Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
2.3. ALP
- Measurements were made at pre and posttreatment BS, showing an increased extent and/or new hot lesion. The interval between examination of BS and ALP was less than 10 days;
- The difference between baseline and follow-up ALP was marked as ∆ALP; ∆ALP = follow-up ALP (at first follow-up BS)—baseline ALP (at pretreatment BS);
- “∆ALP ratio” was defined as the ratio of ∆ALP to baseline ALP; ∆ALP ratio = “∆ALP”/“baseline ALP” × 100 (%);
- Given the Hitachi Automatic Analyzer has 90–110% accuracy, “increased ALP” was defined as an increase in ∆ALP ratio of more than 10% of baseline;
- “Decreased ALP” was defined as a decrease in ∆ALP ratio of more than 10% of baseline;
- “Stable ALP” was defined as the remainder, being neither increased ALP nor decreased ALP.
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
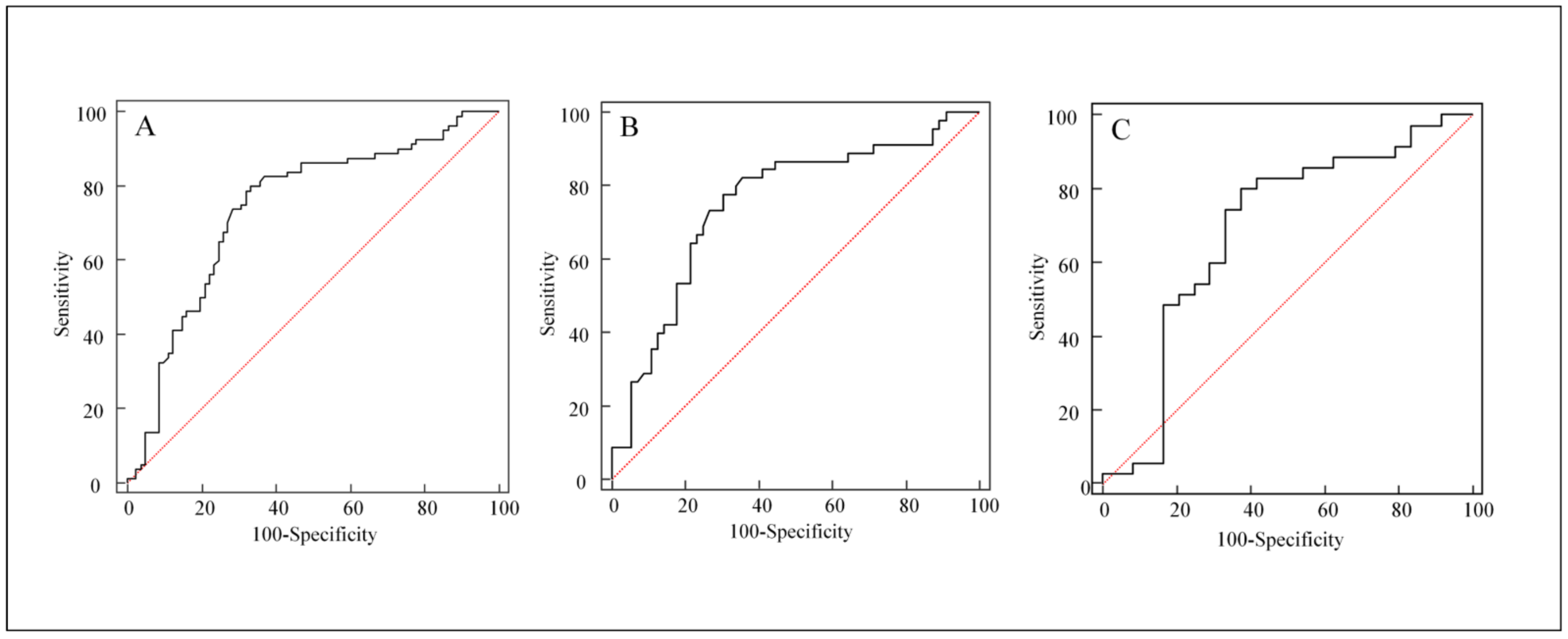
3.2. Univariate Analyses for Distinguishing FP from Disease Progression
3.3. Multivariate Logistic Regression Analysis for Occurrence of FP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.O.; Jacobsen, J.B.; Norgaard, M.; Yong, M.; Fryzek, J.P.; Sorensen, H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Carlin, B.I.; Andriole, G.L. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer 2000, 88, 2989–2994. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Mashiter, G.; Whitaker, K.B.; Moss, D.W.; Rubens, R.D.; Fogelman, I. Bone scan flare predicts successful systemic therapy for bone metastases. J. Nucl. Med. 1988, 29, 1354–1359. [Google Scholar] [PubMed]
- Bauerle, T.; Semmler, W. Imaging response to systemic therapy for bone metastases. Eur. Radiol. 2009, 19, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.S.; Chang, C.P.; Chiu, C.H.; Chu, L.S.; Chen, Y.M.; Tsai, C.M. Bone scan flare phenomenon in non-small-cell lung cancer patients treated with gefitinib. Clin. Nucl. Med. 2009, 34, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Raghavan, M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Control 