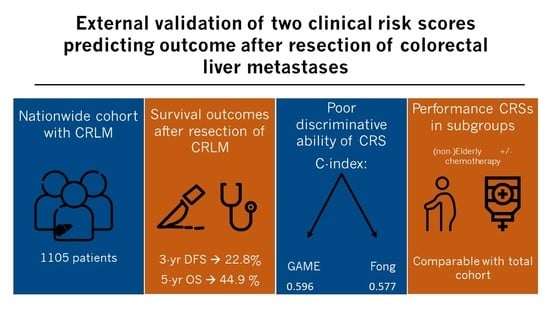
External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort
Abstract
:Simple Summary
Abstract
1. Introduction
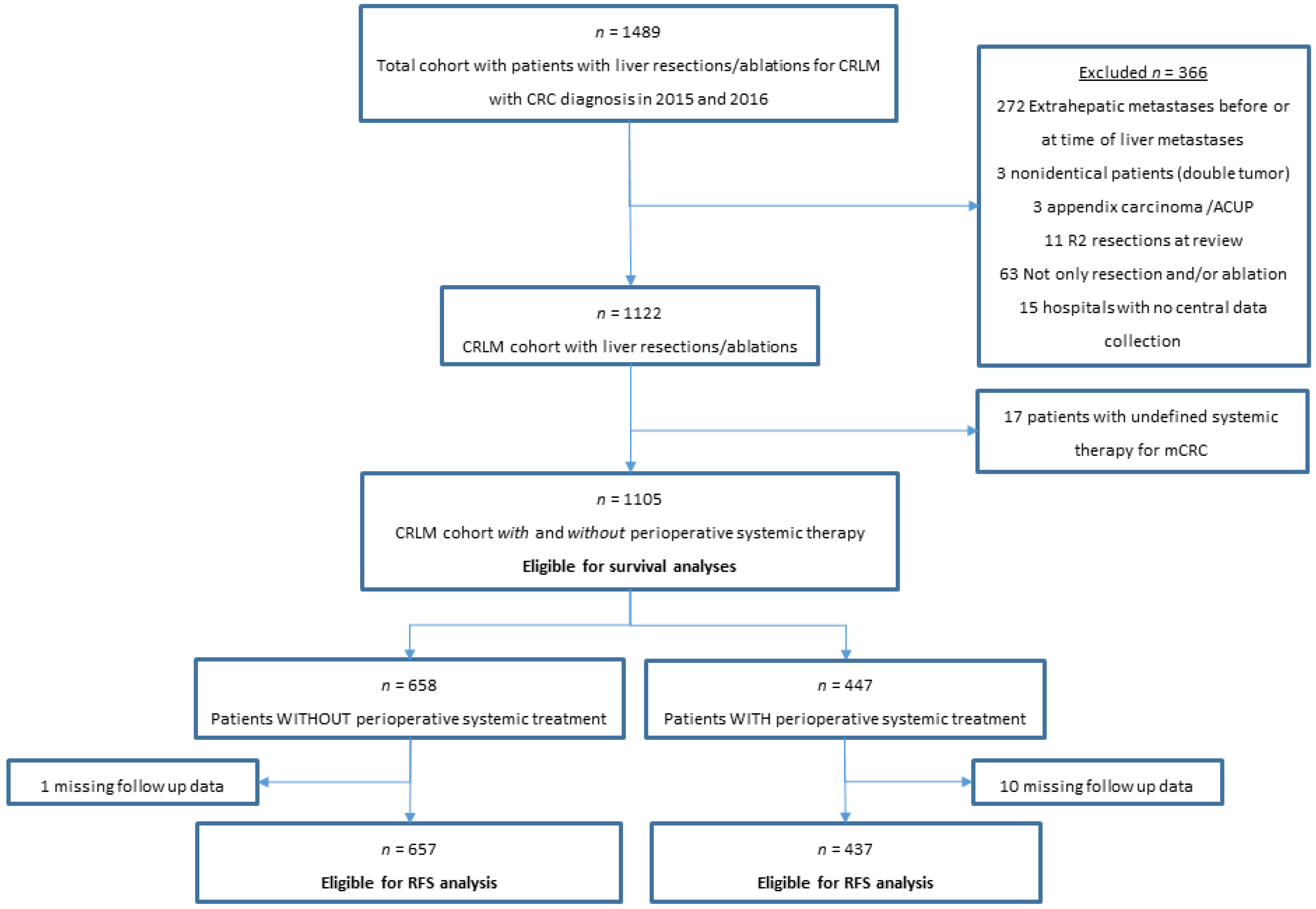
2. Materials and Methods
2.1. Population-Based Cohort
2.2. Clinical Data
2.3. Overall Survival and Disease-Free Survival
2.4. RAS and BRAFV600E Mutational Status
2.5. Statistical Analysis and Handling of Missing Data
2.6. External Validation of CRSs
3. Results
3.1. Patient Characteristics
3.2. Follow-Up and OS and DFS Outcomes in Total Cohort
3.3. External Validation of GAME and Fong CRSs in Total Cohort
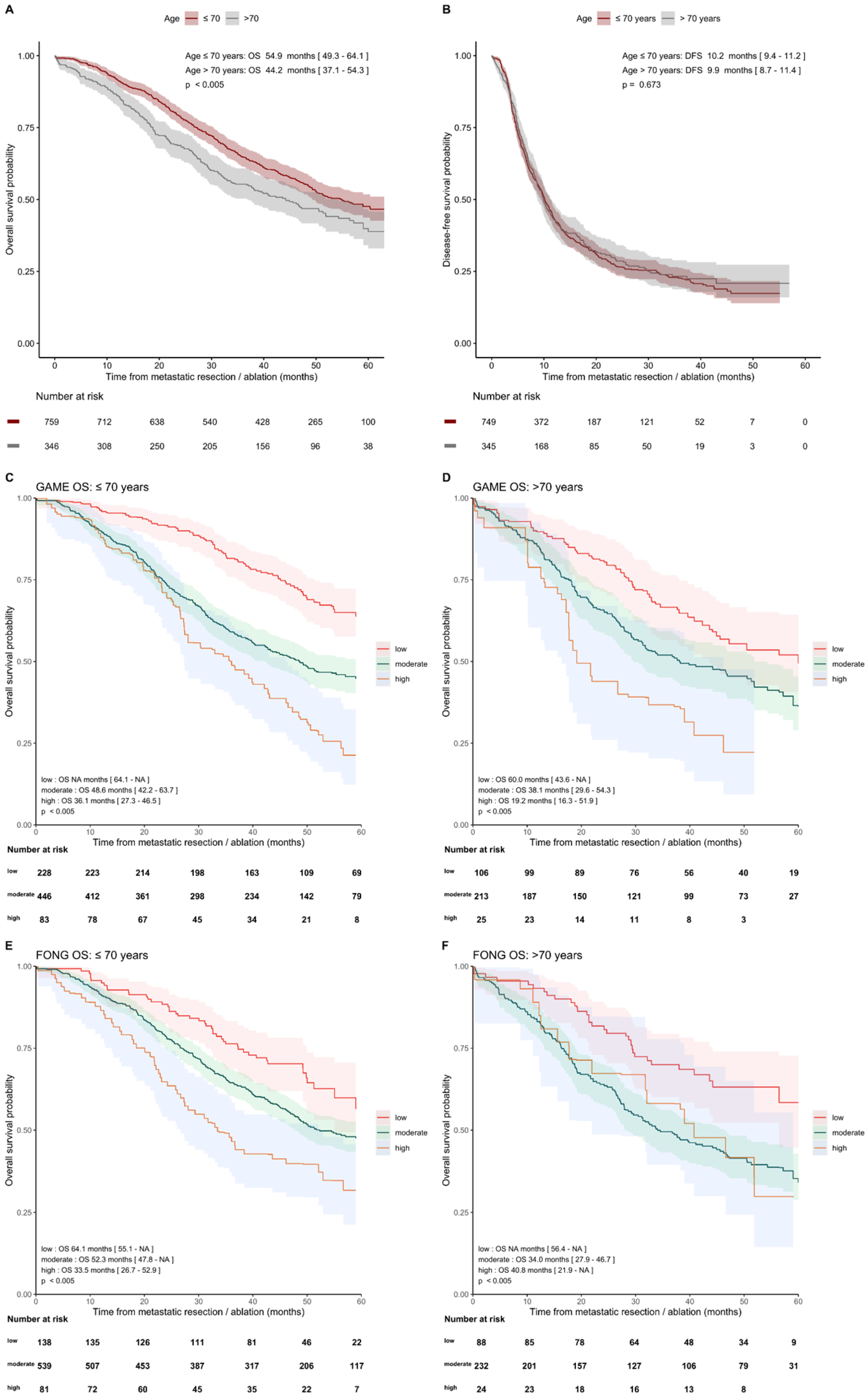
3.4. External Validation of GAME and Fong CRSs in Pre-Specified Subgroups
3.4.1. With and without Perioperative Systemic Therapy
3.4.2. Age ≤ 70 Years and >70 Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Geest, L.G.M.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.G.; De Wilt, J.H.W. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of Long-term Survival After Hepatic Resection for Metastatic Colorectal Cancer: A Multifactorial Model of 929 Patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kanas, G.; Taylor, A.; Primrose, J.N.; Langeberg, W.; Kelsh, M.; Mowat, F.; Alexander, D.; Choti, M.; Poston, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012, 4, 283–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, A.; Aloia, T.A.; Brouquet, A.; Dickson, P.V.; Zimmitti, G.; Maru, D.M.; Kopetz, S.; Loyer, E.M.; Curley, S.A.; Abdalla, E.K.; et al. Margin Status Remains an Important Determinant of Survival After Surgical Resection of Colorectal Liver Metastases in the Era of Modern Chemotherapy. Ann. Surg. 2013, 257, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Chana, P.; Burns, E.M.; Arora, S.; Darzi, A.W.; Faiz, O.D. A Systematic Review of the Impact of Dedicated Emergency Surgical Services on Patient Outcomes. Ann. Surg. 2016, 263, 20–27. [Google Scholar] [CrossRef]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updat. Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated With Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Bolhuis, K.; Kos, M.; van Oijen, M.G.; Swijnenburg, R.-J.; Punt, C.J. Conversion strategies with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: A systematic review. Eur. J. Cancer 2020, 141, 225–238. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- De Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and Patterns of Recurrence Following Curative Intent Surgery for Colorectal Liver Metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.P.; Jackson, R.; Dunne, D.F.J.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J.; Ghaneh, P. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br. J. Surg. 2012, 99, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Allard, M.-A.; Benitez, C.C.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; Bismuth, H.; Baba, H.; Adam, R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist 2016, 21, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Marsh, J.W.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Gholami, S.; Kim, Y.; Andreatos, N.; Rezaee, N.; Deshwar, A.; Buettner, S.; Allen, P.J.; Kingham, T.P.; et al. Genetic and Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br. J. Surg. 2018, 105, 1210–1220. [Google Scholar] [CrossRef]
- Zakaria, S.; Donohue, J.H.; Que, F.G.; Farnell, M.B.; Schleck, C.D.; Ilstrup, D.M.; Nagorney, D.M. Hepatic Resection for Colorectal Metastases: Value for Risk Scoring Systems? Ann. Surg. 2007, 246, 183–191. [Google Scholar] [CrossRef]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Mahar, A.L.; Compton, C.; Halabi, S.; Hess, K.R.; Weiser, M.R.; Groome, P.A. Personalizing prognosis in colorectal cancer: A systematic review of the quality and nature of clinical prognostic tools for survival outcomes. J. Surg. Oncol. 2017, 116, 969–982. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef]
