Implication of Ceramide Kinase/C1P in Cancer Development and Progression
Abstract
:Simple Summary
Abstract
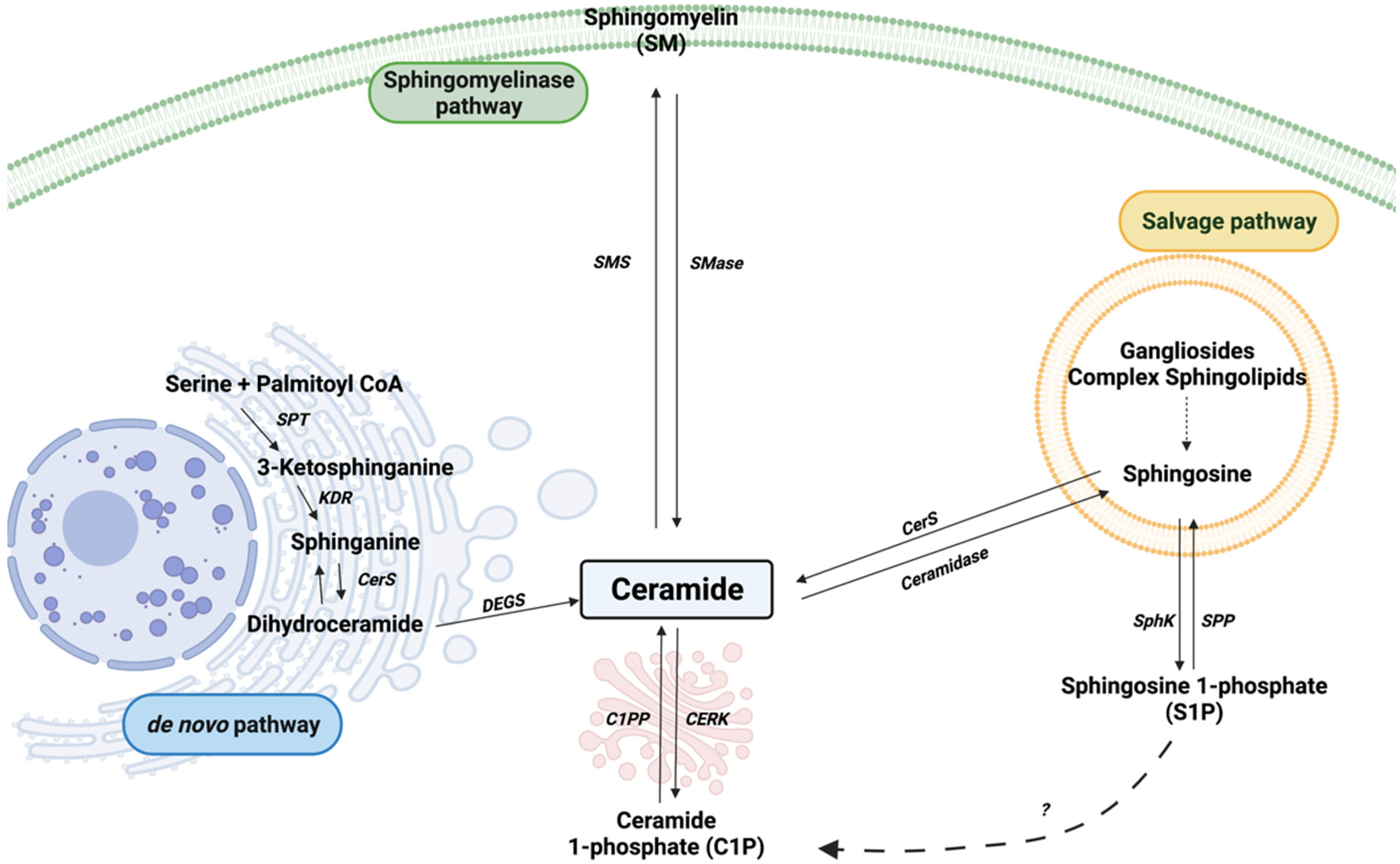
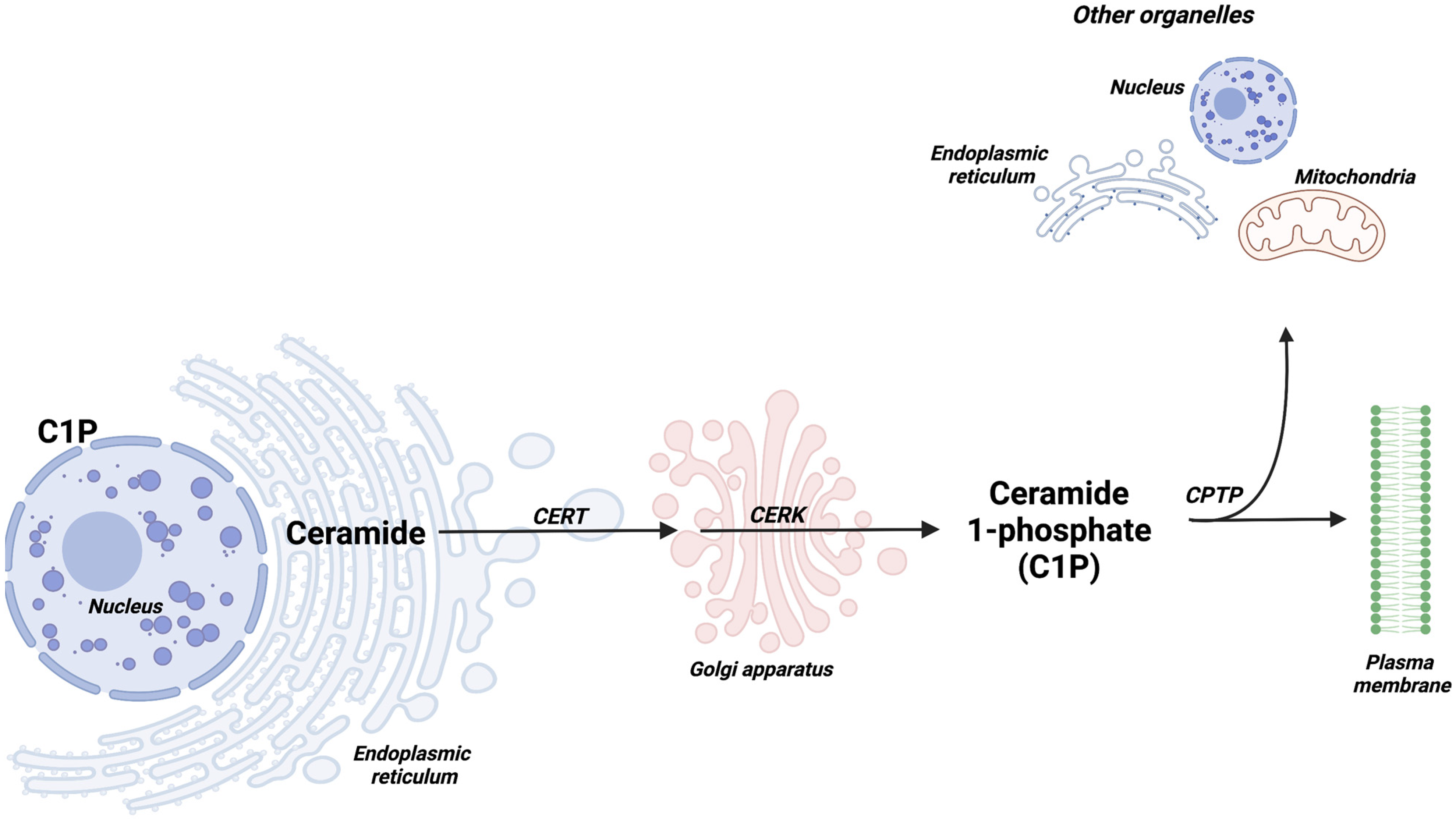
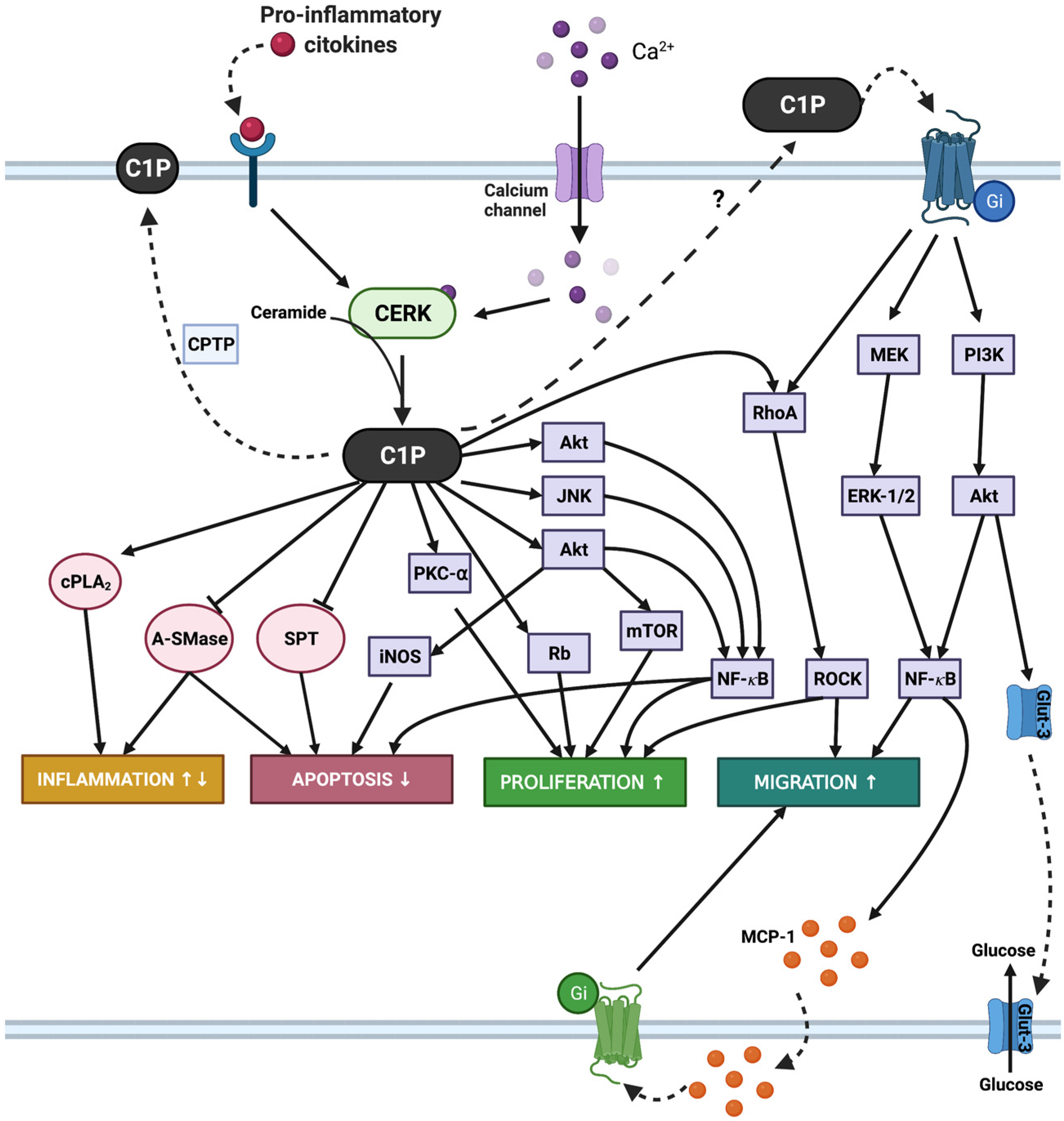
1. Introduction
2. Implication of CERK/C1P in Leukemia Cell Growth and Dissemination
3. Implication of CERK/C1P in Breast Cancer
4. Implication of CERK/C1P in Lung Cancer
5. Implication of CERK/C1P in Neuroblastoma
6. Implication of CERK/C1P in Pancreatic Cancer
7. Implication of CERK/C1P in Prostate Cancer
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bajjalieh, S.M.; Martin, T.F.; Floor, E. Synaptic vesicle ceramide kinase. A calcium-stimulated lipid kinase that co-purifies with brain synaptic vesicles. J. Biol. Chem. 1989, 264, 14354–14360. [Google Scholar] [CrossRef]
- Kolesnick, R.; Hemer, M. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. J. Biol. Chem. 1990, 265, 18803–18808. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, W.-J.; Futerman, A.H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 671–681. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Ventura, A.E.; Kim, J.L.; Silva, L.C.; Futerman, A.H. The role of ceramide in regulating endoplasmic reticulum function. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158489. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.; Kamlekar, R.; Wijesinghe, D.S.; Zou, X.; Zhai, X.; Mishra, S.; Molotkovsky, J.G.; Malinina, L.; Hinchcliffe, E.H.; Chalfant, C.E.; et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 2013, 500, 463–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudker, O.; Futerman, A. Detection and characterization of ceramide-1-phosphate phosphatase activity in rat liver plasma membrane. J. Biol. Chem. 1993, 268, 22150–22155. [Google Scholar] [CrossRef]
- Waggoner, D.W.; Muñoz, A.G.; Dewald, J.; Brindley, D.N. Phosphatidate Phosphohydrolase Catalyzes the Hydrolysis of Ceramide 1-Phosphate, Lysophosphatidate, and Sphingosine 1-Phosphate. J. Biol. Chem. 1996, 271, 16506–16509. [Google Scholar] [CrossRef] [Green Version]
- Mallela, S.K.; Mitrofanova, A.; Merscher, S.; Fornoni, A. Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 158517. [Google Scholar] [CrossRef]
- Muñoz, A.G.; Duffy, P.A.; Martin, A.; O’Brien, L.; Byun, H.S.; Bittman, R.; Brindley, D.N. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: Antagonism by cell-permeable ceramides. Mol. Pharmacol. 1995, 47, 833–839. [Google Scholar]
- Gomez-Muñoz, A.; Frago, L.M.; Alvarez, L.; Varela-Nieto, I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem. J. 1997, 325, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Frago, L.M.; Leon, Y.; de la Rosa, E.; Muñoz, A.G.; Varela-Nieto, I. Nerve growth factor and ceramides modulate cell death in the early developing inner ear. J. Cell Sci. 1998, 111, 549–556. [Google Scholar] [CrossRef]
- Gangoiti, P.; Granado, M.H.; Wang, S.W.; Kong, J.Y.; Steinbrecher, U.P.; Gómez-Muñoz, A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 2008, 20, 726–736. [Google Scholar] [CrossRef]