2012, 19, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.H.; Chen, C.Y.; Ku, C.H.; Janckila, A.J.; Yam, L.T.; Yu, J.C.; Chuang, K.W.; Chao, T.Y. The semiquantitative bone scintigraphy index correlates with serum tartrate-resistant acid phosphatase activity in breast cancer patients with bone metastasis. Mayo. Clin. Proc. 2007, 82, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Matsumoto, S.; Takahashi, S.; Yamashita, T.; Ogata, E. Bone metabolic markers in the evaluation of bone scan flare phenomenon in bone metastases of breast cancer. Clin. Nucl. Med. 1999, 24, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, S.B.; Ceylan, E.; Serter, M.; Karadağ, F.; Demir, E.; Çildağ, O. The clinical importance of bone metabolic markers in detecting bone metastasis of lung cancer. Int. J. Clin. Oncol. 2012, 17, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Pollen, J.J.; Witztum, K.F.; Ashburn, W.L. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am. J. Roentgenol. 1984, 142, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Whitaker, K.B.; Moss, D.W.; Mashiter, G.; Fogelman, I.; Rubens, R.D. Biochemical prediction of response of bone metastases to treatment. Br. J. Cancer 1988, 58, 205–210. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.; Chow, E.; Rowbottom, L.; DeAngelis, C.; Soliman, H. Incidence of pain flare in radiation treatment of bone metastases: A literature review. J. Bone Oncol. 2014, 3, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubley, G.J. Is the flare phenomenon clinically significant? Urology 2001, 58, 5–9. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Andersen, S.; Arnason, S.; Arner, S.; Breivik, H.; Heiskanen, T.; Kalso, E.; Kongsgaard, U.E.; Sjogren, P.; Strang, P.; et al. Breakthrough pain in malignant and non-malignant diseases: A review of prevalence, characteristics and mechanisms. Eur. J. Pain 2005, 9, 195–206. [Google Scholar] [CrossRef]
