Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Two Patient Cohorts
2.2. Comparison of First- and Further-Line Patient Groups
2.3. Pairwise Associations between Predictors and Responses
2.4. Prognostic Markers for Binary Responses (Response3mt and irAE)
2.5. Prognostic Markers for Overall Survival
2.6. Cut-Off Estimation for Continuous Predictors
3. Results
3.1. Comparison of First- and Further-Line Patient Groups
3.2. Pairwise Associations between Predictors and Responses
3.3. Prognostic Markers for Binary Responses (Response3mt and irAE)
3.4. Prognostic Markers for Overall Survival
3.5. Cut-Off Estimation for Continuous Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol. 2018, 36, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Hwang, S.; Kwon, A.Y.; Jeong, J.Y.; Kim, S.; Kang, H.; Park, J.; Kim, J.H.; Han, O.J.; Lim, S.M.; An, H.J. Immune gene signatures for predicting durable clinical benefit of anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci. Rep. 2020, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.Q.; Yu, Y.F.; Ou, Q.Y.; Li, X.Y.; Zhong, R.Z.; Xie, C.M.; Hu, Q.G. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 2016, 7, 13765–13781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Haragan, A.; Field, J.K.; Davies, M.P.A.; Escriu, C.; Gruver, A.; Gosney, J.R. Heterogeneity of PD-L1 expression in non-small cell lung cancer: Implications for specimen sampling in predicting treatment response. Lung Cancer 2019, 134, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lantuejoul, S.; Tsao, M.S.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.Y.; Motoi, N.; et al. PD-L1 Testing for Lung Cancer in 2019: Perspective from the IASLC Pathology Committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef]
- Torlakovic, E.; Lim, H.J.; Adam, J.; Barnes, P.; Bigras, G.; Chan, A.W.H.; Cheung, C.C.; Chung, J.-H.; Couture, C.; Fiset, P.O.; et al. “Interchangeability” of PD-L1 immunohistochemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 2020, 33, 4–17. [Google Scholar] [CrossRef]
