Hyperfractionated Treatment with 177Lu-Octreotate Increases Tumor Response in Human Small-Intestine Neuroendocrine GOT1 Tumor Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiopharmaceutical
2.2. Animal Experiments
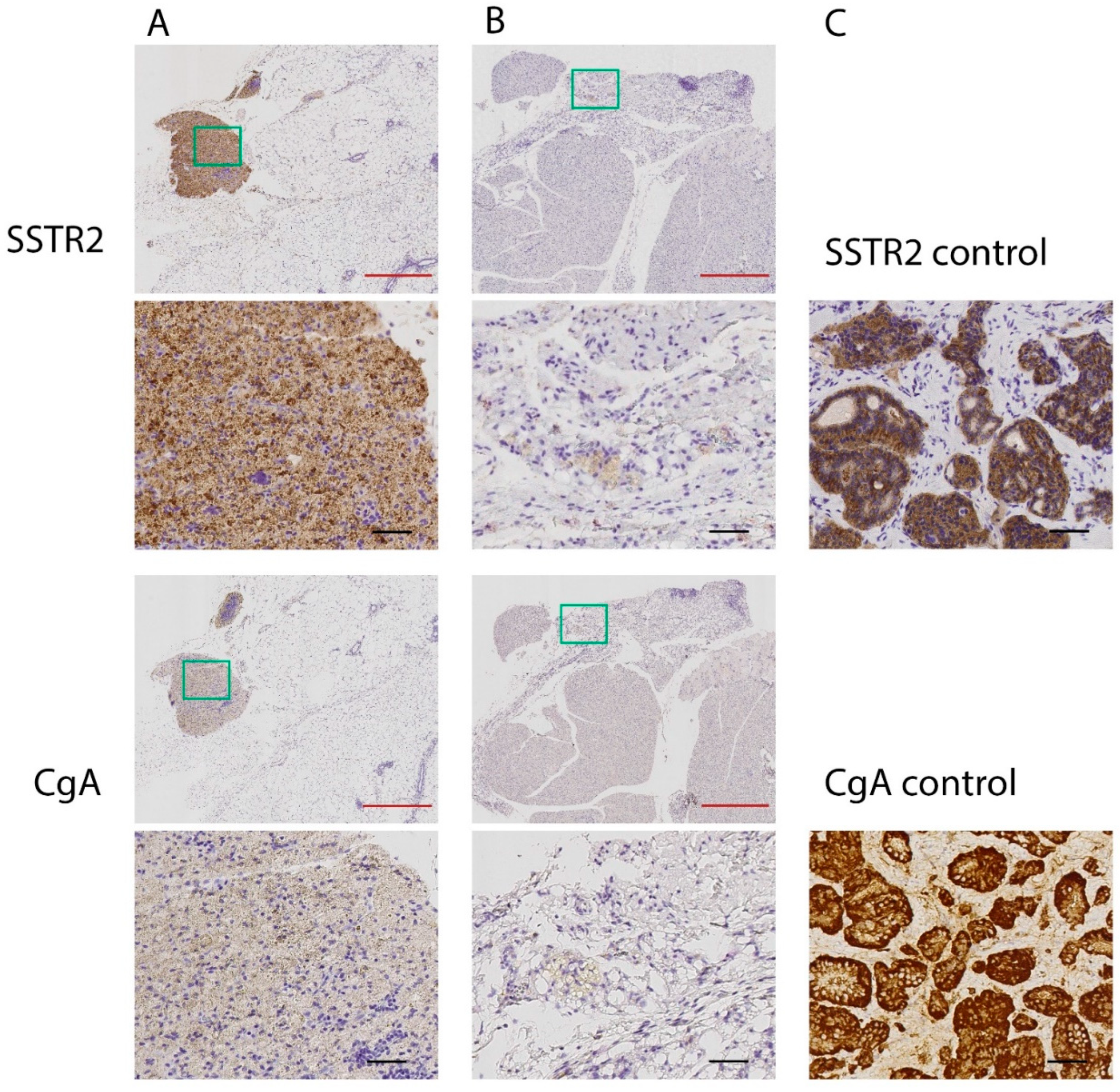
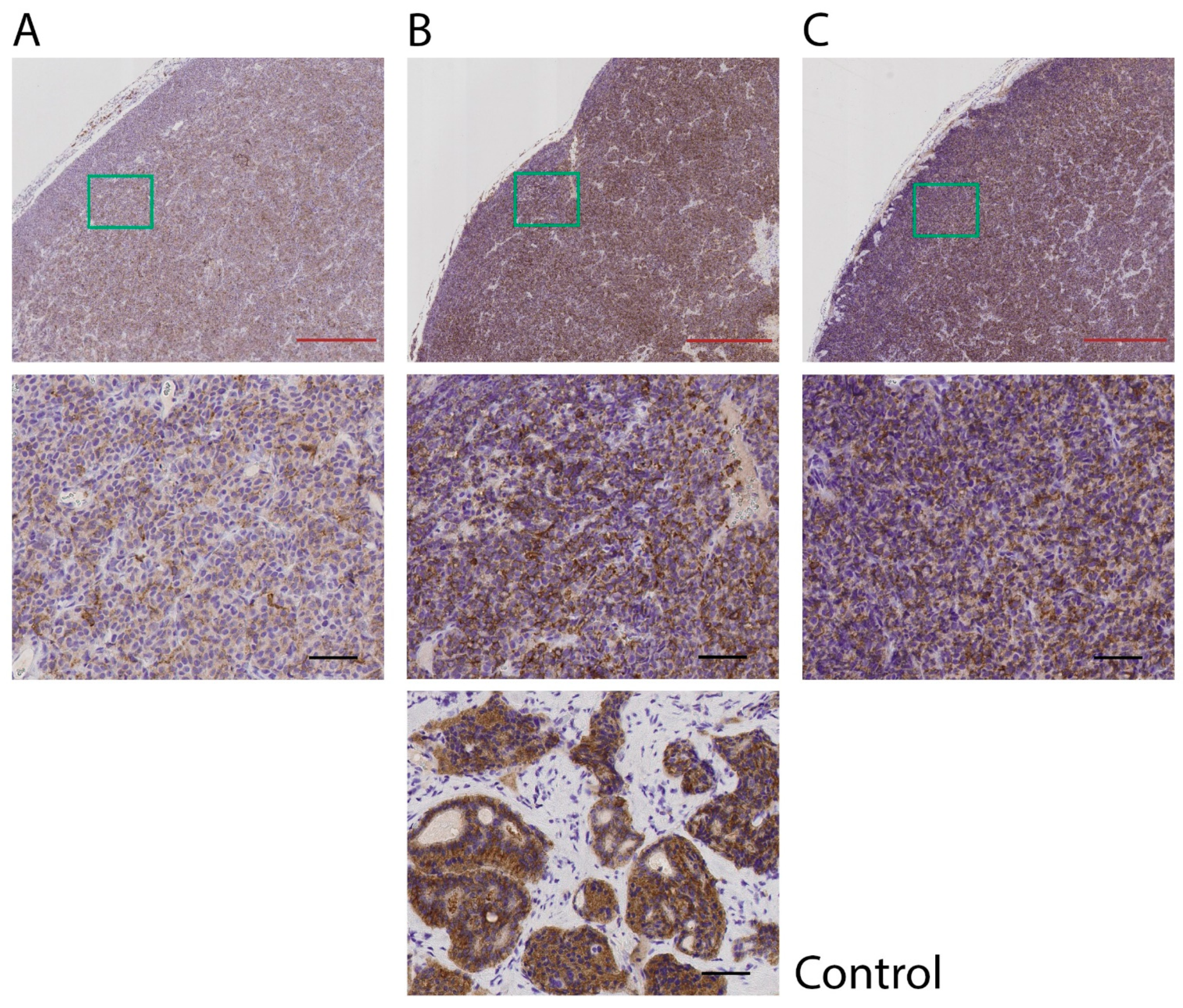
2.3. Immunohistochemical Analyses of SSTR2 and CgA (IHC)
2.4. Data Analysis
