Neuropsychiatric Disorders and Frailty in Older Adults over the Spectrum of Cancer: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction: Cancer in Older Adults
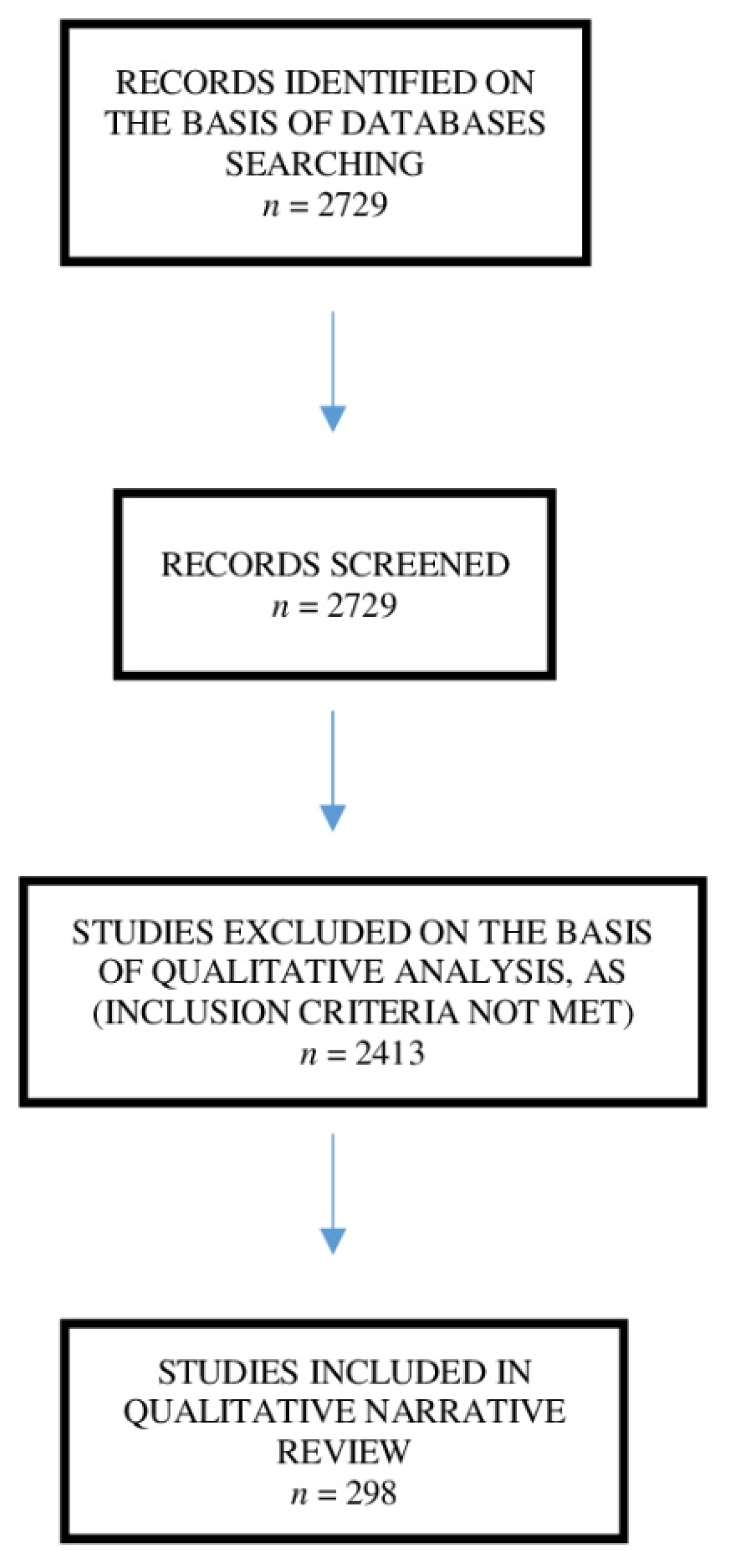
2. Search Strategy and Selection Criteria
2.1. Data Sources
- PubMed
- Ovid Medline
2.2. Search Terms
- Cancer (i.e., any type of solid and/or haematological cancer and any stage).
- Older adults OR old age OR aged (i.e., older adults > 65 years old). In geriatrics, the categorization of aging is based on the following stratification: young old (aged 65–74 years); old persons (75–84 years), and oldest old persons (>85 years).
- Neuropsychiatric disorders OR depression OR anxiety OR sleep disturbances OR attitude OR motivation OR support.
2.3. Study Eligibility Criteria
- Short-term clinical outcomes (i.e., postoperative complications, 30-day mortality; length of stay (LOS) and 30-day readmission and/or cancer-related treatments’ toxicity and/or treatment non-completion and utilization of healthcare services).
- Long-term clinical outcomes (long-term mortality ≥1 year) and/or health-related and self-perceived quality of life along with long-term health-related quality of life (HRQOL) outcomes and/or reduced physical function.
- Late-life symptoms and/or late-life geriatric syndromes and/or identification or progression of late-life frailty.