- Vassiliou, V.; Kalogeropoulou, C.; Giannopoulou, E.; Leotsinidis, M.; Tsota, I.; Kardamakis, D. A novel study investigating the therapeutic outcome of patients with lytic, mixed and sclerotic bone metastases treated with combined radiotherapy and ibandronate. Clin. Exp. Metastasis 2007, 24, 169–178. [Google Scholar] [CrossRef]
- Ryan, C.J.; Shah, S.; Efstathiou, E.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Logothetis, C.J.; Kheoh, T.; Kilian, C.; Haqq, C.M.; et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res. 2011, 17, 4854–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.J.; Autio, K.A.; Basch, E.M.; Danila, D.C.; Larson, S.; Scher, H.I. Monitoring the clinical outcomes in advanced prostate cancer: What imaging modalities and other markers are reliable? Semin. Oncol. 2013, 40, 375–392. [Google Scholar] [CrossRef]
- Clamp, A.; Danson, S.; Nguyen, H.; Cole, D.; Clemons, M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004, 5, 607–616. [Google Scholar] [CrossRef]
- Koizumi, M.; Ogata, E. Bone metabolic markers as gauges of metastasis to bone: A review. Ann. Nucl. Med. 2002, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Delmas, P.D. Markers of bone turnover in bone metastases. Cancer 2000, 88, 2952–2960. [Google Scholar] [CrossRef]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Han, K.S.; Hong, S.J. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J. Cancer Res. Clin. Oncol. 2014, 140, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Mashiter, G.; Fogelman, I.; Whitaker, K.D.; Caleffi, M.; Moss, D.W.; Rubens, R.D. Osteocalcin: A potential marker of metastatic bone disease and response to treatment. Eur. J. Cancer Clin. Oncol. 1988, 24, 1211–1217. [Google Scholar] [CrossRef]
- Wong, K.K.; Piert, M. Dynamic bone imaging with 99mTc-labeled diphosphonates and 18F-NaF: Mechanisms and applications. J. Nucl. Med. 2013, 54, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Aminian, A.; Karimian, F.; Mirsharifi, R.; Alibakhshi, A.; Hasani, S.M.; Dashti, H.; Jahangiri, Y.; Ghaderi, H.; Meysamie, A. Correlation of serum alkaline phosphatase with clinicopathological characteristics of patients with oesophageal cancer. East Mediterr. Health J. 2011, 17, 862–866. [Google Scholar] [CrossRef]