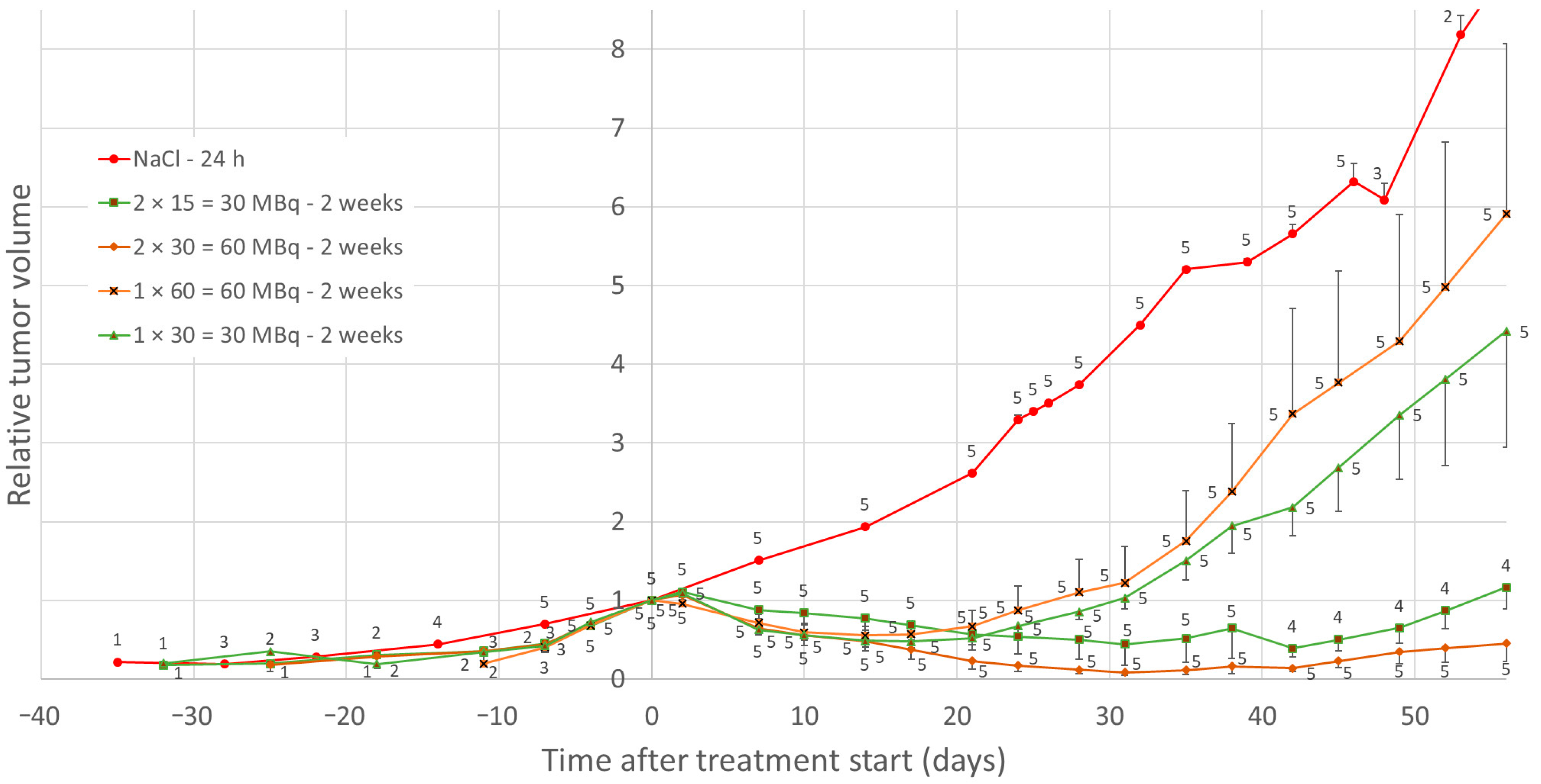
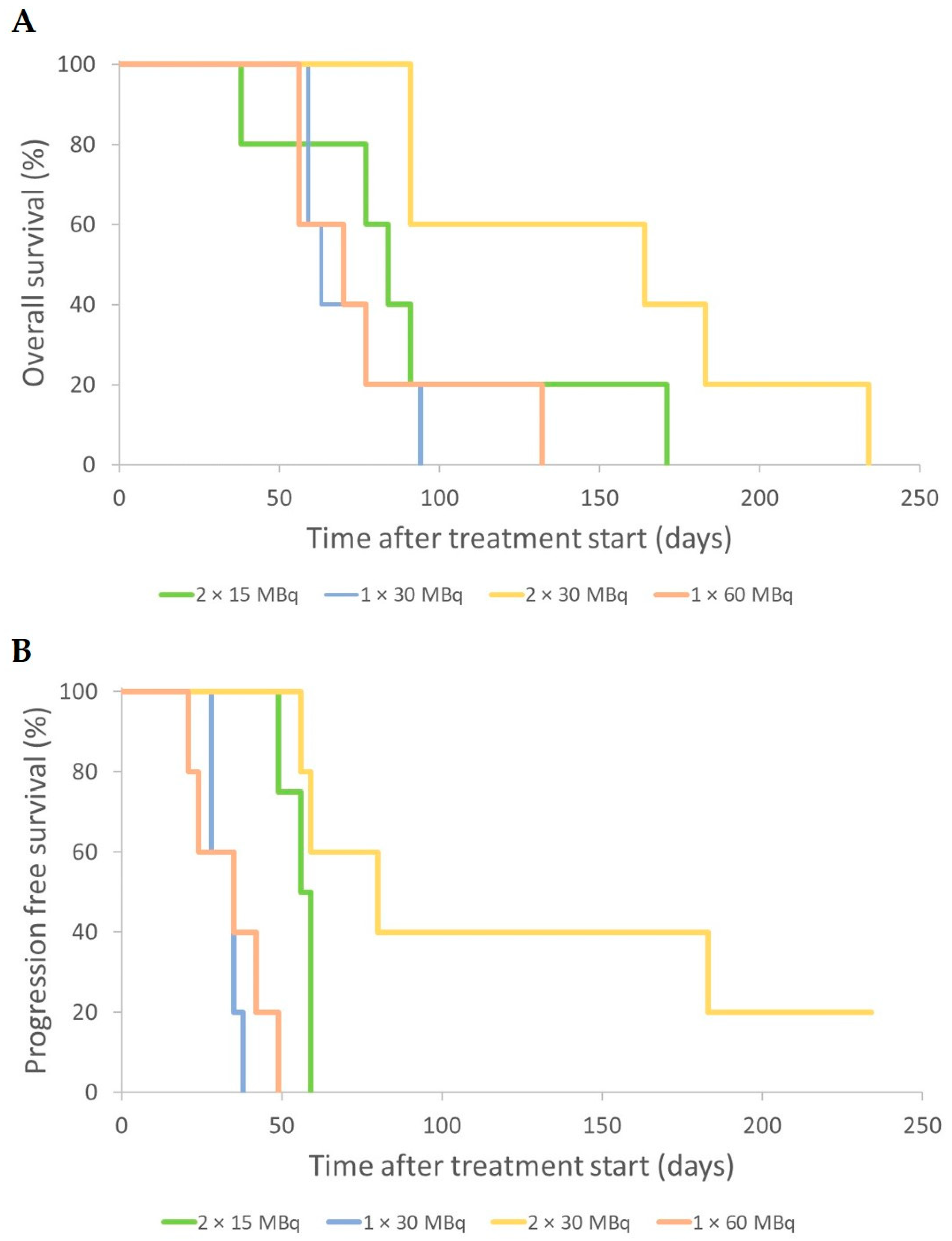
3. Results
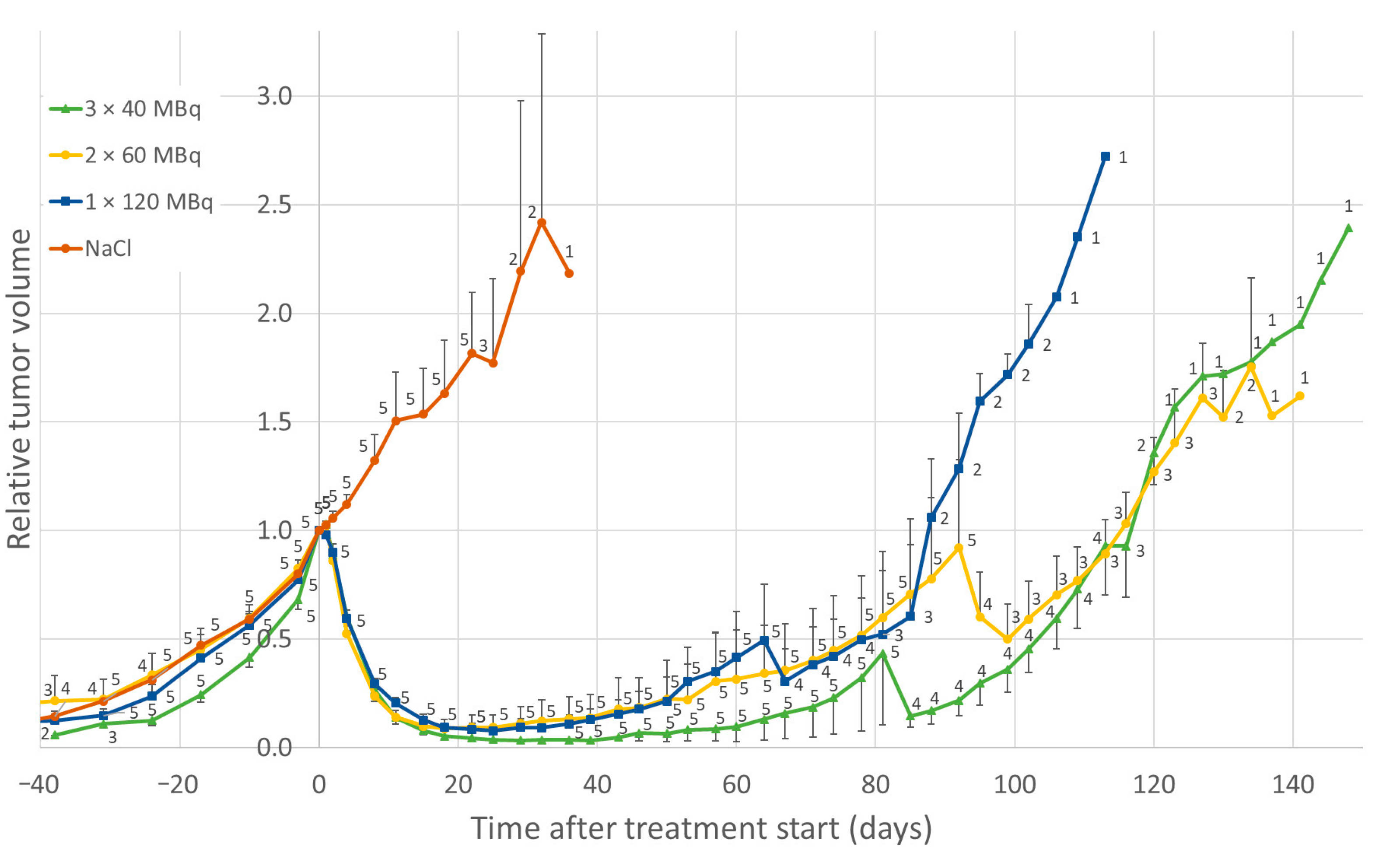
3.1. Effects of Low-Medium Total Amounts of 177Lu-Octreotate (30–60 MBq)
3.2. Effect of High Total Amounts of 177Lu-Octreotate (120 MBq)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LUTATHERA®. Summary of Product Characteristics European Medicines Agency: European Public Assessment Report (EPAR); 2018 [Updated 27 January 2021]. Available online: http://www.ema.europa.eu (accessed on 9 November 2021).