- Abstracts
- Editorials, case studies, score creation studies, pilot studies, and studies with fewer than 50 patients
- Studies without a specific focus on older adults (i.e., age < 65 years or no data about old-age participants)
- Articles related to central nervous system cancer, childhood and adult cancers
- Studies on specific single-disease-affected populations
- Nursing home patients
- Older adults comorbid for neurocognitive conditions, such as all types of dementia (Alzheimer’s dementia, vascular dementia, rapid progressing dementia, Parkinson’s dementia, Lewy body dementia, frontotemporal dementia) according to the current diagnostic criteria and guidelines, and/or medical record and clinical history and/ORCDR scoring) and/or severe depression (as assessed with any validated scale for depression and/or by physician’s clinical judgment and/or DSMV criteria), prior to the diagnosis of cancer
- Older adults with moderate to severe multimorbidity (as assessed by cumulative illness rating scale > 6 or a number of co-occurrent diseases > than 6)
- Palliative cancer patients
- End-stage single-disease patients with cancer such as advanced renal failure, advanced cardiac failure, and advanced lung disease
3. Frailty
3.1. Definition of Frailty
3.2. Frailty and Cancer Outcomes
3.3. Supportive Studies with Respect to Core Studies
3.4. Biomarkers of Frailty and Cancer
3.5. Neuropsychiatric Disorders in Older Adults with Cancer
3.5.1. Depressive Disorders and Suicidal Ideation in Older Adults with Cancer
3.5.2. Cancer and Anxiety Symptoms in Older Adults with Cancer
3.5.3. Sleep Disorders in Older Patients with Cancer
3.5.4. Psychological Conditions: Attitude and Motivation
Attitude
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Danger-associated molecular patterns | DAMPs |
| Senescence-associated secretory phenotype | SASP |
| Tumour necrosis factor-α | TNF-α |
| C-reactive protein | CRP |
| Insulin-like growth factor-1 | IGF-1 |
| Interleukin-6 | IL-6 |
| White blood cells | WBCs |
| T follicular helper cell subsets | Tfh cell |
| Interleukin-1 receptor antagonist | IL-1Ra |
| Soluble endothelial leukocyte adhesion molecule-1 | sE-selectin |
| C-X-C motif chemokine ligand 10 | CXCL10 |
| Transforming growth factor-β | TGF-β |
| Dehydroepiandrosterone | DHEA |
| Insulin-like growth factor binding protein 1–3 | IGFBP 1–3 |
| Glomerular filtration rate | GFR |
References
- Denlinger, C.S.; Carlson, R.W.; Are, M.; Baker, K.S.; Davis, E.; Edge, S.B.; Friedman, D.L.; Goldman, M.; Jones, L.; King, A.; et al. Survivorship: Introduction and Definition. J. Natl. Compr. Cancer Netw. 2014, 12, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armenian, S.H.; Gibson, C.J.; Rockne, R.C.; Ness, K.K. Premature Aging in Young Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Guida, J.L.; Ahles, T.A.; Belsky, D.; Campisi, J.; Cohen, H.J.; DeGregori, J.; Fuldner, R.; Ferrucci, L.; Gallicchio, L.; Gavrilov, L.; et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alfieri, W.; Costanzo, S.; Borgogni, T. Biological resilience of older adults versus frailty. Med. Hypotheses 2011, 76, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Berben, L.; Floris, G.; Wildiers, H.; Hatse, S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers 2021, 13, 1400. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Smith, D.; Sun, C.-L.; Tew, W.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 2016, 122, 3865–3872. [Google Scholar] [CrossRef]
- Williams, G.R.; Dunham, L.; Chang, Y.; Deal, A.M.; Pergolotti, M.; Lund, J.L.; Guerard, E.; Kenzik, K.; Muss, H.B.; Sanoff, H.K. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. J. Oncol. Pract. 2019, 15, e399–e409. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sco. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Magnitsky, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. The Prognostic Importance of Frailty in Cancer Survivors. J. Am. Geriatr. Soc. 2015, 63, 2538–2543. [Google Scholar]
- Margolick, J.B.; Ferrucci, L. Accelerating aging research: How can we measure the rate of biologic aging? Exp. Gerontol. 2015, 64, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [Green Version]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Pamukcuoglu, M.; Bhatia, S.; Weisdorf, D.J.; DeFor, T.E.; Ustun, C.; Nayar, M.; Holtan, S.G.; Jurdi, N.-E.; Thyagarajan, B.; Brunstein, C.G.; et al. Hematopoietic Cell Transplant–Related Toxicities and Mortality in Frail Recipients. Biol. Blood Marrow Transplant. 2019, 25, 2454–2460. [Google Scholar] [CrossRef]
- Murillo, A.; Cronin, A.M.; Laubach, J.P.; Hshieh, T.T.; Tanasijevic, A.M.; Richardson, P.G.; Driver, J.A.; Abel, G.A. Performance of the International Myeloma Working Group myeloma frailty score among patients 75 and older. J. Geriatr. Oncol. 2019, 10, 486–489. [Google Scholar] [CrossRef]
- Tan, K.-Y.; Kawamura, Y.J.; Tokomitsu, A.; Tang, T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am. J. Surg. 2012, 204, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, S.R.; Rønning, B.; Hurria, A.; Skovlund, E.; Jordhøy, M.S.; Nesbakken, A.; Wyller, T.B. A comparison of two pre-operative frailty measures in older surgical cancer patients. J. Geriatr. Oncol. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Burg, M.L.; Clifford, T.G.; Bazargani, S.T.; Lin-Brande, M.; Miranda, G.; Cai, J.; Schuckman, A.K.; Djaladat, H.; Daneshmand, S. Frailty as a predictor of complications after radical cystectomy: A prospective study of various preoperative assessments. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Hohensee, C.; Rosko, A.E.; Anderson, G.L.; Paskett, E.D.; Zaslavsky, O.; Wallace, R.B.; Caan, B.J. Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women’s Health Initiative. JAMA Netw. Open 2020, 3, e2016747. [Google Scholar] [CrossRef]