- Kh, C.; Kpyati, A.; Murthy Ds, J. Significance of Serum Total Alkaline Phosphatase Levels in Breast Cancer. Int. J. Clin. Biomed. Res. 2016, 2, 13–15. [Google Scholar]
- Mayne, P.D.; Thakrar, S.; Rosalki, S.B.; Foo, A.Y.; Parbhoo, S. Identification of bone and liver metastases from breast cancer by measurement of plasma alkaline phosphatase isoenzyme activity. J. Clin. Pathol. 1987, 40, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Demers, L.M.; Costa, L.; Lipton, A. Biochemical markers and skeletal metastases. Cancer 2000, 88, 2919–2926. [Google Scholar] [CrossRef]
- Katagiri, H.; Takahashi, M.; Wakai, K.; Sugiura, H.; Kataoka, T.; Nakanishi, K. Prognostic factors and a scoring system for patients with skeletal metastasis. J. Bone Jt. Surg. Br. 2005, 87, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Anan, K.; Yamamoto, Y.; Higaki, K.; Tanaka, M.; Shibuta, K.; Sagara, Y.; Ohno, S.; Tsuyuki, S.; Mase, T.; et al. Efficacy of goserelin plus anastrozole in premenopausal women with advanced or recurrent breast cancer refractory to an LH-RH analogue with tamoxifen: Results of the JMTO BC08-01 phase II trial. Oncol. Rep. 2013, 29, 1707–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, A.M.; Mennel, R.G.; Georgoulias, V.A.; Nabholtz, J.-M.; Erazo, A.; Lluch, A.; Vogel, C.L.; Kaufmann, M.; von Minckwitz, G.; Henderson, I.C.; et al. Randomized Phase III Trial of Pegylated Liposomal Doxorubicin Versus Vinorelbine or Mitomycin C Plus Vinblastine in Women with Taxane-Refractory Advanced Breast Cancer. J. Clin. Oncol. 2004, 22, 3893–3901. [Google Scholar] [CrossRef]
- Hwang, C. Overcoming docetaxel resistance in prostate cancer: A perspective review. Ther. Adv. Med. Oncol. 2012, 4, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinholes, J.; Coleman, R.; Eastell, R. Effects of bone metastases on bone metabolism: Implications for diagnosis, imaging and assessment of response to cancer treatment. Cancer Treat. Rev. 1996, 22, 289–331. [Google Scholar] [CrossRef]