- Altman, D.G.; De Stavola, B.L.; Love, S.B.; Stepniewska, K.A. Review of survival analyses published in cancer journals. Br. J. Cancer 1995, 72, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Clin. Pract. Urol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Wright, M.N.; Ziegler, A. Ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. J. Stat. Softw. 2017, 77, 17. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- A Package for Survival Analysis in, R. Available online: https://CRAN.R-project.org/package=survival (accessed on 15 November 2021).
- Hothorn, T. Maxstat: Maximally Selected Rank Statistics. R News 2017, 2, 3–5. [Google Scholar]
- Li, S.; Zhang, C.; Pang, G.; Wang, P. Emerging Blood-Based Biomarkers for Predicting Response to Checkpoint Immunotherapy in Non-Small-Cell Lung Cancer. Front. Immunol. 2020, 11, 2731. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 1081. [Google Scholar] [CrossRef]
- Maillet, D.; Corbaux, P.; Stelmes, J.-J.; Dalle, S.; Locatelli-Sanchez, M.; Perier-Muzet, M.; Duruisseaux, M.; Kiakouama-Maleka, L.; Freyer, G.; Boespflug, A.; et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2020, 132, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Khiabany, A.; Nakamura, M.; Pellizzari, G.; Ilieva, K.M.; Lombardi, S.; Gould, H.J.; Corrigan, C.J.; et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells 2020, 9, 1631. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Wang, G.; Zhou, Y.; Luo, M.; Wang, S.; Hong, C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac. J. Clin. Oncol. 2018, 14, e243–e251. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Rosner, S.; Kwong, E.; Shoushtari, A.N.; Friedman, C.F.; Betof, A.S.; Brady, M.S.; Coit, D.G.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018, 7, 690–697. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Wu, F.; Ren, S.; et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar] [CrossRef]
- Prelaj, A.; Rebuzzi, S.E.; Pizzutilo, P.; Bilancia, M.; Montrone, M.; Pesola, F.; Longo, V.; Del Bene, G.; Lapadula, V.; Cassano, F.; et al. EPSILoN: A Prognostic Score Using Clinical and Blood Biomarkers in Advanced Non–Small-cell Lung Cancer Treated With Immunotherapy. Clin. Lung Cancer 2020, 21, 365–377.e5. [Google Scholar] [CrossRef]
