengler, K.; Hatzoglou, V.; Young, R.; Mechalakos, J.; Lee, N. Weekly response assessment of involved lymph nodes to radiotherapy using diffusion-weighted MRI in oropharynx squamous cell carcinoma. Med. Phys. 2016, 43, 137. [Google Scholar] [CrossRef]
- De Perrot, T.; Lenoir, V.; Domingo Ayllon, M.; Dulguerov, N.; Pusztaszeri, M.; Becker, M. Apparent diffusion coefficient histograms of human papillomavirus-positive and human papillomavirus-negative head and neck squamous cell carcinoma: Assessment of tumor heterogeneity and comparison with histopathology. AJNR Am. J. Neuroradiol. 2017, 38, 2153–2610. [Google Scholar] [CrossRef]
- Forastiere, A.; Koch, W.; Trotti, A.; Sidransky, D. Head and neck cancer. N. Engl. J. Med. 2001, 345, 1890–1900. [Google Scholar] [CrossRef]
- Cao, Y.; Popovtzer, A.; Li, D.; Chepeha, D.B.; Moyer, J.S.; Prince, M.E.; Worden, F.; Teknos, T.; Bradford, C.; Mukherji, S.K.; et al. Early prediction of outcome in advanced head-and-neck cancer based on tumor blood volume alterations during therapy: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1287–1290. [Google Scholar] [CrossRef]
- Wang, P.; Popovtzer, A.; Eisbruch, A.; Cao, Y. An approach to identify, from DCE MRI, significant subvolumes of tumors related to outcomes in advanced head-and-neck cancer. Med. Phys. 2012, 39, 5277–5285. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shukla-Dave, A.; Lee, N.Y.; Jansen, J.F.; Thaler, H.T.; Stambuk, H.E.; Fury, M.G.; Patel, S.G.; Moreira, A.L.; Sherman, E.; Karimi, S.; et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Loevner, L.A.; Quon, H.; Kilger, A.; Sherman, E.; Weinstein, G.; Chalian, A.; Poptani, H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2010, 31, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tamaki, T.; Nishio, M.; Beppu, S.; Mukoyama, N.; Hanai, N.; Nishikawa, D.; Koide, Y.; Hasegawa, Y. Peak of standardized uptake value in oral cancer predicts survival adjusting for pathological stage. In Vivo 2018, 32, 1193–1198. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Can imaging parameters provide information regarding histopathology in head and neck squamous cell carcinoma? A meta-analysis. Transl. Oncol. 2018, 11, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.; Pradier, O.; Metges, J.P.; Corcos, L.; Visvikis, D. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef]
- Martens, R.M.; Koopman, T.; Noij, D.P.; Pfaehler, E.; Ubelhor, C.; Sharma, S.; Vergeer, M.R.; Leemans, C.R.; Hoekstra, O.S.; Yaqub, M.; et al. Predictive value of quantitative (18)F-FDG-PET radiomics analysis in patients with head and neck squamous cell carcinoma. EJNMMI Res. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishio, M.; Nakanishi, H.; Hanai, N.; Hirakawa, H.; Kodaira, T.; Tamaki, T.; Hasegawa, Y. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol. Lett. 2016, 12, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Castelli, J.; De Bari, B.; Depeursinge, A.; Simon, A.; Devillers, A.; Roman Jimenez, G.; Prior, J.; Ozsahin, M.; de Crevoisier, R.; Bourhis, J. Overview of the predictive value of quantitative 18 FDG PET in head and neck cancer treated with chemoradiotherapy. Crit. Rev. Oncol. Hematol. 2016, 108, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.M.; Fang, Y.H.D.; Chang, J.T.C.; Huang, C.G.; Tsan, D.L.; Ng, S.H.; Wang, H.M.; Lin, C.Y.; Liao, C.T.; Yen, T.C. Textural features of pretreatment 18F-FDG PET/CT images: Prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]



| Patient Characteristics and Events | |||
|---|---|---|---|
| Age at baseline imaging | Follow-up after treatment | ||
| Median (IQR *) | 63 (56.5–67) | Follow-up in months (IQR *) | 30.7 (17.8–38.7) |
| Sex | Treatment | ||
| Female | 16 | Chemoradiotherapy | 53 |
| Male | 42 | Cisplatin | 49 |
| Tobacco use | Cetuximab | 4 | |
| None (%) | 21 (36.8) | Radiotherapy only | 4 |
| Smoker (%) | 36 (63.2) | No. tumor-related events | |
| HPV positive (%) | 20 (44.4) † | Locoregional recurrence | 18 |
| Primary tumor location | Distant metastases | 20 | |
| Oropharynx | 45 | Tumor-related death | 17 |
| Hypopharynx | 12 | ||
| T stage (n = 58) | |||
| 2 | 18 | ||
| 3 | 15 | ||
| 4 | 25 | ||
| N stage (n = 58) | |||
| 0 | 13 | ||
| 1 | 8 | ||
| 2 | 34 | ||
| 3 | 2 | ||
| Logistic Regression Models | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Features | Patients | Imaging Features | Clinical Parameters + Imaging Features | ||||