- Leeming, D.J.; Koizumi, M.; Byrjalsen, I.; Li, B.; Qvist, P.; Tankó, L.B. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol. Biomark. Prev. 2006, 15, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Garnero, P.; Buchs, N.; Zekri, J.; Rizzoli, R.; Coleman, R.E.; Delmas, P.D. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br. J. Cancer 2000, 82, 858–864. [Google Scholar] [CrossRef]
- Nakajima, K.; Edenbrandt, L.; Mizokami, A. Bone scan index: A new biomarker of bone metastasis in patients with prostate cancer. Int. J. Urol. 2017, 24, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Mizokami, A.; Matsuyama, H.; Ichikawa, T.; Kaneko, G.; Takahashi, S.; Shiina, H.; Horikoshi, H.; Hashine, K.; Sugiyama, Y.; et al. Prognosis of patients with prostate cancer and bone metastasis from the Japanese Prostatic Cancer Registry of Standard Hormonal and Chemotherapy Using Bone Scan Index cohort study. Int. J. Urol. 2021, 28, 955–963. [Google Scholar] [CrossRef]
- Higashiyama, S.; Yoshida, A.; Kawabe, J. Study of the Usefulness of Bone Scan Index Calculated From 99m-Technetium- Hydroxymethylene Diphosphonate ((99m)Tc-HMDP) Bone Scintigraphy for Bone Metastases from Prostate Cancer Using Deep Learning Algorithms. Curr. Med. Imaging 2021, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Anand, A.; Edenbrandt, L.; Bondesson, E.; Bjartell, A.; Widmark, A.; Sternberg, C.N.; Pili, R.; Tuvesson, H.; Nordle, Ö.; et al. Phase 3 Assessment of the Automated Bone Scan Index as a Prognostic Imaging Biomarker of Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 944–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H.; Nakajima, K.; Mizokami, A.; Namiki, M.; Inaki, A.; Taki, J.; Kinuya, S. Bone scintigraphy as a new imaging biomarker: The relationship between bone scan index and bone metabolic markers in prostate cancer patients with bone metastases. Ann. Nucl. Med. 2013, 27, 802–807. [Google Scholar] [CrossRef] [PubMed]