- Arana, L.; Gangoiti, P.; Ouro, A.; Rivera, I.; Ordoñez, M.; Trueba, M.; Lankalapalli, R.; Bittman, R.; Muñoz, A.G. Generation of reactive oxygen species (ROS) is a key factor for stimulation of macrophage proliferation by ceramide 1-phosphate. Exp. Cell Res. 2012, 318, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, H.M.; Kim, K.P.; Son, Y.; Kim, M.-S.; Park, K.-S. Ceramide kinase regulates the migration of bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2019, 508, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Kang, Y.-J.; Lim, Y.; Lee, H.-W.; Bae, K.; Lee, Y.-S.; Yoo, J.-M.; Yoo, H.-S.; Yun, Y.-P. Ceramide 1-phosphate induces neointimal formation via cell proliferation and cell cycle progression upstream of ERK1/2 in vascular smooth muscle cells. Exp. Cell Res. 2011, 317, 2041–2051. [Google Scholar] [CrossRef]
- Miranda, G.E.; Abrahan, C.E.; Agnolazza, D.L.; Politi, L.E.; Rotstein, N.P. Ceramide-1-Phosphate, a New Mediator of Development and Survival in Retina Photoreceptors. Investig. Opthalmology Vis. Sci. 2011, 52, 6580–6588. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.V.; Spalm, F.H.P.; Vera, M.S.; Rotstein, N.P. Sphingolipids as Emerging Mediators in Retina Degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Pastukhov, O.; Schwalm, S.; Römer, I.; Zangemeister-Wittke, U.; Pfeilschifter, J.; Huwiler, A. Ceramide Kinase Contributes to Proliferation but not to Prostaglandin E2 Formation in Renal Mesangial Cells and Fibroblasts. Cell. Physiol. Biochem. 2014, 34, 119–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangoiti, P.; Granado, M.H.; Arana, L.; Ouro, A.; Gomez-Muñoz, A. Activation of protein kinase C-α is essential for stimulation of cell proliferation by ceramide 1-phosphate. FEBS Lett. 2010, 584, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Gangoiti, P.; Arana, L.; Ouro, A.; Granado, M.H.; Trueba, M.; Gómez-Muñoz, A. Activation of mTOR and RhoA is a major mechanism by which ceramide 1-phosphate stimulates macrophage proliferation. Cell. Signal. 2011, 23, 27–34. [Google Scholar] [CrossRef]
- Gangoiti, P.; Bernacchioni, C.; Donati, C.; Cencetti, F.; Ouro, A.; Gómez-Muñoz, A.; Bruni, P. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie 2012, 94, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Ouro, A.; Arana, L.; Riazy, M.; Zhang, P.; Gomez-Larrauri, A.; Steinbrecher, U.; Duronio, V.; Gomez-Muñoz, A. Vascular endothelial growth factor mediates ceramide 1-phosphate-stimulated macrophage proliferation. Exp. Cell Res. 2017, 361, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bernacchioni, C.; Cencetti, F.; Ouro, A.; Bruno, M.; Gomez-Muñoz, A.; Donati, C.; Bruni, P. Lysophosphatidic Acid Signaling Axis Mediates Ceramide 1-Phosphate-Induced Proliferation of C2C12 Myoblasts. Int. J. Mol. Sci. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierucci, F.; Frati, A.; Battistini, C.; Penna, F.; Costelli, P.; Meacci, E. Control of Skeletal Muscle Atrophy Associated to Cancer or Corticosteroids by Ceramide Kinase. Cancers 2021, 13, 3285. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, A.; Kong, J.Y.; Salh, B.; Steinbrecher, U.P. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 2004, 45, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Granado, M.H.; Gangoiti, P.; Ouro, A.; Arana, L.; Gómez-Muñoz, A. Ceramide 1-phosphate inhibits serine palmitoyltransferase and blocks apoptosis in alveolar macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2009, 1791, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, A.; Kong, J.Y.; Parhar, K.; Wang, S.W.; Gangoiti, P.; González, M.; Eivemark, S.; Salh, B.; Duronio, V.; Steinbrecher, U.P. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005, 579, 3744–3750. [Google Scholar] [CrossRef] [Green Version]
- Gangoiti, P.; Granado, M.H.; Arana, L.; Ouro, A.; Gómez-Muñoz, A. Involvement of nitric oxide in the promotion of cell survival by ceramide 1-phosphate. FEBS Lett. 2008, 582, 2263–2269. [Google Scholar] [CrossRef] [Green Version]
- Pascuali, N.; Scotti, L.; Di Pietro, M.; Oubiña, G.; Bas, D.; May, M.; Muñoz, A.G.; Cuasnicú, P.S.; Cohen, D.J.; Tesone, M.; et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 2018, 33, 844–859. [Google Scholar] [CrossRef]
- Le, Q.; Tabuchi, K.; Hara, A. Ceramide-1-phosphate protection of cochlear hair cells against cisplatin ototoxicity. Toxicol. Rep. 2016, 3, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Mena, H.A.; Zubiry, P.R.; Dizier, B.; Mignon, V.; Parborell, F.; Schattner, M.; Boisson-Vidal, C.; Negrotto, S. Ceramide 1-phosphate protects endothelial colony–forming cells from apoptosis and increases vasculogenesis in vitro and in vivo. Arter. Thromb. Vasc. Biol. 2019, 39, e219–e232. [Google Scholar] [CrossRef] [PubMed]
- León, Y.; Magariños, M.; Varela-Nieto, I. Ceramide Kinase Inhibition Blocks IGF-1-Mediated Survival of Otic Neurosensory Progenitors by Impairing AKT Phosphorylation. Front. Cell Dev. Biol. 2021, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.H.; Gangoiti, P.; Ouro, A.; Arana, L.; González, M.; Trueba, M.; Gómez-Muñoz, A. Ceramide 1-phosphate (C1P) promotes cell migration: Involvement of a specific C1P receptor. Cell. Signal. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Arana, L.; Ordoñez, M.; Ouro, A.; Rivera, I.; Gangoiti, P.; Trueba, M.; Muñoz, A.G. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: Involvement in ceramide 1-phosphate-stimulated cell migration. Am. J. Physiol. Metab. 2013, 304, E1213–E1226. [Google Scholar] [CrossRef]
- Ouro, A.; Arana, L.; Rivera, I.-G.; Ordoñez, M.; Gomez-Larrauri, A.; Presa, N.; Simón, J.; Trueba, M.; Gangoiti, P.; Bittman, R.; et al. Phosphatidic acid inhibits ceramide 1-phosphate-stimulated macrophage migration. Biochem. Pharmacol. 2014, 92, 642–650. [Google Scholar] [CrossRef]
- Kim, C.; Schneider, G.; Abdel-Latif, A.; Mierzejewska, K.; Sunkara, M.; Borkowska, S.; Ratajczak, J.; Morris, A.J.; Kucia, M.; Ratajczak, M.Z. Ceramide-1-Phosphate Regulates Migration of Multipotent Stromal Cells and Endothelial Progenitor Cells—Implications for Tissue Regeneration. Stem Cells 2012, 31, 500–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapetyan, A.V.; Klyachkin, Y.M.; Selim, S.; Sunkara, M.; Ziada, K.M.; Cohen, D.A.; Zuba-Surma, E.K.; Ratajczak, J.; Smyth, S.S.; Ratajczak, M.Z.; et al. Bioactive Lipids and Cationic Antimicrobial Peptides as New Potential Regulators for Trafficking of Bone Marrow-Derived Stem Cells in Patients with Acute Myocardial Infarction. Stem Cells Dev. 2013, 22, 1645–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankins, J.L.; Ward, K.E.; Linton, S.; Barth, B.; Stahelin, R.; Fox, T.E.; Kester, M. Ceramide 1-Phosphate Mediates Endothelial Cell Invasion via the Annexin a2-p11 Heterotetrameric Protein Complex. J. Biol. Chem. 2013, 288, 19726–19738. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.S.; Simón, M.V.; Spalm, F.H.P.; Ayala-Peña, V.B.; German, O.L.; Politi, L.E.; Valtierra, F.X.S.; Rotstein, N.P. Ceramide-1-phosphate promotes the migration of retina Müller glial cells. Exp. Eye Res. 2021, 202, 108359. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Regulation of cell growth, survival and migration by ceramide 1-phosphate—implications in lung cancer progression and inflammation. Cell. Signal. 2021, 83, 109980. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef]
- Presa, N.; Gomez-Larrauri, A.; Dominguez-Herrera, A.; Trueba, M.; Gomez-Muñoz, A. Novel signaling aspects of ceramide 1-phosphate. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158630. [Google Scholar] [CrossRef]
- Graf, C.; Klumpp, M.; Habig, M.; Rovina, P.; Billich, A.; Baumruker, T.; Oberhauser, B.; Bornancin, F. Targeting Ceramide Metabolism with a Potent and Specific Ceramide Kinase Inhibitor. Mol. Pharmacol. 2008, 74, 925–932. [Google Scholar] [CrossRef]
- Pastukhov, O.; Schwalm, S.; Zangemeister-Wittke, U.; Fabbro, D.; Bornancin, F.; Japtok, L.; Kleuser, B.; Pfeilschifter, J.; Huwiler, A. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br. J. Pharmacol. 2014, 171, 5829–5844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumada, H.; Mitsutake, S.; Inagaki, Y.; Mitsunaga, S.; Tsuchikawa, H.; Katsumura, S.; Igarashi, Y. Kinetics of the Ceramide Kinase Inhibitor K1, a Suppressor of Mast-Cell Activation. Biosci. Biotechnol. Biochem. 2007, 71, 2581–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Larrauri, A.; Das Adhikari, U.; Aramburu-Nuñez, M.; Custodia, A.; Ouro, A. Ceramide Metabolism Enzymes—Therapeutic Targets against Cancer. Medicina 2021, 57, 729. [Google Scholar] [CrossRef]
- Chalfant, C.E.; Spiegel, S. Sphingosine 1-phosphate and ceramide 1-phosphate: Expanding roles in cell signaling. J. Cell Sci. 2005, 118, 4605–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berwick, M.L.; Dudley, B.A.; Maus, K.; Chalfant, C.E. The Role of Ceramide 1-Phosphate in Inflammation, Cellular Proliferation, and Wound Healing. Adv. Exp. Med. Biol. 2019, 1159, 65–77. [Google Scholar] [CrossRef]
- Ordoñez, M.; Presa, N.; Trueba, M.; Gomez-Muñoz, A. Implication of Ceramide Kinase in Adipogenesis. Mediat. Inflamm. 2017, 2017, 9374563. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez, M.; Presa, N.; Dominguez-Herrera, A.; Trueba, M.; Gomez-Muñoz, A. Regulation of adipogenesis by ceramide 1-phosphate. Exp. Cell Res. 2018, 372, 150–157. [Google Scholar] [CrossRef]