- Runzer-Colmenares, F.M.; Urrunaga-Pastor, D.; Aguirre, L.G.; Reategui-Rivera, C.M.; Parodi, J.F.; Taype-Rondan, A. Frailty and vulnerability as predictors of radiotoxicity in older adults: A longitudinal study in Peru. Med. Clínica (Engl. Ed.) 2017, 149, 325–330. [Google Scholar] [CrossRef]
- Runzer-Colmenares, F.M.; Urrunaga-Pastor, D.; Roca-Moscoso, M.A.; De Noriega, J.; Rosas-Carrasco, O.; Parodi, J.F. Frailty and Vulnerability as Predictors of Chemotherapy Toxicity in Older Adults: A Longitudinal Study in Peru. J. Nutr. Health Aging 2020, 24, 966–972. [Google Scholar] [CrossRef]
- Hay, C.M.; Donovan, H.S.; Campbell, G.B.; Taylor, S.E.; Wang, L.; Courtney-Brooks, M. Chemotherapy in older adult gynecologic oncology patients: Can a phenotypic frailty score predict tolerance? Gynecol. Oncol. 2019, 152, 304–309. [Google Scholar] [CrossRef]
- El Haddad, K.; Rolland, Y.; Gérard, S.; Mourey, L.; Sourdet, S.; Vellas, B.; Stephan, E.; Van Kan, G.A.; Barreto, P.D.S.; Balardy, L. No Difference in the Phenotypic Expression of Frailty among Elderly Patients Recently Diagnosed with Cancer vs. Cancer Free Patients. J. Nutr. Health Aging 2020, 24, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Inci, M.G.; Anders, L.; Woopen, H.; Richter, R.; Guzel, D.; Armbrust, R.; Sehouli, J. Frailty Index for prediction of surgical outcome in ovarian cancer: Results of a prospective study. Gynecol. Oncol. 2021, 161, 396–401. [Google Scholar] [CrossRef]
- Giannotti, C.; Sambuceti, S.; Signori, A.; Ballestrero, A.; Murialdo, R.; Romairone, E.; Scabini, S.; Caffa, I.; Odetti, P.; Nencioni, A.; et al. Frailty assessment in elective gastrointestinal oncogeriatric surgery: Predictors of one-year mortality and functional status. J. Geriatr. Oncol. 2019, 10, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, A.; Hammoud, Z.T.; Velanovich, V.; Hodari, A.; Borgi, J.; Rubinfeld, I. A modified frailty index to assess morbidity and mortality after lobectomy. J. Surg. Res. 2013, 183, 40–46. [Google Scholar] [CrossRef]
- Tatar, C.; Benlice, C.; Delaney, C.P.; Holubar, S.D.; Liska, D.; Steele, S.R.; Gorgun, E. Modified frailty index predicts high-risk patients for readmission after colorectal surgery for cancer. Am. J. Surg. 2020, 220, 187–190. [Google Scholar] [CrossRef]
- Hodari, A.; Hammoud, Z.T.; Borgi, J.F.; Tsiouris, A.; Rubinfeld, I.S. Assessment of Morbidity and Mortality After Esophagectomy Using a Modified Frailty Index. Ann. Thorac. Surg. 2013, 96, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Vermillion, S.A.; Hsu, F.-C.; Dorrell, R.D.; Shen, P.; Clark, C.J. Modified frailty index predicts postoperative outcomes in older gastrointestinal cancer patients. J. Surg. Oncol. 2017, 115, 997–1003. [Google Scholar] [CrossRef]
- Pandit, V.; Khan, M.; Martinez, C.; Jehan, F.; Zeeshan, M.; Koblinski, J.; Hamidi, M.; Omesieta, P.; Osuchukwu, O.; Nfonsam, V. A modified frailty index predicts adverse outcomes among patients with colon cancer undergoing surgical intervention. Am. J. Surg. 2018, 216, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Mogal, H.; Bs, S.A.V.; Dodson, R.; Hsu, F.-C.; Howerton, R.; Shen, P.; Clark, C.J. Modified Frailty Index Predicts Morbidity and Mortality After Pancreaticoduodenectomy. Ann. Surg. Oncol. 2017, 24, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Igwe, E.; Rice, L.W.; Spencer, R.J.; Rose, S.L. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol. Oncol. 2015, 137, 98–101. [Google Scholar] [CrossRef]
- Di Donato, V.; Di Pinto, A.; Giannini, A.; Caruso, G.; D’Oria, O.; Tomao, F.; Fischetti, M.; Perniola, G.; Palaia, I.; Muzii, L.; et al. Modified fragility index and surgical complexity score are able to predict postoperative morbidity and mortality after cytoreductive surgery for advanced ovarian cancer. Gynecol. Oncol. 2021, 161, 4–10. [Google Scholar] [CrossRef]
- Pitts, K.D.; Arteaga, A.A.; Stevens, B.P.; White, W.C.; Su, D.; Spankovich, C.; Jefferson, G.D.; Jackson, L.L. Frailty as a Predictor of Postoperative Outcomes among Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 2019, 160, 664–671. [Google Scholar] [CrossRef]
- Abt, N.B.; Richmon, J.D.; Koch, W.M.; Eisele, D.W.; Agrawal, N. Assessment of the Predictive Value of the Modified Frailty Index for Clavien-Dindo Grade IV Critical Care Complications in Major Head and Neck Cancer Operations. JAMA Otolaryngol. Neck Surg. 2016, 142, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Levy, I.; Finkelstein, M.; Bilal, K.H.; Palese, M. Modified frailty index associated with Clavien-Dindo IV complications in robot-assisted radical prostatectomies: A retrospective study. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 425–431. [Google Scholar] [CrossRef]
- Pearl, J.A.; Patil, D.; Filson, C.; Arya, S.; Alemozaffar, M.; Master, V.A.; Ogan, K. Patient Frailty and Discharge Disposition Following Radical Cystectomy. Clin. Genitourin. Cancer 2017, 15, e615–e621. [Google Scholar] [CrossRef]