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.; van Eijck, C.H.; Valkema, R.; de Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr.-Relat. Cancer 2010, 17, R53–R73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; de Herder, W.W.; Feelders, R.A.; van Eijck, C.H.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol. 2005, 23, 2754–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of (177)Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Bernhardt, P.; Svensson, J.B.; Wängberg, B.; Ahlman, H.; Forssell-Aronsson, E. Estimation of absorbed dose to the kidneys in patients after treatment with 177Lu-octreotate: Comparison between methods based on planar scintigraphy. EJNMMI Res. 2012, 2, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swärd, C.; Bernhardt, P.; Ahlman, H.; Wängberg, B.; Forssell-Aronsson, E.; Larsson, M.; Svensson, J.; Rossi-Norrlund, R.; Kölby, L. [177Lu-DOTA 0-Tyr 3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: The value of measuring absorbed dose to the kidney. World J. Surg. 2010, 34, 1368–1372. [Google Scholar] [CrossRef]
- De Jong, M.; Breeman, W.A.P.; Bernard, B.F.; Bakker, W.H.; Schaar, M.; van Gameren, A.; Bugaj, J.E.; Erion, J.; Schmidt, M.; Srinivasan, A.; et al. [177Lu-DOTA0,Tyr3]octreotate for somatostatin receptor-targeted radionuclide therapy. Int. J. Cancer 2001, 92, 628–633. [Google Scholar] [CrossRef]
- Kölby, L.; Bernhardt, P.; Johanson, V.; Schmitt, A.; Ahlman, H.; Forssell-Aronsson, E.; Macke, H.; Nilsson, O. Successful receptor-mediated radiation therapy of xenografted human midgut carcinoid tumour. Br. J. Cancer 2005, 93, 1144–1151. [Google Scholar] [CrossRef] [Green Version]
- Elf, A.K.; Bernhardt, P.; Hofving, T.; Arvidsson, Y.; Forssell-Aronsson, E.; Wängberg, B.; Nilsson, O.; Johanson, V. NAMPT Inhibitor GMX1778 Enhances the Efficacy of 177Lu-DOTATATE Treatment of Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kölby, L.; Maecke, H.R.; Forssell-Aronsson, E. Radiation therapy of small cell lung cancer with 177Lu-DOTA-Tyr3-octreotate in an animal model. J. Nucl. Med. 2004, 45, 1542–1548. [Google Scholar] [PubMed]
- Nilsson, O.N.; Arvidsson, Y.; Johanson, V.; Forssell-Aronsson, E.; Ahlman, H. New medical strategies for midgut carcinoids. Anti-Cancer Agents Med. Chem. 2010, 10, 250–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forssell-Aronsson, E.; Spetz, J.; Ahlman, H. Radionuclide therapy via SSTR: Future aspects from experimental animal studies. Neuroendocrinology 2013, 97, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Österlund, A.; Forssell-Aronsson, E. The amount of injected 177Lu-octreotate strongly influences biodistribution and dosimetry in C57BL/6N mice. Acta Oncol. 2016, 55, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, P.; Kölby, L.; Johanson, V.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Biodistribution of 111in-DTPA-D-Phe1-octreotide in tumor-bearing nude mice: Influence of amount injected and route of administration. Nucl. Med. Biol. 2003, 30, 253–260. [Google Scholar] [CrossRef]
- Andersson, P.; Forssell-Aronsson, E.; Johanson, V.; Wängberg, B.; Nilsson, O.; Fjälling, M.; Ahlman, H. Internalization of indium-111 into human neuroendocrine tumor cells after incubation with indium-111-DTPA-D-Phe1-octreotide. J. Nucl. Med. 1996, 37, 2002–2006. [Google Scholar]
- Lesche, S.; Lehmann, D.; Nagel, F.; Schmid, H.A.; Schulz, S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J. Clin. Endocrinol. Metab. 2009, 94, 654–661. [Google Scholar] [CrossRef]
- Spetz, J.; Dalmo, J.; Nilsson, O.; Wängberg, B.; Ahlman, H.; Forssell-Aronsson, E. Specific binding and uptake of 131I-MIBG and 111In-octreotide in metastatic paraganglioma--tools for choice of radionuclide therapy. Horm. Metab. Res. 2012, 44, 400–404. [Google Scholar] [CrossRef]