- Lankalapalli, R.; Ouro, A.; Arana, L.; Muñoz, A.G.; Bittman, R. Caged Ceramide 1-Phosphate Analogues: Synthesis and Properties. J. Org. Chem. 2009, 74, 8844–8847. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Muñoz, A.; Gangoiti, P.; Rivera, I.-G.; Presa, N.; Gomez-Larrauri, A.; Ordoñez, M. Caged ceramide 1-phosphate (C1P) analogs: Novel tools for studying C1P biology. Chem. Phys. Lipids 2016, 194, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kim, C.H.; Abdel-Latif, A.; Schneider, G.; Kucia, M.; Morris, A.J.; Laughlin, M.J.; Ratajczak, J. A novel perspective on stem cell homing and mobilization: Review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 2012, 26, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, L.-P.; Morad, S.A.; Davis, T.S.; MacDougall, M.R.; Kassai, M.; Abdelmageed, N.; Fox, T.E.; Kester, M.; Loughran, T.P.; Abad, J.L.; et al. Chemotherapy selection pressure alters sphingolipid composition and mitochondrial bioenergetics in resistant HL-60 cells. J. Lipid Res. 2019, 60, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Gangoiti, P.; Presa, N.; Dominguez-Herrera, A.; Donati, C.; Bruni, P.; Trueba, M.; Gomez-Muñoz, A.; Ouro, A. Phosphatidic Acid Stimulates Myoblast Proliferation through Interaction with LPA1 and LPA2 Receptors. Int. J. Mol. Sci. 2021, 22, 1452. [Google Scholar] [CrossRef]
- Fukami, K.; Furuhashi, K.; Inagaki, M.; Endo, T.; Hatano, S.; Takenawa, T. Requirement of phosphatidylinositol 4,5-bisphosphate for α-actinin function. Nature 1992, 359, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.N.; Fox, T.E.; Kester, M.; Jefferson, L.S.; Kimball, S.R. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am. J. Physiol. Physiol. 2010, 299, C335–C344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sliva, D.; Harvey, K.; Mason, R.; Lloyd, F.; English, D. Effect of Phosphatidic Acid on Human Breast Cancer Cells Exposed to Doxorubicin. Cancer Investig. 2001, 19, 783–790. [Google Scholar] [CrossRef]
- Foster, D.A.; Salloum, D.; Menon, D.; Frias, M.A. Phospholipase D and the Maintenance of Phosphatidic Acid Levels for Regulation of Mammalian Target of Rapamycin (mTOR). J. Biol. Chem. 2014, 289, 22583–22588. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Cambronero, J. Phosphatidic acid, phospholipase D and tumorigenesis. Adv. Biol. Regul. 2014, 54, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Andresen, B.; Rizzo, M.A.; Shome, K.; Romero, G. The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade. FEBS Lett. 2002, 531, 65–68. [Google Scholar] [CrossRef]
- Kim, S.C.; Wang, X. Essays in Biochemistry; Portland Press Ltd.: London, UK, 2020; pp. 533–546. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Payne, A.W.; Pant, D.K.; Pan, T.-C.; Chodosh, L.A. Ceramide Kinase Promotes Tumor Cell Survival and Mammary Tumor Recurrence. Cancer Res. 2014, 74, 6352–6363. [Google Scholar] [CrossRef] [Green Version]
- Bhadwal, P.; Dahiya, D.; Shinde, D.; Vaiphei, K.; Math, R.G.H.; Randhawa, V.; Agnihotri, N. LC-HRMS based approach to identify novel sphingolipid biomarkers in breast cancer patients. Sci. Rep. 2020, 10, 4668. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, S.; Erhardt, M.; Römer, I.; Pfeilschifter, J.; Zangemeister-Wittke, U.; Huwiler, A. Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt. Int. J. Mol. Sci. 2020, 21, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruckhäberle, E.; Karn, T.; Rody, A.; Hanker, L.; Gätje, R.; Metzler, D.; Holtrich, U.; Kaufmann, M. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 1005–1013. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, Y.; Wang, L.; Liao, S.; Wang, Y.; Shi, M.; Tu, Y.; Zhou, Y.; Wei, W. Ceramide kinase mediates intrinsic resistance and inferior response to chemotherapy in triple-negative breast cancer by upregulating Ras/ERK and PI3K/Akt pathways. Cancer Cell Int. 2021, 21, 42. [Google Scholar] [CrossRef]