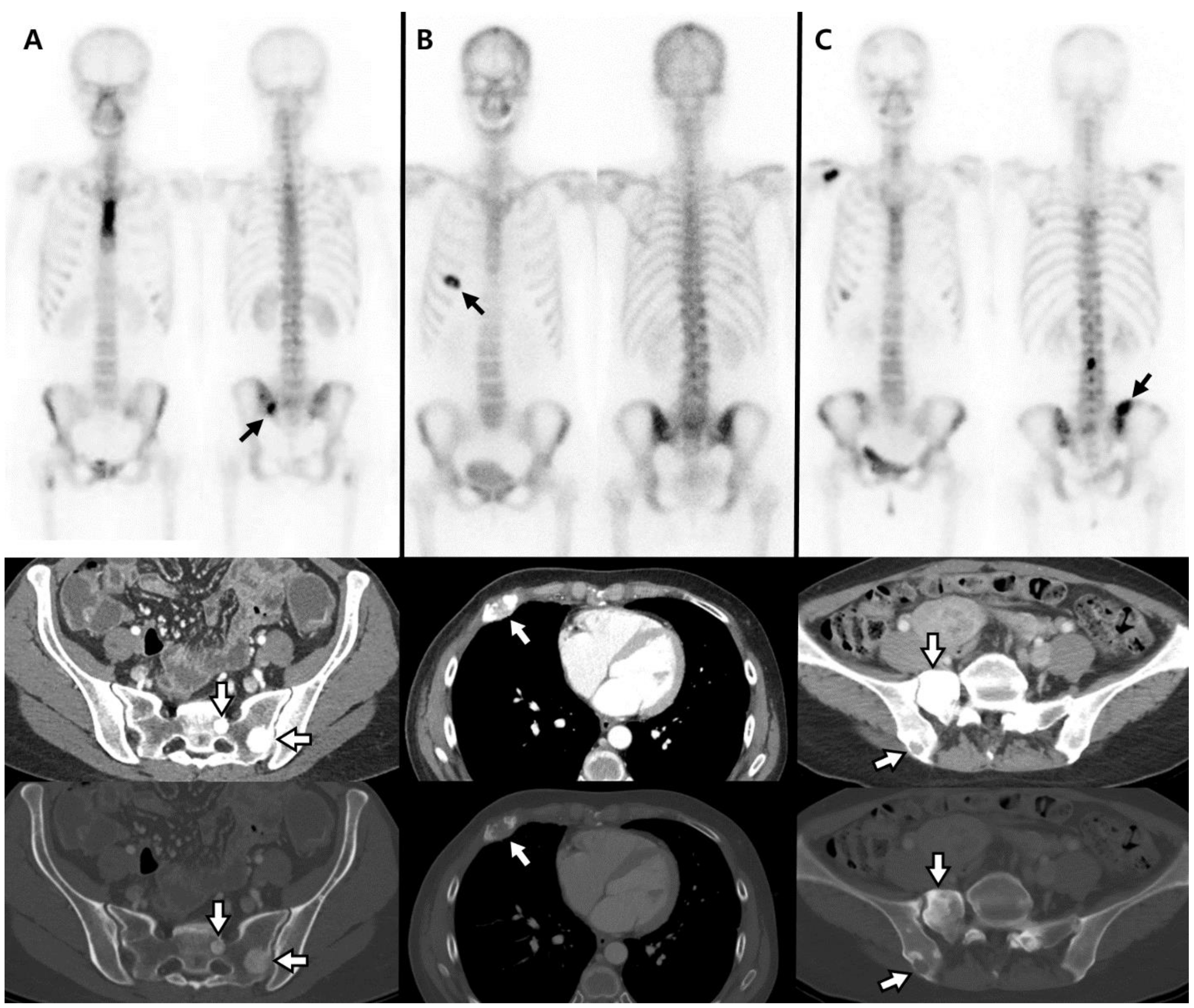
| Characteristic | Total N = 160 | Breast N = 101 | Prostate N = 59 | p Value |
|---|---|---|---|---|
| Age (years, range) | 58 (32–84) | 54 (32–82) | 69 (47–84) | p < 0.0001 |
| Clinical factor (%) | ||||
| At diagnosis of primary tumor | 30 (18.6) | 18 (17.8) | 12 (20.3) | p = 0.7322 |
| Extraosseous metastasis | 59 (36.6) | 51 (50.5) | 8 (13.6) | p < 0.0001 |
| On study therapy (%) | ||||
| Chemotherapy | 98 (61.3) | 61 (60.4) | 37 (62.7) | p = 0.7724 |
| Endocrine therapy | 64 (40.0) | 37 (36.6) | 27 (45.8) | p = 0.2569 |
| Bisphosphonate | 57 (35.6) | 48 (47.5) | 9 (15.3) | p < 0.0001 |
| HER2-targeted therapy | 22 (21.8) | – | ||
| Time interval (months, range) | ||||
| From pretreatment BS to treatment | 1 (0–4) | 1 (0–4) | 1 (0–4) | p = 0.7798 |
| From treatment to posttreatment BS | 2 (0–6) | 2 (0–6) | 2 (0–6) | p = 0.3793 |
| Radiological pattern (%) | ||||
| Osteosclerotic | 113 (70.6) | 63 (62.4) | 50 (84.7) | p = 0.0086 |
| Osteolytic | 15 (9.4) | 11 (10.9) | 4 (6.8) | |
| Mixed | 32 (20.2) | 27 (26.7) | 5 (8.5) | |
| Response to treatment (%) | ||||
| Flare phenomenon | 80 (50.0) | 45 (44.6) | 35 (59.3) | p = 0.0724 |
| Progression | 80 (50.0) | 56 (55.4) | 24 (40.7) | |
| ALP (IU/L, range) | ||||
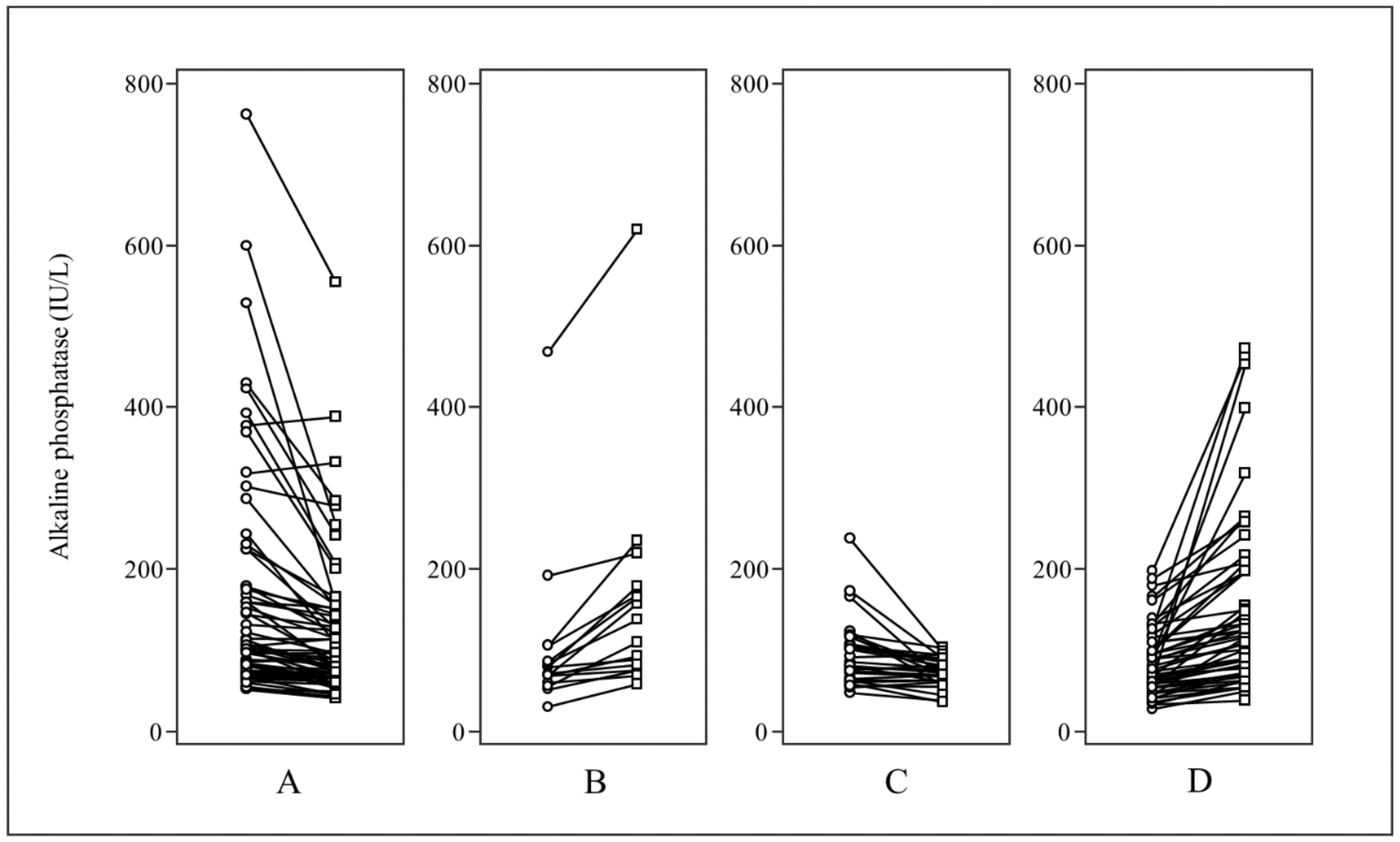
| Baseline ALP | 89.5 (28–762) | 75 (28–600) | 113 (61–762) | p < 0.0001 |
| Follow-up ALP | 89.5 (36–619) | 77 (36–475) | 114 (56–619) | p < 0.0001 |
| Receptor (%) | ||||
| Estrogen receptor-positive | 76 (75.2) | – | ||
| Progesterone receptor-positive | 57 (56.4) | – | ||
| HER2-positive | 29 (28.7) | – | ||
| Gleason score (%) | ||||
| 6 | – | 4 (6.8) | ||
| 7 | – | 5 (8.5) | ||
| 8 | – | 17 (28.8) | ||
| 9 | – | 22 (37.3) | ||
| 10 | – | 7 (11.9) | ||
| Unknown | – | 4 (6.8) |
| Variables | Flare | Progression | p Value |
|---|---|---|---|
| N = 80 | N = 80 | ||
| Clinical factors | |||