- Hamaker, M.; Seynaeve, C.; Wymenga, A.; van Tinteren, H.; Nortier, J.; Maartense, E.; de Graaf, H.; de Jongh, F.; Braun, J.; Los, M.; et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: Results from the OMEGA study of the Dutch Breast Cancer Trialists’ Group. Breast 2014, 23, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wildes, T.M.; Ruwe, A.P.; Fournier, C.; Gao, F.; Carson, K.R.; Piccirillo, J.F.; Tan, B.; Colditz, G.A. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J. Geriatr. Oncol. 2013, 4, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Jauhari, Y.; Gannon, M.R.; Dodwell, D.; Horgan, K.; Tsang, C.; Clements, K.; Medina, J.; Tang, S.; Pettengell, R.; Cromwell, D.A. Addressing frailty in patients with breast cancer: A review of the literature. Eur. J. Surg. Oncol. 2020, 46, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Extermann, M.; Meyer, J.; McGinnis, M.; Crocker, T.T.; Corcoran, M.-B.; Yoder, J.; Haley, W.E.; Chen, H.; Boulware, D.; Balducci, L. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit. Rev. Oncol./Hematol. 2004, 49, 69–75. [Google Scholar] [CrossRef]
- Scheepers, E.R.; Vondeling, A.M.; Thielen, N.; Van Der Griend, R.; Stauder, R.; Hamaker, M.E. Geriatric assessment in older patients with a hematologic malignancy: A systematic review. Haematologica 2020, 105, 1484–1493. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Domm, A.S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Geessink, N.; Schoon, Y.; van Goor, H.; Rikkert, M.O.; Melis, R. On behalf of the TOPICS-MDS consortium Frailty and quality of life among older people with and without a cancer diagnosis: Findings from TOPICS-MDS. PLoS ONE 2017, 12, e0189648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.R.; Deal, A.M.; Sanoff, H.K.; Nyrop, K.A.; Guerard, E.J.; Pergolotti, M.; Shachar, S.S.; Reeve, B.B.; Bensen, J.T.; Choi, S.K.; et al. Frailty and health-related quality of life in older women with breast cancer. Support. Care Cancer 2019, 27, 2693–2698. [Google Scholar] [CrossRef]
- Cook, G.; Larocca, A.; Facon, T.; Zweegman, S.; Engelhardt, M. Defining the vulnerable patient with myeloma—A frailty position paper of the European Myeloma Network. Leukemia 2020, 34, 2285–2294. [Google Scholar] [CrossRef]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and Aging—The Inflammatory Connection. Aging Dis. 2017, 8, 611–627. [Google Scholar] [CrossRef] [Green Version]
- Al Saedi, A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 2019, 14, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- William, D.; Daisy, W.; Elizabeth, S. Frailty and the Immune System. J. Aging Res. Healthc. 2017, 2, 1–14. [Google Scholar]
- Castro-Herrera, V.M.; Lown, M.; Fisk, H.L.; Owen-Jones, E.; Lau, M.; Lowe, R.; Hood, K.; Gillespie, D.; Hobbs, F.D.R.; Little, P.; et al. Relationships Between Age, Frailty, Length of Care Home Residence and Biomarkers of Immunity and Inflammation in Older Care Home Residents in the United Kingdom. Front. Aging 2021, 2, 599084. [Google Scholar] [CrossRef]
- Mitnitski, A.; Collerton, J.; Martin-Ruiz, C.; Jagger, C.; Von Zglinicki, T.; Rockwood, K.; Kirkwood, T.B.L. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015, 13, 161. [Google Scholar] [CrossRef] [Green Version]
- Bürkle, A.; Moreno-Villanueva, M.; Bernhard, J.; Blasco, M.A.; Zondag, G.; Hoeijmakers, J.H.; Toussaint, O.; Grubeck-Loebenstein, B.; Mocchegiani, E.; Collino, S.; et al. MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 2015, 151, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Wogksch, M.D. Frailty and aging in cancer survivors. Transl. Res. 2020, 221, 65–82. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.N.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Matjusaitis, M.; Chin, G.; Sarnoski, E.A.; Stolzing, A. Biomarkers to identify and isolate senescent cells. Ageing Res. Rev. 2016, 29, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, J.; Shen, Y.; Yang, Y.; Huang, P.; Chen, S.; Zou, C.; Dong, B. The association between telomere length and frailty: A systematic review and meta-analysis. Exp. Gerontol. 2018, 106, 16–20. [Google Scholar] [CrossRef]
- Codari, M.; Zanardo, M.; Di Sabato, M.E.; Nocerino, E.; Messina, C.; Sconfienza, L.M.; Sardanelli, F. MRI-Derived Biomarkers Related to Sarcopenia: A Systematic Review. J. Magn. Reson. Imaging 2020, 51, 1117–1127. [Google Scholar] [CrossRef]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Sánchez-Flores, M.; Marcos-Pérez, D.; Lorenzo-López, L.; Maseda, A.; Millán-Calenti, J.C.; Pasaro, E.; Laffon, B. Exploring Genetic Outcomes as Frailty Biomarkers. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 74, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Tang, N.; Suen, E.; Leung, J.; Leung, P. Telomeres and frailty. Mech. Ageing Dev. 2008, 129, 642–648. [Google Scholar] [CrossRef]