- Gomez-Muñoz, A. The Role of Ceramide 1-Phosphate in Tumor Cell Survival and Dissemination. Adv. Cancer Res. 2018, 140, 217–234. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Tu, M.; Xu, L.; Wei, X.; Miao, Y. How to Establish a Solitary and Localized VX2 Lung Cancer Rabbit Model? A Simple and Effective Intrapulmonary Tumor Implantation Technique. J. Surg. Res. 2009, 154, 284–292. [Google Scholar] [CrossRef]
- Schiller, J.H.; Harrington, D.; Belani, C.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Nakashima, S.; Banno, Y.; Yamakawa, H.; Hayashi, K.; Takenaka, K.; Nishimura, Y.; Sakai, N.; Nozawa, Y. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000, 7, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-H.; Chen, H.H.-W.; Chang, J.-Y.; Luo, S.-J.; Li, C.-L.; Chen, C.-L.; Su, W.-C.; Lin, C.-F. Inhibiting glucosylceramide synthase facilitates the radiosensitizing effects of vinorelbine in lung adenocarcinoma cells. Cancer Lett. 2014, 349, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.G.; Han, T.-Y.; Liu, Y.Y.; Hansen, N.; Giuliano, A.E.; Cabot, M.C. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother. Pharmacol. 2001, 47, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Sandalcioglu, I.E.; Wagner, M.; Weller, M.; Gulbins, E. Lysosomal ceramide mediates gemcitabine-induced death of glioma cells. J. Mol. Med. 2009, 87, 1123–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Yoshimura, S.-I.; Sawada, M.; Naganawa, T.; Iwama, T.; Nakashima, S.; Sakai, N. Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells. J. Neuro-Oncol. 2001, 52, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Maceyka, M.; Payne, S.G.; Lamour, N.; Milstien, S.; Chalfant, C.E.; Spiegel, S. Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett. 2007, 581, 735–740. [Google Scholar] [CrossRef]
- Schneider, G.; Sellers, Z.P.; Bujko, K.; Kakar, S.S.; Kucia, M.; Ratajczak, M.Z. Novel pleiotropic effects of bioactive phospholipids in human lung cancer metastasis. Oncotarget 2017, 8, 58247–58263. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, S.; Tamori, M.; Tanaka, A.; Utsumi, N.; Sato, H.; Hatakeyama, H.; Hisaka, A.; Kohama, T.; Yamagata, K.; Honda, T.; et al. Inhibitory effects of ceramide kinase on Rac1 activation, lamellipodium formation, cell migration, and metastasis of A549 lung cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158675. [Google Scholar] [CrossRef]
- Kato, N.; Harada, M.; Yamashiro, K. Kaposi’s Sarcoma Associated with Lung Cancer and Immunosuppression. J. Dermatol. 1996, 23, 564–571. [Google Scholar] [CrossRef]