| Age (years, range) | 60 (32–81) | 56 (33–84) | p = 0.2836 |
| Extraosseous metastasis (%) | 33 (41.3) | 26 (32.5) | p = 0.2529 |
| Prior therapy (%) | 66 (82.5) | 65 (81.3) | p = 0.8379 |
| Combination therapy (%) | 45 (56.3) | 46 (57.5) | p = 0.8736 |
| Treatment regimen (%) | |||
| Chemotherapy | 50 (62.5) | 48 (60.0) | p = 0.7463 |
| Endocrine therapy | 29 (36.3) | 35 (43.8) | p = 0.3344 |
| Bisphosphonate | 26 (32.5) | 31 (38.8) | p = 0.4106 |
| Patterns of scintigraphic change (%) | |||
| Extent | 67 (83.8) | 64 (80.0) | p = 0.5394 |
| New lesion | 44 (55.0) | 41 (51.3) | p = 0.6357 |
| Number of aggravated lesions (%) | |||
| Multiple change | 50 (62.5) | 46 (57.5) | p = 0.5199 |
| Location of aggravated lesions (%) | |||
| Skull | 9 (11.3) | 15 (18.8) | p = 0.1854 |
| Spines | 45 (56.3) | 48 (60.0) | p = 0.6318 |
| Chest cage | 49 (61.3) | 41 (51.3) | p = 0.2038 |
| Pelvis | 52 (65.0) | 40 (50.0) | p = 0.0557 |
| Extremities | 16 (20.0) | 16 (20.0) | p = 1.0000 |
| Radiologic patterns (%) | |||
| Osteosclerotic | 55 (68.8) | 58 (72.5) | p = 0.7239 |
| Osteolytic | 7 (8.8) | 8 (10.0) | |
| Mixed | 18 (22.5) | 14 (17.5) | |
| ALP | |||
| Baseline ALP (IU/L, range) | 100 (30 –762) | 81 (28–239) | p = 0.0013 |
| Follow-up ALP (IU/L, range) | 90 (42–619) | 88 (36–475) | p = 0.6159 |
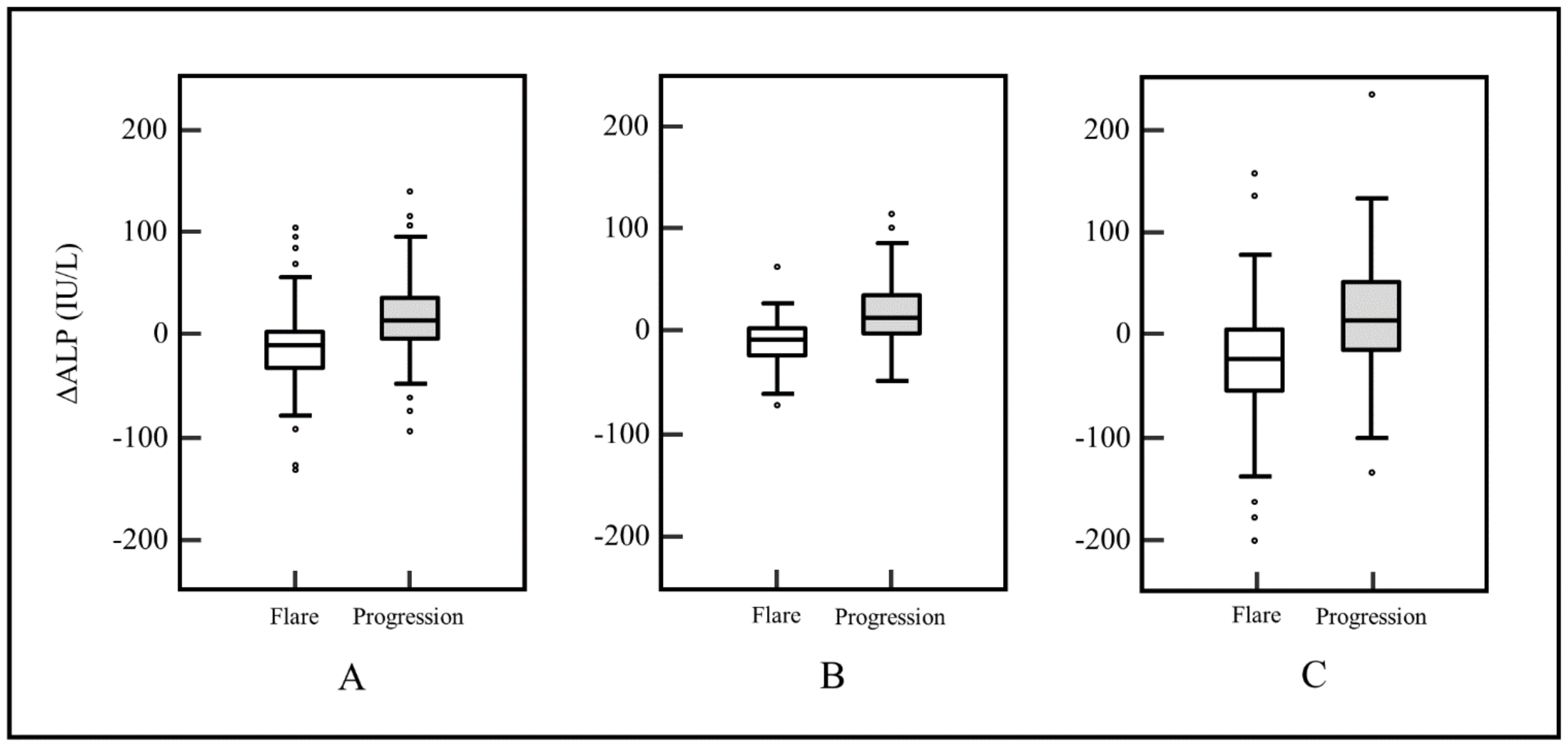
| ∆ALP (IU/L, range) | −11 (−373–150) | 15 (−140–393) | p < 0.0001 |
| ∆ALP ratio (range) | −10% (−71–132%) | 19% (−60–644%) | p < 0.0001 |
| Stable or decreased ALP level (%) | 64 (80.0) | 29 (36.3) | p < 0.0001 |
| Variable | Estimate | Standard Error | Wald χ2 | p Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Clinical factor | ||||||
| Age > 60 | 0.32492 | 0.43791 | 0.5505 | 0.4581 | 1.3839 | 0.5866–3.2649 |
| Extraosseous metastasis | 0.60738 | 0.42946 | 2.0002 | 0.1573 | 1.8356 | 0.7911–4.2593 |
| Prior therapy | 0.17686 | 0.49504 | 0.1276 | 0.7209 | 1.1935 | 0.4523–3.1492 |
| Combination therapy | −0.26917 | 0.55802 | 0.2327 | 0.6295 | 0.7640 | 0.2559–2.2808 |
| Treatment regimen | ||||||