- Waser, B.; Tamma, M.L.; Cescato, R.; Maecke, H.R.; Reubi, J.C. Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J. Nucl. Med. 2009, 50, 936–941. [Google Scholar] [CrossRef] [Green Version]
- Hofland, L.J.; Lamberts, S.W. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr. Rev. 2003, 24, 28–47. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kolby, L.; Forssell-Aronsson, E. Differences in biodistribution between 99mTc-depreotide, 111In-DTPA-octreotide, and 177Lu-DOTA-Tyr3-octreotate in a small cell lung cancer animal model. Cancer Biother. Radiopharm. 2005, 20, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breeman, W.A.; Kwekkeboom, D.J.; Kooij, P.P.; Bakker, W.H.; Hofland, L.J.; Visser, T.J.; Ensing, G.J.; Lamberts, S.W.; Krenning, E.P. Effect of dose and specific activity on tissue distribution of indium-111-pentetreotide in rats. J. Nucl. Med. 1995, 36, 623–627. [Google Scholar] [PubMed]
- Dalmo, J.; Rudqvist, N.; Spetz, J.; Laverman, P.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Biodistribution of 177Lu-octreotate and 111In-minigastrin in female nude mice transplanted with human medullary thyroid carcinoma GOT2. Oncol. Rep. 2012, 27, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabet, A.; Nagarajah, J.; Dogan, A.S.; Biersack, H.J.; Sabet, A.; Guhlke, S.; Ezziddin, S. Does PRRT with standard activities of 177Lu-octreotate really achieve relevant somatostatin receptor saturation in target tumor lesions? Insights from intra-therapeutic receptor imaging in patients with metastatic gastroenteropancreatic neuroendocrine tumors. EJNMMI Res. 2013, 3, 82. [Google Scholar]
- Andersson, P.; Forssell-Aronsson, E.; Grétarsdóttir, J.; Johanson, V.; Wängberg, B.; Nilsson, O.; Fjälling, M.; Ahlman, H. Biokinetics and dosimetry after repeated injections of 111In-DTPA-D-Phe1-octreotide. In Proceedings of the Sixth International Radiopharmaceutical Dosimetry Symposium, ORISE 99-0164, Gatlinburg, TN, USA, 7–10 May 1996; Volume 1, pp. 126–136. [Google Scholar]
- Bernhardt, P.; Oddstig, J.; Kölby, L.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Effects of treatment with (177)Lu-DOTA-Tyr(3)-octreotate on uptake of subsequent injection in carcinoid-bearing nude mice. Cancer Biother. Radiopharm. 2007, 22, 644–653. [Google Scholar] [CrossRef]
- Oddstig, J.; Bernhardt, P.; Lizana, H.; Nilsson, O.; Ahlman, H.; Kölby, L.; Forssell-Aronsson, E. Inhomogeneous activity distribution of 177Lu-DOTA0-Tyr3-octreotate and effects on somatostatin receptor expression in human carcinoid GOT1 tumors in nude mice. Tumour Biol. 2012, 33, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Oddstig, J.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Radiation induces up-regulation of somatostatin receptors 1, 2, and 5 in small cell lung cancer in vitro also at low absorbed doses. Cancer Biother. Radiopharm. 2011, 26, 759–765. [Google Scholar] [CrossRef]
- Oddstig, J.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl. Med. Biol. 2006, 33, 841–846. [Google Scholar] [CrossRef]
- Dalmo, J.; Spetz, J.; Montelius, M.; Langen, B.; Arvidsson, Y.; Johansson, H.; Parris, T.Z.; Helou, K.; Wängberg, B.; Nilsson, O.; et al. Priming increases the anti-tumor effect and therapeutic window of (177)Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1. EJNMMI Res. 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Spetz, J.; Langen, B.; Rudqvist, N.P.; Parris, T.Z.; Shubbar, E.; Dalmo, J.; Wängberg, B.; Nilsson, O.; Helou, K.; Forssell-Aronsson, E. Transcriptional effects of (177)Lu-octreotate therapy using a priming treatment schedule on GOT1 tumor in nude mice. EJNMMI Res. 2019, 9, 28. [Google Scholar] [CrossRef]