- Nwabudike, S.M.; Hemmings, S.; Paul, Y.; Habtegebriel, Y.; Polk, O.; Mehari, A. Pulmonary Kaposi Sarcoma: An Uncommon Cause of Respiratory Failure in the Era of Highly Active Antiretroviral Therapy—Case Report and Review of the Literature. Case Rep. Infect. Dis. 2016, 2016, 9354136. [Google Scholar] [CrossRef] [Green Version]
- Hadi, L.A.; Calcaterra, F.; Brambilla, L.; Carenza, C.; Marfia, G.; Della Bella, S.; Riboni, L. Enhanced phosphorylation of sphingosine and ceramide sustains the exuberant proliferation of endothelial progenitors in Kaposi sarcoma. J. Leukoc. Biol. 2018, 103, 525–533. [Google Scholar] [CrossRef]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Chiarugi, P.; Fiaschi, T. Redox signalling in anchorage-dependent cell growth. Cell. Signal. 2007, 19, 672–682. [Google Scholar] [CrossRef]
- Leslie, N.R. The Redox Regulation of PI 3-Kinase–Dependent Signaling. Antioxid. Redox Signal. 2006, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Blanchetot, C.; Boonstra, J. The ROS-NOX Connection in Cancer and Angiogenesis. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Bollinger, N.; Weber, T.J.; Tan, R.M.; Markillie, L.M.; Karin, N.J. Reactive oxygen species alter autocrine and paracrine signaling. Free Radic. Biol. Med. 2011, 51, 2041–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, B.M.; Gustafson, S.J.; Hankins, J.L.; Kaiser, J.M.; Haakenson, J.K.; Kester, M.; Kuhn, T.B. Ceramide kinase regulates TNFα-stimulated NADPH oxidase activity and eicosanoid biosynthesis in neuroblastoma cells. Cell. Signal. 2012, 24, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ito, H.; Hagiwara, K.; Yoshida, K.; Sobue, S.; Ichihara, M.; Takagi, A.; Kojima, T.; Tanaka, K.; Tamiya-Koizumi, K.; et al. ATRA inhibits ceramide kinase transcription in a human neuroblastoma cell line, SH-SY5Y cells: The role of COUP-TFI. J. Neurochem. 2010, 112, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, S.; Yokose, U.; Kato, M.; Matsuoka, I.; Yoo, J.-M.; Kim, T.-J.; Yoo, H.-S.; Fujimoto, K.; Ando, Y.; Sugiura, M.; et al. The generation and behavioral analysis of ceramide kinase-null mice, indicating a function in cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 2007, 363, 519–524. [Google Scholar] [CrossRef]
- Bini, F.; Frati, A.; Garcia-Gil, M.; Battistini, C.; Granado, M.; Martinesi, M.; Mainardi, M.; Vannini, E.; Luzzati, F.; Caleo, M.; et al. New signalling pathway involved in the anti-proliferative action of vitamin D3 and its analogues in human neuroblastoma cells. A role for ceramide kinase. Neuropharmacology 2012, 63, 524–537. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. In Proceedings of the The Lancet; Elsevier: Amsterdam, The Netherlands, 2011; Volume 378, pp. 607–620. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.; Ordoñez, M.; Presa, N.; Gangoiti, P.; Gomez-Larrauri, A.; Trueba, M.; Fox, T.; Kester, M.; Muñoz, A.G. Ceramide 1-phosphate regulates cell migration and invasion of human pancreatic cancer cells. Biochem. Pharmacol. 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Stoica, A.-F.; Chang, C.-H.; Pauklin, S. Molecular Therapeutics of Pancreatic Ductal Adenocarcinoma: Targeted Pathways and the Role of Cancer Stem Cells. Trends Pharmacol. Sci. 2020, 41, 977–993. [Google Scholar] [CrossRef]