| Chemotherapy | −0.78687 | 1.01881 | 0.5965 | 0.4399 | 0.4553 | 0.0618–3.3535 |
| Hormone therapy | −0.77529 | 1.08234 | 0.5131 | 0.4738 | 0.4606 | 0.0552–3.8424 |
| Bisphosphonate | −0.15488 | 0.53587 | 0.08354 | 0.7726 | 0.8565 | 0.2996–2.4484 |
| Bone scan pattern | ||||||
| Extent | 0.52625 | 0.62111 | 0.7179 | 0.3968 | 1.6926 | 0.5010–5.7180 |
| New lesion | 0.49799 | 0.51028 | 0.9524 | 0.3291 | 1.6454 | 0.6052–4.4733 |
| Number of aggravated lesions | ||||||
| Multiple change | −0.55776 | 0.61424 | 0.8246 | 0.3639 | 0.5725 | 0.1718–1.9082 |
| Location of aggravated lesions | ||||||
| Chest cage | 0.88091 | 0.46871 | 3.5323 | 0.0602 | 2.4131 | 0.9629–6.0471 |
| Spines | 0.28031 | 0.47020 | 0.3554 | 0.5511 | 1.3235 | 0.5266–3.3264 |
| Pelvis | 0.65158 | 0.48411 | 1.8116 | 0.1783 | 1.9186 | 0.7428–4.9552 |
| Extremities | 0.69215 | 0.56456 | 1.5030 | 0.2202 | 1.9980 | 0.6607–6.0417 |
| Skull | −1.05102 | 0.63274 | 2.7591 | 0.0967 | 0.3496 | 0.1011–1.2082 |
| Radiologic pattern | 0.47916 | 0.36753 | 1.6997 | 0.1923 | 1.6147 | 0.7857–3.3185 |
| Stable or decreased ALP | 2.36373 | 0.44600 | 28.0881 | <0.0001 | 10.6305 | 4.4352–25.4800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-h.; Hong, C.-M.; Jo, I.; Jeong, S.-Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy. Cancers 2022, 14, 254. https://doi.org/10.3390/cancers14010254
Jung J-h, Hong C-M, Jo I, Jeong S-Y, Lee S-W, Lee J, Ahn B-C. Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy. Cancers. 2022; 14(1):254. https://doi.org/10.3390/cancers14010254
Chicago/Turabian StyleJung, Ji-hoon, Chae-Moon Hong, Il Jo, Shin-Young Jeong, Sang-Woo Lee, Jaetae Lee, and Byeong-Cheol Ahn. 2022. "Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy" Cancers 14, no. 1: 254. https://doi.org/10.3390/cancers14010254
APA StyleJung, J.-h., Hong, C.-M., Jo, I., Jeong, S.-Y., Lee, S.-W., Lee, J., & Ahn, B.-C. (2022). Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy. Cancers, 14(1), 254. https://doi.org/10.3390/cancers14010254