- Kölby, L.; Bernhardt, P.; Ahlman, H.; Wängberg, B.; Johanson, V.; Wigander, A.; Forssell-Aronsson, E.; Karlsson, S.; Ahrén, B.; Stenman, G.; et al. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am. J. Pathol. 2001, 158, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Cullinane, C.; Jeffery, C.M.; Roselt, P.D.; van Dam, E.M.; Jackson, S.; Kuan, K.; Jackson, P.; Binns, D.; van Zuylekom, J.; Harris, M.J.; et al. Peptide Receptor Radionuclide Therapy with (67)Cu-CuSarTATE Is Highly Efficacious Against a Somatostatin-Positive Neuroendocrine Tumor Model. J. Nucl. Med. 2020, 61, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Romiani, A.; Spetz, J.; Shubbar, E.; Lind, D.E.; Hallberg, B.; Palmer, R.H.; Forssell-Aronsson, E. Neuroblastoma xenograft models demonstrate the therapeutic potential of (177)Lu-octreotate. BMC Cancer 2021, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Forrer, F.; Capello, A.; Bijster, M.; Bernard, B.F.; Reubi, J.C.; Krenning, E.P.; de Jong, M. Up-regulation of somatostatin receptor density on rat CA20948 tumors escaped from low dose [177Lu-DOTA0,Tyr3]octreotate therapy. Q. J. Nucl. Med. Mol. Imaging 2007, 51, 324–333. [Google Scholar] [PubMed]
- Pencharz, D.; Walker, M.; Yalchin, M.; Quigley, A.-M.; Caplin, M.; Toumpanakis, C.; Navalkissoor, S. Early efficacy of and toxicity from lutetium-177-DOTATATE treatment in patients with progressive metastatic NET. Nucl. Med. Commun. 2017, 38, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Carpizo, D.R.; Harris, C.R. Genetic Drivers of Ileal Neuroendocrine Tumors. Cancers 2021, 13, 5070. [Google Scholar] [CrossRef]
- Nilsson, O.; Kölby, L.; Bernhardt, P.; Forssell-Aronsson, E.; Johanson, V.; Ahlman, H. GOT1 xenografted to nude mice: A unique model for in vivo studies on SSTR-mediated radiation therapy of carcinoid tumors. Ann. N. Y. Acad. Sci. 2004, 1014, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Montelius, M.; Jalnefjord, O.; Spetz, J.; Nilsson, O.; Forssell-Aronsson, E.; Ljungberg, M. Multiparametric MR for non-invasive evaluation of tumour tissue histological characteristics after radionuclide therapy. NMR Biomed. 2019, 32, e4060. [Google Scholar] [CrossRef]


| Total Activity | Activity | Time Apart | Tumor Volume [mm3] | Group Size | ||
|---|---|---|---|---|---|---|
| 1st Injection | 2nd Injection | 3rd Injection | ||||
| 30 MBq | 15 MBq | 15 MBq | - | 2 weeks | 520 ± 70 | n = 5 |
| 30 MBq | 30 MBq | NaCl | - | 2 weeks | 530 ± 50 | n = 5 |
| 60 MBq | 30 MBq | 30 MBq | - | 2 weeks | 400 ± 30 | n = 5 |
| 60 MBq | 60 MBq | NaCl | - | 2 weeks | 350 ± 30 | n = 5 |
| 120 MBq | 60 MBq | 60 MBq | NaCl | 1 week | 1100 ± 40 | n = 3 |
| 120 MBq | 40 MBq | 40 MBq | 40 MBq | 24 h | 1400 ± 90 | n = 5 |
| 120 MBq | 60 MBq | 60 MBq | NaCl | 24 h | 1300 ± 80 | n = 5 |
| 120 MBq | 120 MBq | NaCl | NaCl | 24 h | 1600 ± 90 | n = 5 |
| Mock treatment | NaCl | NaCl | NaCl | 24 h | 1300 ± 90 | n = 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvborn, M.; Shubbar, E.; Forssell-Aronsson, E. Hyperfractionated Treatment with 177Lu-Octreotate Increases Tumor Response in Human Small-Intestine Neuroendocrine GOT1 Tumor Model. Cancers 2022, 14, 235. https://doi.org/10.3390/cancers14010235
Elvborn M, Shubbar E, Forssell-Aronsson E. Hyperfractionated Treatment with 177Lu-Octreotate Increases Tumor Response in Human Small-Intestine Neuroendocrine GOT1 Tumor Model. Cancers. 2022; 14(1):235. https://doi.org/10.3390/cancers14010235
Chicago/Turabian StyleElvborn, Mikael, Emman Shubbar, and Eva Forssell-Aronsson. 2022. "Hyperfractionated Treatment with 177Lu-Octreotate Increases Tumor Response in Human Small-Intestine Neuroendocrine GOT1 Tumor Model" Cancers 14, no. 1: 235. https://doi.org/10.3390/cancers14010235