| Features | AUC | SD | Features | AUC | SD | |||
| Locoregional recurrence-free survival | PRE | 47 | 15 | 0.79 | 0.16 | 22 | 0.79 | 0.16 |
| INTRA | 47 | 15 | 0.49 | 0.09 | 22 | 0.47 | 0.09 | |
| Delta | 47 | 15 | 0.76 | 0.14 | 22 | 0.77 | 0.14 | |
| ALL | 47 | 45 | 0.81 | 0.14 | 52 | 0.80 | 0.15 | |
| Distant metastasis | PRE | 57 | 15 | 0.79 | 0.12 | 22 | 0.84 | 0.18 |
| INTRA | 57 | 15 | 0.63 | 0.15 | 22 | 0.81 | 0.17 | |
| Delta | 57 | 15 | 0.52 | 0.12 | 22 | 0.82 | 0.15 | |
| ALL | 57 | 45 | 0.86 | 0.15 | 52 | 0.88 | 0.13 | |
| Overall survival | PRE | 57 | 15 | 0.62 | 0.18 | 22 | 0.82 | 0.12 |
| INTRA | 57 | 15 | 0.46 | 0.13 | 22 | 0.64 | 0.15 | |
| Delta | 57 | 15 | 0.48 | 0.11 | 22 | 0.66 | 0.15 | |
| ALL | 57 | 45 | 0.53 | 0.17 | 52 | 0.69 | 0.16 | |
| Cox Regression Models | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Features | Patients | Imaging Features | Clinical Parameters + Imaging Features | ||||
| Features | C index | SD | Features | C index | SD | |||
| Locoregional recurrence-free survival | PRE | 57 | 15 | 0.70 | 0.18 | 22 | 0.69 | 0.15 |
| INTRA | 57 | 15 | 0.48 | 0.10 | 22 | 0.48 | 0.10 | |
| Delta | 57 | 15 | 0.75 | 0.14 | 22 | 0.73 | 0.15 | |
| ALL | 57 | 45 | 0.72 | 0.15 | 52 | 0.72 | 0.16 | |
| Distant metastasis | PRE | 57 | 15 | 0.79 | 0.13 | 22 | 0.75 | 0.15 |
| INTRA | 57 | 15 | 0.64 | 0.16 | 22 | 0.64 | 0.14 | |
| Delta | 57 | 15 | 0.52 | 0.14 | 22 | 0.58 | 0.16 | |
| ALL | 57 | 45 | 0.77 | 0.14 | 52 | 0.75 | 0.14 | |
| Overall survival | PRE | 57 | 15 | 0.65 | 0.18 | 22 | 0.75 | 0.15 |
| INTRA | 57 | 15 | 0.48 | 0.12 | 22 | 0.55 | 0.14 | |
| Delta | 57 | 15 | 0.49 | 0.10 | 22 | 0.66 | 0.10 | |
| ALL | 57 | 45 | 0.53 | 0.16 | 52 | 0.62 | 0.15 | |
| Locoregional Recurrence Risk Calculator | Metastasis Risk Calculator | Death Risk Calculator | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Fill-In | Formula | Result | Predictor | Fill-In | Formula | Result | Predictor | Fill-In | Formula | Result |
| PRE-ADC_kurtosis | x | 0.317326 | A | PRE-ADC_kurtosis | x | 1.233603 | A | Gender | x | −0.685436 | A |
| PRE-Kep | x | 0.168844 | B | PRE-SUV_peak | x | 0.167255 | B | HPV | x | −3.196345 | B |
| PRE-SUV_peak | x | 1.469971 | C | N-stage | x | 0.908168 | C | ||||
| Δ-ADC_skewness | x | 0.010764 | D | PRE-ADC_skewness | x | 0.925759 | D | ||||
| Δ-f | x | −0.036035 | E | PRE-D | x | −0.138775 | E | ||||
| Δ-SUV_peak | x | −0.190617 | F | PRE-f | x | −0.277885 | F | ||||
| Δ-TLG | x | −0.008579 | G | PRE-MATV | x | 8.640004 | G | ||||
| PRE-SUV_mean | x | −0.836001 | H | ||||||||
| PRE-SUV_peak | x | 1.885920 | I | ||||||||
| Linear probability | Y = −2.173498 + (Sum A + B + C + D + E + F + G) | Linear probability | Y = −5.950312 + (Sum A + B) | Linear probability | Y = −1.387043+ (Sum A + B + C + D + E + F + G + H + I) | ||||||
| Probability formula | 1/(1 + exp(−Y)) = | Probability formula | 1/(1 + exp(−Y)) = | Probability formula | 1/(1 + exp(−Y)) = | ||||||
| Risk | % | Risk | % | Risk | % | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, R.M.; Koopman, T.; Lavini, C.; Brug, T.v.d.; Zwezerijnen, G.J.C.; Marcus, J.T.; Vergeer, M.R.; Leemans, C.R.; Bree, R.d.; Graaf, P.d.; et al. Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation. Cancers 2022, 14, 216. https://doi.org/10.3390/cancers14010216
Martens RM, Koopman T, Lavini C, Brug Tvd, Zwezerijnen GJC, Marcus JT, Vergeer MR, Leemans CR, Bree Rd, Graaf Pd, et al. Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation. Cancers. 2022; 14(1):216. https://doi.org/10.3390/cancers14010216
Chicago/Turabian StyleMartens, Roland M., Thomas Koopman, Cristina Lavini, Tim van de Brug, Gerben J. C. Zwezerijnen, J. Tim Marcus, Marije R. Vergeer, C. René Leemans, Remco de Bree, Pim de Graaf, and et al. 2022. "Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation" Cancers 14, no. 1: 216. https://doi.org/10.3390/cancers14010216
APA StyleMartens, R. M., Koopman, T., Lavini, C., Brug, T. v. d., Zwezerijnen, G. J. C., Marcus, J. T., Vergeer, M. R., Leemans, C. R., Bree, R. d., Graaf, P. d., Boellaard, R., & Castelijns, J. A. (2022). Early Response Prediction of Multiparametric Functional MRI and 18F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation. Cancers, 14(1), 216. https://doi.org/10.3390/cancers14010216