- Kuc, N.; Doermann, A.; Shirey, C.; Lee, D.D.; Lowe, C.-W.; Awasthi, N.; Schwarz, R.E.; Stahelin, R.V.; Schwarz, M.A. Pancreatic ductal adenocarcinoma cell secreted extracellular vesicles containing ceramide-1-phosphate promote pancreatic cancer stem cell motility. Biochem. Pharmacol. 2018, 156, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culig, Z.; Santer, F. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014, 33, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Saranyutanon, S.; Srivastava, S.K.; Pai, S.; Singh, S.; Singh, A.P. Therapies Targeted to Androgen Receptor Signaling Axis in Prostate Cancer: Progress, Challenges, and Hope. Cancers 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansinho, A.; Macedo, D.; Fernandes, I.; Costa, L. Castration-Resistant Prostate Cancer: Mechanisms, Targets and Treatment. In Retinal Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1096, pp. 117–133. [Google Scholar]
- Eto, M.; Bennouna, J.; Hunter, O.C.; Hershberger, P.A.; Kanto, T.; Johnson, C.S.; Lotze, M.T.; Amoscato, A.A. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate 2003, 57, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, J.; Wittka, A.; Leonetti, D.; Roy, V.V.; Estephan, H.; Maier, P.; Reis, H.; Herskind, C.; Jendrossek, V.; Paris, F.; et al. Caveolin-1 regulates the ASMase/ceramide-mediated radiation response of endothelial cells in the context of tumor–stroma interactions. Cell Death Dis. 2020, 11, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsel, L.; Zaidel, G.; Drumgoole, H.M.; Jelovac, D.; Drachenberg, C.; Rhee, J.G.; Brodie, A.M.; Bielawska, A.; Smyth, M.J. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate 2004, 58, 382–393. [Google Scholar] [CrossRef]
- Camacho, L.; Zabala-Letona, A.; Cortazar, A.R.; Astobiza, I.; Dominguez-Herrera, A.; Ercilla, A.; Crespo, J.; Viera, C.; Fernández-Ruiz, S.; Martinez-Gonzalez, A.; et al. Identification of Androgen Receptor Metabolic Correlome Reveals the Repression of Ceramide Kinase by Androgens. Cancers 2021, 13, 4307. [Google Scholar] [CrossRef]
- Wilson, S.; Qi, J.; Filipp, F.V. Refinement of the androgen response element based on ChIP-Seq in androgen-insensitive and androgen-responsive prostate cancer cell lines. Sci. Rep. 2016, 6, 32611. [Google Scholar] [CrossRef]
- Wu, L.; Runkle, C.; Jin, H.; Li, J.; Yang, X.; Kuzel, T.; Lee, C.; Yu, J. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene 2013, 33, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho, L.; Ouro, A.; Gomez-Larrauri, A.; Carracedo, A.; Gomez-Muñoz, A. Implication of Ceramide Kinase/C1P in Cancer Development and Progression. Cancers 2022, 14, 227. https://doi.org/10.3390/cancers14010227
Camacho L, Ouro A, Gomez-Larrauri A, Carracedo A, Gomez-Muñoz A. Implication of Ceramide Kinase/C1P in Cancer Development and Progression. Cancers. 2022; 14(1):227. https://doi.org/10.3390/cancers14010227
Chicago/Turabian StyleCamacho, Laura, Alberto Ouro, Ana Gomez-Larrauri, Arkaitz Carracedo, and Antonio Gomez-Muñoz. 2022. "Implication of Ceramide Kinase/C1P in Cancer Development and Progression" Cancers 14, no. 1: 227. https://doi.org/10.3390/cancers14010227