- Cortellini, A.; Tiseo, M.; Banna, G.L.; Cappuzzo, F.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Cortinovis, D.; Grossi, F.; et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥50%. Cancer Immunol. Immunother. 2020, 69, 2209–2221. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.S.; Kim, B.J. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Oncotarget 2017, 8, 93149–93155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef] [PubMed]
- Ottonello, S.; Genova, C.; Cossu, I.; Fontana, V.; Rijavec, E.; Rossi, G.; Biello, F.; Dal Bello, M.G.; Tagliamento, M.; Alama, A.; et al. Association Between Response to Nivolumab Treatment and Peripheral Blood Lymphocyte Subsets in Patients With Non-small Cell Lung Cancer. Front. Immunol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Karantanos, T.; Karanika, S.; Seth, B.; Gignac, G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: A clinical study. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2019, 21, 206–212. [Google Scholar] [CrossRef]
- Moghanaki, D.; Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.; Giuste, F.; Burns, A.; Sebastian, N.; Ramalingam, S.; Sukhatme, V.; et al. Association of concomitant NSAID and immunotherapy on outcomes in patients with non-small cell lung cancer: Analysis of the National Veterans Health Administration Database. J. Clin. Oncol. 2021, 39, 9107. [Google Scholar] [CrossRef]
- Wang, D.Y.; McQuade, J.L.; Rai, R.R.; Park, J.J.; Zhao, S.; Ye, F.; Beckermann, K.E.; Rubinstein, S.M.; Johnpulle, R.; Long, G.V.; et al. The Impact of Nonsteroidal Anti-Inflammatory Drugs, Beta Blockers, and Metformin on the Efficacy of Anti-PD-1 Therapy in Advanced Melanoma. Oncologist 2020, 25, e602–e605. [Google Scholar] [CrossRef]