- Lang, H.; Baumgart, J.; Heinrich, S.; Tripke, V.; Passalaqua, M.; Maderer, A.; Galle, P.R.; Roth, W.; Kloth, M.; Moehler, M. Extended Molecular Profiling Improves Stratification and Prediction of Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 270, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, W.; Ren, L.; Chen, J.; Tang, W.; Liu, T.; Jian, M.; Liu, Y.; Wei, Y.; Xu, J. Comprehensive Evaluation of Relapse Risk (CERR) Score for Colorectal Liver Metastases: Development and Validation. Oncologist 2020, 25, e1031–e1041. [Google Scholar] [CrossRef] [Green Version]
- Dupré, A.; Berhane, S.; Chan, A.; Rivoire, M.; Chong, C.; Lai, P.; Cucchetti, A.; Poston, G.J.; Malik, H.; Johnson, P. Multicentre validation of a clinical prognostic score integrating the systemic inflammatory response to the host for patients treated with curative-intent for colorectal liver metastases: The Liverpool score. Eur. J. Surg. Oncol. 2019, 45, 999–1004. [Google Scholar] [CrossRef]
- Paredes, A.Z.; Ms, J.M.H.; Tsilimigras, D.I.; Moro, A.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Makris, E.A.; Poultsides, G.A.; et al. A Novel Machine-Learning Approach to Predict Recurrence After Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2020, 27, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-Ticket” Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.M. Generalizing the Results of Cancer Clinical Trials. J. Clin. Oncol. 2010, 28, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Nanji, S.; Wei, X.; Mackillop, W.J. Management and Outcome of Colorectal Cancer Liver Metastases in Elderly Patients. JAMA Oncol. 2015, 1, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Adam, R.; Frilling, A.; Elias, D.; Laurent, C.; Ramos, E.; Capussotti, L.; Poston, G.J.; Wicherts, D.A.; de Haas, R.J. Liver resection of colorectal metastases in elderly patients. Br. J. Surg. 2010, 97, 366–376. [Google Scholar] [CrossRef]
- Papamichael, D.; Audisio, R.A.; Glimelius, B.; de Gramont, A.; Glynne-Jones, R.; Haller, D.; Köhne, C.-H.; Rostoft, S.; Lemmens, V.; Mitry, E.; et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann. Oncol. 2015, 26, 463–476. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Colon Cancer (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 18 June 2021).