- Nagasawa, M.; Takami, Y.; Akasaka, H.; Kabayama, M.; Maeda, S.; Yokoyama, S.; Fujimoto, T.; Nozato, Y.; Imaizumi, Y.; Takeda, M.; et al. High plasma adiponectin levels are associated with frailty in a general old-old population: The Septuagenarians, Octogenarians, Nonagenarians Investigation with Centenarians study. Geriatr. Gerontol. Int. 2018, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-S.; Wu, C.-H.; Chen, S.-C.; Huang, K.-C.; Chen, C.-Y.; Chang, C.-I.; Chuang, L.-M.; Chen, C.-Y. Plasma Adiponectin Levels Correlate Positively with an Increasing Number of Components of Frailty in Male Elders. PLoS ONE 2013, 8, e56250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardene, P.; Al Saedi, A.; Singh, L.; Bermeo, S.; Vogrin, S.; Phu, S.; Suriyaarachchi, P.; Pignolo, R.J.; Duque, G. Age, gender, and percentage of circulating osteoprogenitor (COP) cells: The COP Study. Exp. Gerontol. 2017, 96, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-J.; Xiong, Y.-Z.; Xu, X.-J.; Huang, L.-F.; Zhang, Y.; Wang, X.-J.; Xiu, L.-C.; Huang, J.-X.; Lian, T.-Y.; Liang, D.-M.; et al. Tfh cell subset biomarkers and inflammatory markers are associated with frailty status and frailty subtypes in the community-dwelling older population: A cross-sectional study. Aging 2020, 12, 2952–2973. [Google Scholar] [CrossRef]
- Hekmatimoghaddam, S.; Firoozabadi, A.D.; Zare-Khormizi, M.R.; Pourrajab, F. Sirt1 and Parp1 as epigenome safeguards and microRNAs as SASP-associated signals, in cellular senescence and aging. Ageing Res. Rev. 2017, 40, 120–141. [Google Scholar] [CrossRef]
- Boreskie, K.F.; Oldfield, C.J.; Hay, J.L.; Moffatt, T.L.; Hiebert, B.M.; Arora, R.C.; Duhamel, T.A. Myokines as biomarkers of frailty and cardiovascular disease risk in females. Exp. Gerontol. 2020, 133, 110859. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Hwang, H.; Kim, S.-K.; Choi, J.Y.; Lee, S.-M.; Bang, H.; Kwon, E.-S.; Lee, K.-P.; Chung, S.G.; Kwon, K.-S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018, 8, 8574. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.K.Y.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Labisi, O. Suicide Risk Assessment in the Depressed Elderly Patient with Cancer. J. Gerontol. Soc. Work 2006, 47, 17–25. [Google Scholar] [CrossRef]
- Ravi, P.; Karakiewicz, P.I.; Roghmann, F.; Gandaglia, G.; Choueiri, T.K.; Menon, M.; McKay, R.R.; Nguyen, P.L.; Sammon, J.D.; Sukumar, S.; et al. Mental health outcomes in elderly men with prostate cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2014; Volume 32, pp. 1333–1340. [Google Scholar] [CrossRef]
- Kissane, D.W. Unrecognised and untreated depression in cancer care. Lancet Psychiatry 2014, 1, 320–321. [Google Scholar] [CrossRef]
- Passik, S.D.; Dugan, W.; McDonald, M.V.; Rosenfeld, B.; Theobald, D.E.; Edgerton, S. Oncologists’ recognition of depression in their patients with cancer. J. Clin. Oncol. 1998, 16, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Kok, R.M.; Reynolds, C.F., 3rd. Management of Depression in Older Adults: A Review. Jama 2017, 317, 2114–2122. [Google Scholar] [CrossRef]
- Blazer, D.G. Depression in Late Life: Review and Commentary. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M249–M265. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Hung, H.; Chen, H.; Chang, Y.; Yang, Y.; Liu, C.; Hsieh, R.; Leu, Y.; Chen, Y.; Wang, T.; Tsai, L. Evaluation of the reliability and validity of the Mandarin Version of Demoralization Scale for cancer patients. J. Intern. Med. Taiwan 2010, 21, 427–435. [Google Scholar]
- Shaw, J.; Sethi, S.; Vaccaro, L.; Beatty, L.; Kirsten, L.; Kissane, D.; Kelly, B.; Mitchell, G.; Sherman, K.; Turner, J. Is care really shared? A systematic review of collaborative care (shared care) interventions for adult cancer patients with depression. BMC Health Serv. Res. 2019, 19, 120. [Google Scholar] [CrossRef] [Green Version]
- Cole, T.B.; Bowling, J.M.; Patetta, M.J.; Blazer, D.G. Risk factors for suicide among older adults with cancer. Aging Ment. Health 2014, 18, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Park, E.-C. Suicide risk after cancer diagnosis among older adults: A nationwide retrospective cohort study. J. Geriatr. Oncol. 2020, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Misono, S.; Weiss, N.S.; Fann, J.R.; Redman, M.; Yueh, B. Incidence of Suicide in Persons with Cancer. J. Clin. Oncol. 2008, 26, 4731–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miccinesi, G.; Crocetti, E.; Benvenuti, A.; Paci, E. Suicide mortality is decreasing among cancer patients in Central Italy. Eur. J. Cancer 2004, 40, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Arniani, S.; Acciai, S.; Barchielli, A.; Buiatti, E. High suicide mortality soon after diagnosis among cancer patients in central Italy. Br. J. Cancer 1998, 77, 1194–1196. [Google Scholar] [CrossRef]
- Levi, F.; Bulliard, J.-L.; La Vecchia, C. Suicide Risk among Incident Cases of Cancer in the Swiss Canton of Vaud. Oncology 1991, 48, 44–47. [Google Scholar] [CrossRef]
- Miller, M.; Mogun, H.; Azrael, D.; Hempstead, K.; Solomon, D.H. Cancer and the Risk of Suicide in Older Americans. J. Clin. Oncol. 2008, 26, 4720–4724. [Google Scholar] [CrossRef]
- Dormer, N.R.C.; McCaul, K.A.; Kristjanson, L.J. Risk of suicide in cancer patients in Western Australia, 1981-2002. Med. J. Aust. 2008, 188, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Akechi, T.; Kugaya, A.; Okamura, H.; Nakano, T.; Okuyama, T.; Mikami, I.; Shima, Y.; Yamawaki, S.; Uchitomi, Y. Suicidal thoughts in cancer patients: Clinical experience in psycho-oncology. Psychiatry Clin. Neurosci. 1999, 53, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.E.; Brock, R.; Charnock, J.; Wickramasinghe, B.; Will, O.; Pitman, A. Risk of Suicide After Cancer Diagnosis in England. JAMA Psychiatry 2019, 76, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.; Renshaw, C.; Okello, C.; Moller, H.; Davies, E.A. Suicide in cancer patients in South East England from 1996 to 2005: A population-based study. Br. J. Cancer 2009, 101, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, U.; Christensen, M.-L.; Engholm, G.; Storm, H. Suicides among Danish cancer patients 1971–1999. Br. J. Cancer 2005, 92, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.V.D.C.; dos Santos, S.L.; Ceballos, A.G.D.C.D.; Furtado, B.M.A.S.M.; Bonfim, C.V.D. Risk factors for suicide in individuals with cancer: An integrative literature review. Rev. Bras. Enferm. 2021, 74, e20190889. [Google Scholar] [CrossRef]