- Pennock, N.D.; Martinson, H.A.; Guo, Q.; Betts, C.B.; Jindal, S.; Tsujikawa, T.; Coussens, L.M.; Borges, V.F.; Schedin, P. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J. Immuno Ther. Cancer 2018, 6, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talmadge, J.E.; Hood, K.C.; Zobel, L.C.; Shafer, L.R.; Coles, M.; Toth, B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 2007, 7, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Pelly, V.S.; Moeini, A.; Roelofsen, L.M.; Bonavita, E.; Bell, C.R.; Hutton, C.; Blanco-Gomez, A.; Banyard, A.; Bromley, C.P.; Flanagan, E.; et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. J. Cancer Discov. 2021, 11, 2602–2619. [Google Scholar] [CrossRef] [PubMed]

| Total | Further-Line Patient Group | First-Line Patient Group | |
|---|---|---|---|
| n = 111 | n = 71 | ||
| Age (yrs) | median (range) | 65 (36–89) | 67 (43–82) |
| standard deviation | 9.7 | 9.3 | |
| Sex | female | 35 (31.5%) | 22 (31%) |
| male | 76 (68.5%) | 49 (69%) | |
| Smoker | yes | 100 (90.1%) | 63 (88.7%) |
| no | 11 (9.9%) | 8 (11.3%) | |
| BMI | median (range) | 24 (17.1–42.5) | 24.5 (16.2–35.2) |
| Histologic subtype | Adenocarcinoma | 84 (75.7%) | 59 (83.1%) |
| Squamous cell carcinoma | 27 (24.3%) | 12 (16.9%) | |
| Immune-rel. Adverse Events | yes | 31 (27.9%) | 24 (33.8%) |
| no | 80 (72.1%) | 47 (66.2%) | |
| Lymphocytes (G/l) | median | 1.09 (0.17–3.75) | 1.47 (0.31–4.7) |
| standard deviation | 0.7183 | 0.7579 | |
| Normal range (1.5–4) | 34 (30.6%) | 34 (47.9%) | |
| Not in normal range | 77 (69.4%) | 37 (52.1%) | |
| Monocytes (G/l) | median | 0.81 (0.09–1.98) | 0.69 (0.02–3.1) |
| standard deviation | 0.3308 | 0.4058 | |
| Normal range (0.16–0.95) | 80 (72.1%) | 54 (76.1%) | |
| Not in normal range | 31 (27.9%) | 17 (23.9%) | |
| Neutrophils (G/l) | median | 4.76 (0.3–13.89) | 5.1 (1.25–16.7) |
| standard deviation | 2.598 | 2.7834 | |
| normal range (1.4–8) | 95 (85.6%) | 58 (81.7%) | |
| Not in normal range | 16 (14.14%) | 13 (18.3%) | |
| Eosinophils (G/l) | median | 0.12 (0–1.09) | 0.16 (0.01–0.5) |
| standard deviation | 0.2069 | 0.1295 | |
| Normal range (0–0.7) | 107 (96.4%) | 71 (100%) | |
| Not in normal range | 4 (3.6%) | 0 (0%) | |
| Basophils (G/l) | median | 0.02 (0.01–0.1) | 0.04 (0.01–0.12) |
| standard deviation | 0.0217 | 0.0223 | |
| Normal range (0–0.15) | 111 (100%) | 71 (100%) | |