- Li, M.; Kennedy, E.B.; Byrne, N.; Gérin-Lajoie, C.; Katz, M.R.; Keshavarz, H.; Sellick, S.; Green, E. Management of Depression in Patients with Cancer: A Clinical Practice Guideline. J. Oncol. Pract. 2016, 12, 747–756. [Google Scholar] [CrossRef]
- Andersen, B.L.; DeRubeis, R.; Berman, B.S.; Gruman, J.; Champion, V.; Massie, M.J.; Holland, J.C.; Partridge, A.H.; Bak, K.; Somerfield, M.R.; et al. Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults with Cancer: An American Society of Clinical Oncology Guideline Adaptation. J. Clin. Oncol. 2014, 32, 1605–1619. [Google Scholar] [CrossRef]
- Soleimani, M.A.; Sharif, S.P.; Yaghoobzadeh, A.; Allen, K.-A.; Nia, H.S. An Examination of Psychometric Characteristics and Factor Structure of Death Anxiety Scale Within a Sample of Iranian Patients with Heart Disease. Int. J. Epidemiol. Res. 2017, 4, 260–266. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Fairclough, D.L.; Wolfe, P.; Emanuel, L.L. Talking with terminally ill patients and their caregivers about death, dying, and bereavement: Is it stressful? Is it helpful? Arch. Intern. Med. 2004, 164, 1999–2004. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, A.; Panahipour, H.; Allen, K.A.; Hosseinian, S. Effects of Death Anxiety on Perceived Stress in Individuals with Multiple Sclerosis and the Role of Self-Transcendence. Omega J. Death Dying 2021, 84, 91–102. [Google Scholar] [CrossRef]
- Solomon, S.; Greenberg, J.; Pyszczynski, T. Pride and prejudice: Fear of death and social behavior. Curr. Dir. Psychol. Sci. 2000, 9, 200–204. [Google Scholar] [CrossRef]
- Bahrami, N.; Moradi, M.; Soleimani, M.; Kalantari, Z.; Hosseini, F. Death anxiety and its relationship with quality of life in women with cancer. Iran J. Nurs. 2013, 26, 51–61. [Google Scholar]
- Fuller-Thomson, E.; West, K. Flourishing despite a cancer diagnosis: Factors associated with complete mental health in a nationally-representative sample of cancer patients aged 50 years and older. Aging Ment. Health 2018, 23, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.A.; Bahrami, N.; Allen, K.-A.; Alimoradi, Z. Death anxiety in patients with cancer: A systematic review and meta-analysis. Eur. J. Oncol. Nurs. 2020, 48, 101803. [Google Scholar] [CrossRef]
- Mehnert, A.; Brähler, E.; Faller, H.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Weis, J.; Boehncke, A.; Hund, B.; et al. Four-Week Prevalence of Mental Disorders in Patients with Cancer across Major Tumor Entities. J. Clin. Oncol. 2014, 32, 3540–3546. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessur, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Thielking, P.D. Cancer pain and anxiety. Curr. Pain Headache Rep. 2003, 7, 249–261. [Google Scholar] [CrossRef]
- Smith, H.R. Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.N.; Kamath, A. Quality of Life among Cancer Patients. Indian J. Palliat. Care 2017, 23, 445–450. [Google Scholar] [CrossRef]
- Maddock, C.; Pariante, C.M. How does stress affect you? An overview of stress, immunity, depression and disease. Epidemiol. Psichiatr. Soc. 2001, 10, 153–162. [Google Scholar] [CrossRef]
- Spiegel, D.; Giese-Davis, J. Depression and cancer: Mechanisms and disease progression. Biol. Psychiatry 2003, 54, 269–282. [Google Scholar] [CrossRef]
- Strine, T.W.; Mokdad, A.H.; Dube, S.R.; Balluz, L.S.; Gonzalez, O.; Berry, J.T.; Manderscheid, R.; Kroenke, K. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen. Hosp. Psychiatry 2008, 30, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kisely, S.; Crowe, E.; Lawrence, D. Cancer-Related Mortality in People with Mental Illness. JAMA Psychiatry 2013, 70, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.; Pearce, A.; Lopez, A.-L.; Price, M.A. Clinical anxiety disorders in the context of cancer: A scoping review of impact on resource use and healthcare costs. Eur. J. Cancer Care 2018, 27, e12893. [Google Scholar] [CrossRef]
- Meier, C.; Taubenheim, S.; Lordick, F.; Mehnert-Theuerkauf, A.; Götze, H. Depression and anxiety in older patients with haematologicalcancer (70+)—Geriatric, social, cancer- and treatment-related associations. J. Geriatr. Oncol. 2020, 11, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.B.; Phillips, T.; Smith, T.J. Using the New ASCO Clinical Practice Guideline for Palliative Care Concurrent with Oncology Care Using the TEAM Approach. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annu. Meet. 2017, 37, 714–723. [Google Scholar]
- Bloom, J.R. Surviving and thriving? Psycho-Oncology 2002, 11, 89–92. [Google Scholar] [CrossRef]
- Deimling, G.T.; Bowman, K.F.; Sterns, S.; Wagner, L.J.; Kahana, B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psycho-Oncology 2006, 15, 306–320. [Google Scholar] [CrossRef]
- Otte, J.L.; Carpenter, J.S.; Manchanda, S.; Rand, K.L.; Skaar, T.C.; Weaver, M.T.; Chernyak, Y.; Zhong, X.; Igega, C.; Landis, C. Systematic review of sleep disorders in cancer patients: Can the prevalence of sleep disorders be ascertained? Cancer Med. 2014, 4, 183–200. [Google Scholar] [CrossRef] [Green Version]