| Not in normal range | 0 (0%) | 0 (0%) | |
| PDL1-TC | <1% | 45 (40.5%) | 23 (32.4%) |
| 1–50% | 33 (29.8%) | 28 (39.4%) | |
| >50% | 15 (13.5%) | 19 (26.8%) | |
| not available | 18 (16.2%) | 1 (1.4%) | |
| Response (CR, PR) at 3 months | yes | 36 (32.4%) | 36 (50.7%) |
| no | 75 (67.6%) | 35 (49.3%) | |
| Antibiotics | yes | 55 (49.5%) | 42 (59.2%) |
| no | 56 (50.5%) | 29 (40.8%) | |
| NSAR | yes | 42 (37.8%) | 20 (28.2%) |
| no | 69 (62.2%) | 51 (71.8%) | |
| Steroids | yes | 52 (46.8%) | 43 (60.6%) |
| no | 59 (53.2%) | 28 (39.4%) | |
| Metformin | yes | 9 (8.1%) | 4 (5.6%) |
| no | 102 (91.9%) | 67 (94.4%) | |
| Treatment | ICI & Chemo | 11 (9.9%) | 42 (59.2%) |
| ICI | 100 (90.1%) | 29 (40.8%) | |
| OS (days) | median (range) | 340 (9–1807) | 461 (61–1198) |
| standard deviation | 463.3 | 290.2 |
| Univariate Methods | Multivariate Methods | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Logistic Regressions | Additional Tests | Classification Random Forest | ||||||||
| Patient Group | Response Variable | Predictor | Estimate (OR) | 95% CI | Raw p-Value (LR/Fisher) | Adjusted p-Value (Holm) | Raw p-Value (Wilcoxon) | Adjusted p-Value (Holm) | Raw Impurity Importance p-Value (RF) | Adjusted p-Value (Holm) |
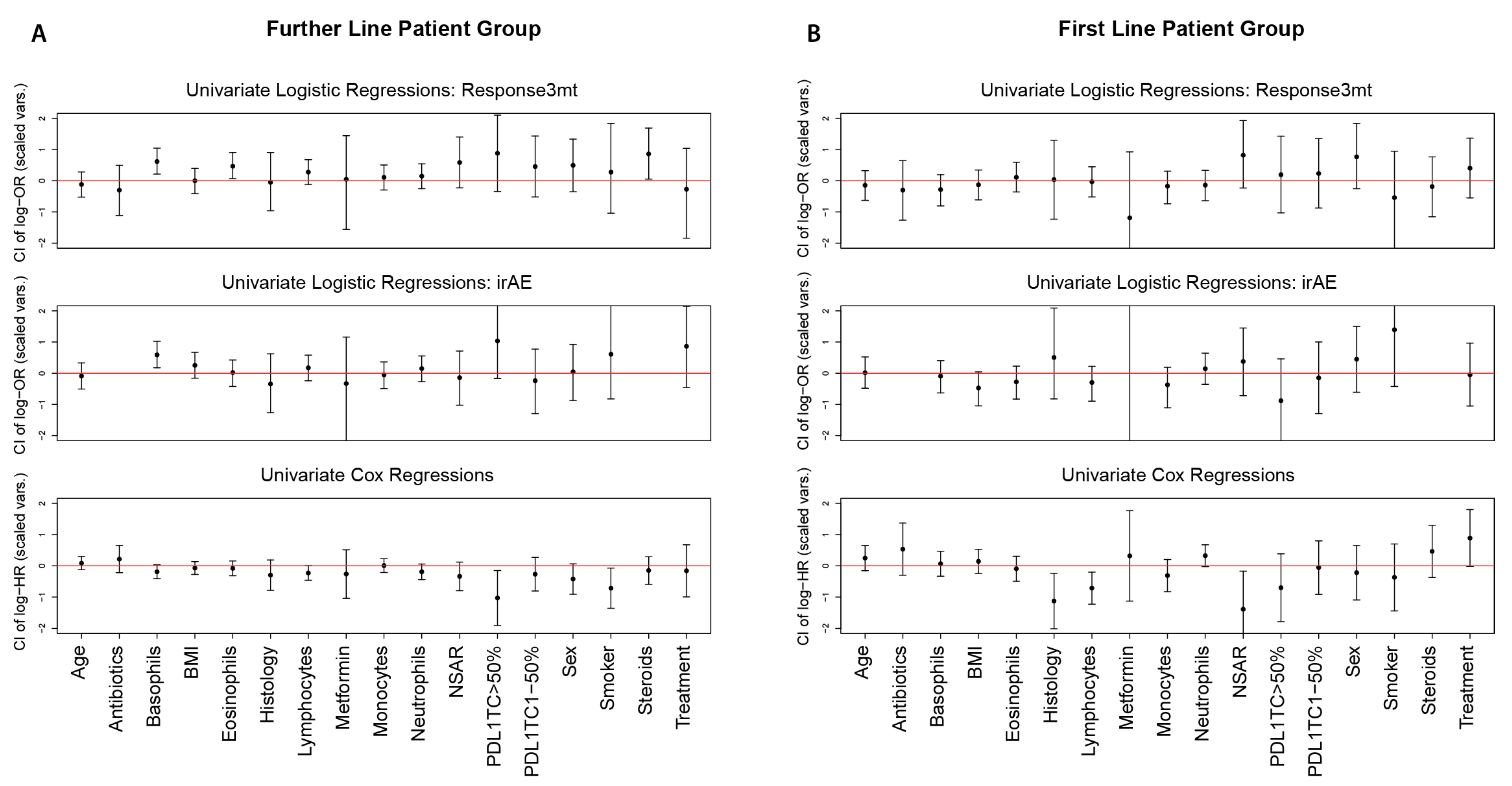
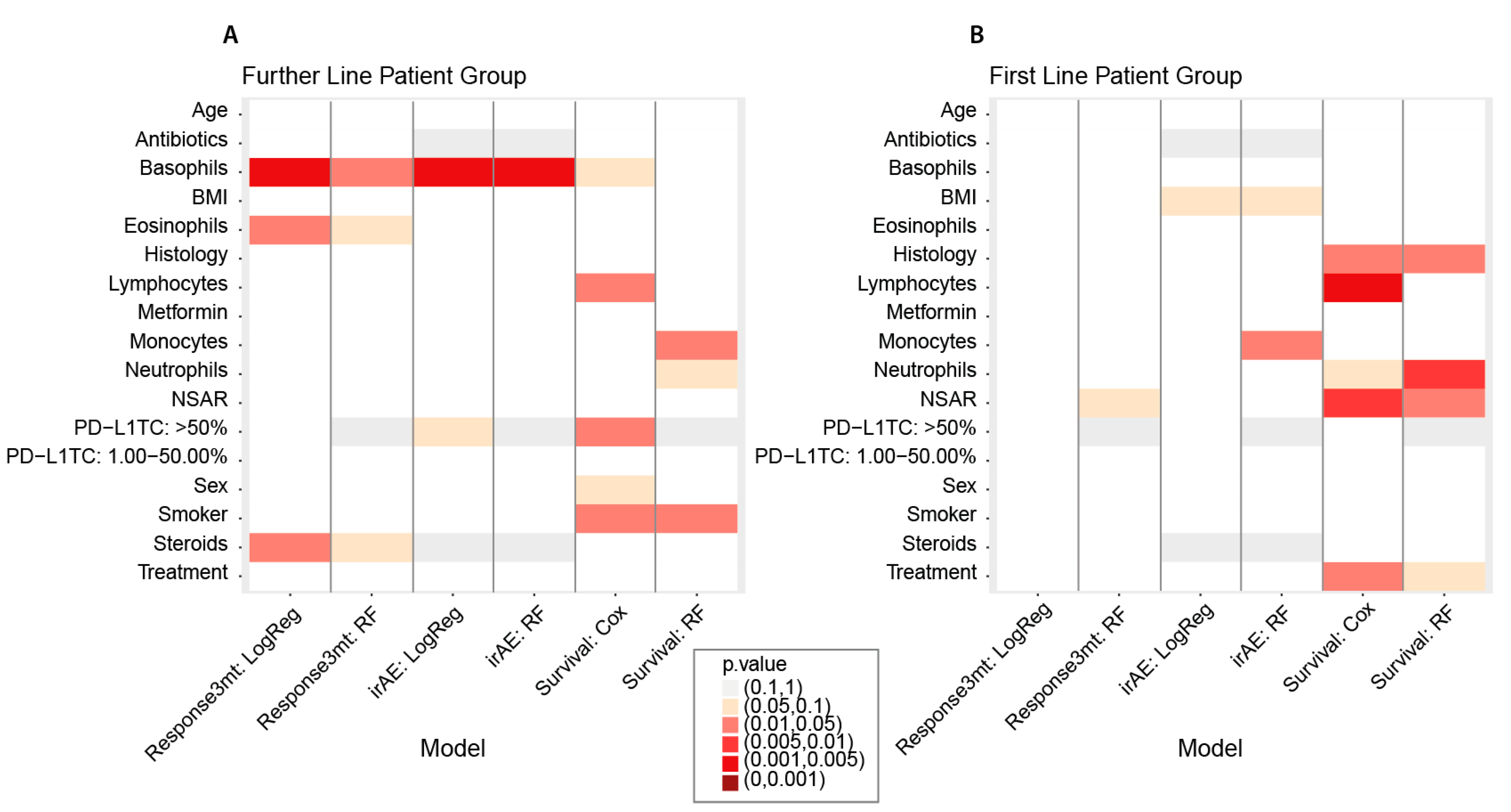
| Further Line | Response3mt | Basophils (0.01 G/l) | 1.3258 | (1.1017,1.6152) | 0.0027 | 0.0453 | 0.0014 | 0.001 | 0.0177 | 0.2832 |
| Eosinophils (0.01 G/l) | 1.0227 | (1.0032,1.0446) | 0.0215 | 0.3443 | 0.0446 | 0.2679 | 0.0978 | 1 | ||
| Steroids: TRUE | 2.3571 | (1.0547,5.4146) | 0.0438 | 0.6564 | - | - | 0.0515 | 0.7725 | ||
| irAE | Basophils (0.01 G/l) | 1.3108 | (1.0849,1.6008) | 0.0049 | 0.0839 | 0.0112 | 0.0781 | 0.0040 | 0.056 | |
| First Line | irAE | Monocytes (1 G/l) | 0.4011 | (0.0652,1.5982) | 0.2167 | 1 | 0.1849 | 1 | 0.0235 | 0.329 |
| Univariate Methods | Multivariate Methods | ||||||
|---|---|---|---|---|---|---|---|
| Univariate Cox Proportional Hazard Regressions | Survival Random Forest | ||||||