- Vasbinder, A.; Reding, K.W.; Wang, D.; Han, C.J.; Zaslavsky, O.; Langford, D.J.; Feliciano, E.M.C.; Barrington, W.E.; Paskett, E.D. Postdiagnosis Physical Activity: Association with Long-Term Fatigue and Sleep Disturbance in Older Adult Breast Cancer Survivors. Clin. J. Oncol. Nurs. 2020, 24, 381–391. [Google Scholar] [CrossRef]
- Loh, K.P.; Burhenn, P.; Hurria, A.; Zachariah, F.; Mohile, S.G. How do I best manage insomnia and other sleep disorders in older adults with cancer? J. Geriatr. Oncol. 2016, 7, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Waldman, L.; Morrison, L.J. Sleep disorders and fatigue: Special issues in the older adult with cancer. Cancer J. (Sudbury Mass.) 2014, 20, 352–357. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lee, D.T. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit. Rev. Oncol./Hematol. 2011, 78, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Grov, E.K.; Fosså, S.D.; Dahl, A.A. Insomnia in elderly cancer survivors—A population-based controlled study of associations with lifestyle, morbidity, and psychosocial factors. Results from the Health Survey of North-Trøndelag County (HUNT-2). Insomnia in elderly cancer survivors. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2011, 19, 1319–1326. [Google Scholar] [CrossRef]
- Mao, J.J.; Armstrong, K.; Bowman, M.; Xie, S.X.; Kadakia, R.; Farrar, J.T. Symptom Burden Among Cancer Survivors: Impact of Age and Comorbidity. J. Am. Board Fam. Med. 2007, 20, 434–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzecka, A.; Sarul, K.; Dyła, T.; Avila-Rodriguez, M.; Cabezas-Perez, R.; Chubarev, V.N.; Minyaeva, N.N.; Klochkov, S.G.; Neganova, M.E.; Mikhaleva, L.M.; et al. The Association of Sleep Disorders, Obesity and Sleep-Related Hypoxia with Cancer. Curr. Genom. 2020, 21, 444–453. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Borniger, J.C. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef] [Green Version]
- López, E.; De La Torre-Luque, A.; Lazo, A.; Álvarez, J.; Buela-Casal, G. Assessment of sleep disturbances in patients with cancer: Cross-sectional study in a radiotherapy department. Eur. J. Oncol. Nurs. 2016, 20, 71–76. [Google Scholar] [CrossRef]
- Phillips, K.M.; Jim, H.S.; Donovan, K.A.; Pinder-Schenck, M.C.; Jacobsen, P. Characteristics and correlates of sleep disturbances in cancer patients. Support. Care Cancer 2011, 20, 357–365. [Google Scholar] [CrossRef]
- Lowery-Allison, A.E.; Passik, S.D.; Cribbet, M.R.; Reinsel, R.A.; O’Sullivan, B.; Norton, L.; Kirsh, K.L.; Kavey, N.B. Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliat. Support. Care 2018, 16, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-T.; Lu, Y.-H.; Yang, H.; Guo, R.-X.; Wang, Y.; Wen, L.-H.; Zhang, Y.-R.; Sun, H.-Y. The Knowledge and Attitude Towards Advance Care Planning Among Chinese Patients with Advanced Cancer. J. Cancer Educ. 2021, 36, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Rakowski, W.; Clough-Gorr, K.M.; Silliman, R.A. The Getting-Out-of-Bed (GoB) scale: A measure of motivation and life outlook in older adults with cancer. J. Psychosoc. Oncol. 2009, 27, 454–468. [Google Scholar] [CrossRef]
- Usta, Y.Y. Importance of social support in cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 3569–3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nausheen, B.; Gidron, Y.; Peveler, R.; Moss-Morris, R. Social support and cancer progression: A systematic review. J. Psychosom. Res. 2009, 67, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.K.; Schüz, B.; Ziegelmann, J.P.; Warner, L.M.; Wurm, S. Short-Term Buffers, but Long-Term Suffers? Differential Effects of Negative Self-Perceptions of Aging Following Serious Health Events. J. Gerontol. Ser. B 2015, 72, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, B.R.; Bavishi, A. Survival Advantage Mechanism: Inflammation as a Mediator of Positive Self-Perceptions of Aging on Longevity. J. Gerontol. Ser. B 2016, 73, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Eglit, G.M.L.; Maldonado, Y.; Daly, R.; Liu, J.; Tu, X.; Jeste, D.V. Attitude Toward Own Aging Among Older Adults: Implications for Cancer Prevention. Gerontologist 2019, 59, S38–S49. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.K.; Nielsen, D.L.; Vinther, A.; Lund, C.M.; Jarden, M. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment—A qualitative interview study. Eur. J. Oncol. Nurs. 2019, 41, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kreling, B.; Figueiredo, M.I.; Sheppard, V.L.; Mandelblatt, J.S. A qualitative study of factors affecting chemotherapy use in older women with breast cancer: Barriers, promoters, and implications for intervention. Psycho-Oncology 2006, 15, 1065–1076. [Google Scholar] [CrossRef]
- Dumontier, C.; Clough-Gorr, K.M.; Silliman, R.A.; Stuck, A.E.; Moser, A. Motivation and mortality in older women with early stage breast cancer: A longitudinal study with ten years of follow-up. J. Geriatr. Oncol. 2016, 8, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A.J.; Modi, R. Psychiatric issues in older cancer patients. Crit. Rev. Oncol. 2003, 48, 185–197. [Google Scholar] [CrossRef]
- Social Support National Cancer Institute. NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/social-support (accessed on 23 June 2021).