| Patient Group | Predictor | Estimate (HR) | 95% CI | Raw p-Value (LR) | Adjusted p-Value (Holm) | Raw Impurity Importance p-Value (RF) | Adjusted p-Value (Holm) |
| Further Line | Lymphocytes (G/l) | 0.7268 | (0.524, 1.008) | 0.0475 | 0.7119 | 0.6327 | 1 |
| PD-L1TC: >50% | 0.3579 | (0.1494, 0.8575) | 0.0101 | 0.1717 | 0.2144 (for whole predictor PD-L1TC) | 1 | |
| Smoker: TRUE | 0.4883 | (0.2571, 0.9274) | 0.0441 | 1 | 0.0490 | 0.7350 | |
| Monocytes (G/l) | 1.0227 | (0.5245, 1.9944) | 0.9474 | 1 | 0.0453 | 0.728 | |
| First Line | Histology: Adenocarcinoma | 0.3273 | (0.135, 0.7934) | 0.0239 | 0.3579 | 0.0202 | 0.303 |
| Lymphocytes (G/l) | 0.3912 | (0.1984,0.7714) | 0.0031 | 0.0535 | 0.1721 | 1 | |
| NSAR: TRUE | 0.2478 | (0.0738, 0.8325) | 0.0078 | 0.1256 | 0.0336 | 0.4708 | |
| Treatment: ICI & Chemo | 2.4608 | (0.9909, 6.1112) | 0.0420 | 0.5886 | 0.0989 | 1 | |
| Neutrophils (G/l) | 1.1255 | (0.9943, 1.274) | 0.081 | 1 | 0.0088 | 0.1408 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiltbrunner, S.; Spohn, M.-L.; Wechsler, R.; Akhoundova, D.; Bankel, L.; Kasser, S.; Bihr, S.; Britschgi, C.; Maathuis, M.H.; Curioni-Fontecedro, A. Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer. Cancers 2022, 14, 75. https://doi.org/10.3390/cancers14010075
Hiltbrunner S, Spohn M-L, Wechsler R, Akhoundova D, Bankel L, Kasser S, Bihr S, Britschgi C, Maathuis MH, Curioni-Fontecedro A. Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer. Cancers. 2022; 14(1):75. https://doi.org/10.3390/cancers14010075
Chicago/Turabian StyleHiltbrunner, Stefanie, Meta-Lina Spohn, Ramona Wechsler, Dilara Akhoundova, Lorenz Bankel, Sabrina Kasser, Svenja Bihr, Christian Britschgi, Marloes H. Maathuis, and Alessandra Curioni-Fontecedro. 2022. "Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer" Cancers 14, no. 1: 75. https://doi.org/10.3390/cancers14010075
APA StyleHiltbrunner, S., Spohn, M.-L., Wechsler, R., Akhoundova, D., Bankel, L., Kasser, S., Bihr, S., Britschgi, C., Maathuis, M. H., & Curioni-Fontecedro, A. (2022). Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer. Cancers, 14(1), 75. https://doi.org/10.3390/cancers14010075