- Pinquart, M.; Duberstein, P. Associations of social networks with cancer mortality: A meta-analysis. Crit. Rev. Oncol. 2010, 75, 122–137. [Google Scholar] [CrossRef] [Green Version]
- Osann, K.; Wilford, J.; Wenzel, L.; Hsieh, S.; Tucker, J.A.; Wahi, A.; Monk, B.J.; Nelson, E.L. Relationship between social support, quality of life, and Th2 cytokines in a biobehavioral cancer survivorship trial. Support. Care Cancer 2019, 27, 3301–3310. [Google Scholar] [CrossRef] [Green Version]
- Durá-Ferrandis, E.; Mandelblatt, J.S.; Clapp, J.; Luta, G.; Faul, L.; Kimmick, G.; Cohen, H.J.; Yung, R.L.; Hurria, A. Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psycho-Oncology 2017, 26, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.; Azuero, A.; Halilova, K.I.; Mph, C.P.W.; Kenzik, K.M.; Kvale, E.A.; Williams, G.; Meneses, K.; Rn, M.S.; Mph, S.K.Y.; et al. Most impactful factors on the health-related quality of life of a geriatric population with cancer. Cancer 2018, 124, 596–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrokni, A.; Tin, A.; Alexander, K.; Sarraf, S.; Afonso, A.; Filippova, O.; Harris, J.; Downey, R.J.; Vickers, A.J.; Korc-Grodzicki, B. Development and Evaluation of a New Frailty Index for Older Surgical Patients with Cancer. JAMA Netw. Open 2019, 2, e193545. [Google Scholar] [CrossRef] [PubMed]
- Wulff-Burchfield, E.; Dietrich, M.S.; Ridner, S.; Murphy, B.A. Late systemic symptoms in head and neck cancer survivors. Support. Care Cancer 2019, 27, 2893–2902. [Google Scholar] [CrossRef] [Green Version]
- Rostoft, S.; O’Donovan, A.; Soubeyran, P.; Alibhai, S.M.H.; Hamaker, M.E. Geriatric Assessment and Management in Cancer. J. Clin. Oncol. 2021, 39, 2058–2067. [Google Scholar] [CrossRef]


| Reference | Cancer Type/Surgery | Frailty Assessment | Outcome |
|---|---|---|---|
| Pamukcuoglu et al. [18] | HCT | Physical frailty phenotype | Short term: high-grade non-haematological toxicity |
| Long term: long-term mortality (1 year) | |||
| Kristjansson et al. [21] | Colorectal cancer/elective surgery | Physical frailty phenotype CGA | Short term: postoperative complications and mortality |
| - | - | Long term: mortality (median follow-up 20 months) | |
| Tan et al. [20] | Colorectal cancer/elective surgery | Physical frailty phenotype | Short term: postoperative major complications |
| Burg et al. [22] | Bladder cancer/radical cystectomy | Physical frailty phenotype | Short term: high-grade 30- and 90-day complications |
| Cespedes Feliciano et al. [23] | Different types of cancer | Physical frailty phenotype | Long term: mortality after cancer diagnosis (median follow-up 5.8 years) |
| Runzer-Colmenares et al. [24] | Different types of cancer treated with radiotherapy | Physical frailty phenotype, VES-13 ∗, G-8 ∗ | Short term: Radiotherapy toxicity of grade III, IV, or V |
| Runzer-Colmenares et al. [25] | Different types of cancer treated with chemotherapy | Physical frailty phenotype, VES-13 ∗, G-8 ∗ | Short term: Chemotherapy toxicity of grade III, IV, or V |
| Hay et al. [26] | Gynaecologic cancer | Physical frailty phenotype | Short term: administration and completion of chemotherapy |
| Murillo et al. [19] | Multiple myeloma | Physical frailty phenotype | Long term: mortality (median follow-up 10.6 months) |
| Inci et al. [28] | Ovarian cancer/cytoreductive surgery | The deficit accumulation model of Rockwood | Short term: severe postoperative complications |
| Long term: overall survival ( median follow-up 37.6 months) | |||
| Giannotti et al. [29] | Gastrointestinal cancer/surgery | The deficit accumulation model of Rockwood and CGA | Short term: postoperative complications; 30-day mortality |
| Long term: 1-year mortality |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzyka, M.; Tagliafico, L.; Serafini, G.; Baiardini, I.; Braido, F.; Nencioni, A.; Monacelli, F. Neuropsychiatric Disorders and Frailty in Older Adults over the Spectrum of Cancer: A Narrative Review. Cancers 2022, 14, 258. https://doi.org/10.3390/cancers14010258
Muzyka M, Tagliafico L, Serafini G, Baiardini I, Braido F, Nencioni A, Monacelli F. Neuropsychiatric Disorders and Frailty in Older Adults over the Spectrum of Cancer: A Narrative Review. Cancers. 2022; 14(1):258. https://doi.org/10.3390/cancers14010258
Chicago/Turabian StyleMuzyka, Mariya, Luca Tagliafico, Gianluca Serafini, Ilaria Baiardini, Fulvio Braido, Alessio Nencioni, and Fiammetta Monacelli. 2022. "Neuropsychiatric Disorders and Frailty in Older Adults over the Spectrum of Cancer: A Narrative Review" Cancers 14, no. 1: 258. https://doi.org/10.3390/cancers14010258
APA StyleMuzyka, M., Tagliafico, L., Serafini, G., Baiardini, I., Braido, F., Nencioni, A., & Monacelli, F. (2022). Neuropsychiatric Disorders and Frailty in Older Adults over the Spectrum of Cancer: A Narrative Review. Cancers, 14(1), 258. https://doi.org/10.3390/cancers14010258