- Nederlandse Vereniging voor Heelkunde Colorectaal Carcinoom (CRC) (Version 2019). Available online: https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Hele%20richtlijn%20CRC_Commentaarronde.pdf (accessed on 18 June 2021).
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Casparie, M.; Tiebosch, A.T.M.G.; Burger, G.; Blauwgeers, H.; van de Pol, A.; van Krieken, J.H.J.M.; Meijer, G.A. Pathology Databanking and Biobanking in The Netherlands, a Central Role for PALGA, the Nationwide Histopathology and Cytopathology Data Network and Archive. Cell. Oncol. 2007, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Steel, J.L.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Clary, B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB 2011, 13, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Mekenkamp, L.J.M.; Koopman, M.; Teerenstra, S.; Van Krieken, J.H.J.M.; Mol, L.; Nagtegaal, I.; Punt, C.J.A. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br. J. Cancer 2010, 103, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Beelen, K.; Opdam, M.; Severson, T.M.; Koornstra, R.H.T.; Vincent, A.D.; Wesseling, J.; Muris, J.J.; Berns, E.M.J.J.; Vermorken, J.B.; Van Diest, P.J.; et al. PIK3CA mutations, phosphatase and tensin homolog, human epidermal growth factor receptor 2, and insulin-like growth factor 1 receptor and adjuvant tamoxifen resistance in postmenopausal breast cancer patients. Breast Cancer Res. 2014, 16, R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.W.; Seaman, S.R.; White, I.R.; Carpenter, J.R.; Alzheimer’s Disease Neuroimaging Ingitiative. Multiple imputation of covariates by fully conditional specification: Accommodating the substantive model. Stat. Methods Med. Res. 2015, 24, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Morisot, A.; Bessaoud, F.; Landais, P.; Rébillard, X.; Trétarre, B.; Daurès, J.-P. Prostate cancer: Net survival and cause-specific survival rates after multiple imputation. BMC Med. Res. Methodol. 2015, 15, 54. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.; Altman, D.G.; Holder, R.L.; Royston, P. Combining estimates of interest in prognostic modelling studies after multiple imputation: Current practice and guidelines. BMC Med. Res. Methodol. 2009, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A. WebPlotDigitizer Version 4.4. Available online: https://automeris.io/WebPlotDigitizer (accessed on 3 November 2020).
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the Yield of Medical Tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Pitroda, S.P.; Khodarev, N.N.; Huang, L.; Uppal, A.; Wightman, S.C.; Ganai, S.; Joseph, N.; Pitt, J.; Brown, M.; Forde, M.; et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat. Commun. 2018, 9, 1793. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Bagante, F.; Moris, D.; Cloyd, J.; Spartalis, E.; Pawlik, T.M. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg. Oncol. 2018, 27, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.M.; Magni, E.; Amatu, A.; Mauri, G.; Bencardino, K.; Truini, M.; Veronese, S.M.; De Carlis, L.; Ferrari, G.; Nichelatti, M.; et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2017, 16, e153–e163. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Chun, Y.S.; Kopetz, S.; Vauthey, J. Biomarkers in colorectal liver metastases. Br. J. Surg. 2018, 105, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Reissfelder, C.; Schulze-Bergkamen, H.; Jäger, D.; Büchler, M.W.; Weitz, J.; Koch, M. Adjuvant therapy after resection of colorectal liver metastases: The predictive value of the MSKCC clinical risk score in the era of modern chemotherapy. BMC Cancer 2014, 14, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayez, N.; Lalmahomed, Z.S.; Van Der Pool, A.E.M.; Vergouwe, Y.; Van Montfort, K.; De Jonge, J.; Eggermont, A.M.M.; Ijzermans, J.N.M.; Verhoef, C. Is the Clinical Risk Score for Patients with Colorectal Liver Metastases Still Useable in the Era of Effective Neoadjuvant Chemotherapy? Ann. Surg. Oncol. 2011, 18, 2757–2763. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K. Reconsideration of the Indications for Adjuvant Chemotherapy for Liver Metastases from Colorectal Cancer After Initial Hepatectomy. Ann. Surg. Oncol. 2013, 21, 139–146. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone for liver metastasis from colorectal cancer: JCOG0603 study. J. Clin. Oncol. 2020, 38, 4005. [Google Scholar] [CrossRef]
- Ayez, N.; Van Der Stok, E.P.; De Wilt, H.; Radema, S.A.; Van Hillegersberg, R.; Roumen, R.M.; Vreugdenhil, G.; Tanis, P.J.; Punt, C.J.; Dejong, C.H.; et al. Neo-adjuvant chemotherapy followed by surgery versus surgery alone in high-risk patients with resectable colorectal liver metastases: The CHARISMA randomized multicenter clinical trial. BMC Cancer 2015, 15, 180. [Google Scholar] [CrossRef] [Green Version]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]


| NCR Cohort (n = 1105) | Patients without Systemic Therapy (n = 658) | Patients withSystemic Therapy (n = 447) | p-Value | Patients ≤ 70 Years (n = 759) | Patients > 70 Years (n = 346) | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age Median (IQI) | 66 (59–72) | 68 (61–74) | 63 (56–70) | < 0.001 | 62 (56–66) | 75 (72–78) | <0.001 | |
| Sex | 0.60 | <0.008 | ||||||
| Male | 690 (62) | 415 (63) | 275 (62) | 454 (60) | 236 (68) | |||
| Female | 415 (38) | 243 (37) | 172 (39) | 305 (40) | 110 (32) | |||
| Side primary tumor | 0.92 | 0.09 | ||||||
| Right | 261 (24) | 154 (23) | 107 (24) | 167 (22) | 94 (27) | |||
| Left | 473 (43) | 285 (43) | 188 (42) | 324 (43) | 149 (43) | |||
| Rectum | 371 (34) | 219 (33) | 152 (34) | 268 (35) | 103 (30) | |||
| Chemoradiotherapy primary tumor | <0.001 | 0.67 | ||||||
| No | 977 (88) | 556 (85) | 421 (94) | 669 (88) | 308 (89) | |||
| Yes | 128 (12) | 102 (15) | 26 (6) | 90 (12) | 38 (11) | |||
| T-status primary tumor | 0.01 | 0.97 | ||||||
| 1 | 27 (3) | 21 (3) | 6 (1) | 19 (3) | 8 (2) | |||
| 2 | 128 (12) | 86 (13) | 42 (10) | 89 (12) | 39 (11) | |||
| 3 | 757 (69) | 455 (69) | 302 (69) | 516 (69) | 241 (70) | |||
| 4 | 185 (17) | 96 (15) | 89 (20) | 129 (17) | 56 (16) | |||
| Missing | 8 (-) | 0 (-) | 8 (-) | 6 (-) | 2 (-) | |||
| Nodal status primary tumor | 0.22 | 0.71 | ||||||
| N0 | 408 (37) | 257 (39) | 151 (34) | 277 (37) | 131 (38) | |||
| N1 | 389 (35) | 224 (34) | 165 (37) | 265 (35) | 124 (36) | |||
| N2 | 306(28) | 177 (27) | 129 (29) | 216 (29) | 90 (26) | |||
| Missing | 2 (-) | 0 (-) | 2 (-) | 1 (-) | 1 (-) | |||
| Stage of disease at diagnosis | < 0.001 | 0.40 | ||||||
| I | 25 (2) | 20 (3) | 5 (1) | 16 (2) | 9 (3) | |||
| II | 102 (9) | 87 (13) | 15 (3) | 65 (9) | 37 (11) | |||
| III | 187 (17) | 162 (25) | 25 (6) | 123 (16) | 64 (19) | |||
| IV | 791 (72) | 389 (59) | 402 (90) | 555 (73) | 236 (68) | |||
| Differentiation grade of CRC | 0.12 | 0.77 | ||||||
| Low | 17 (2) | 7 (1) | 10 (3) | 13 (2) | 4 (1) | |||
| Intermediate | 936 (92) | 577 (93) | 359 (90) | 642 (92) | 294 (92) | |||
| High | 68 (7) | 37 (6) | 31 (8) | 46 (7) | 22 (7) | |||
| Missing | 84 (-) | 37 (-) | 47 (11) | 58 (-) | 26 (-) | |||
| Time to metastases | <0.001 | 0.20 | ||||||
| Synchronous | 823 (75) | 412 (63) | 411 (92) | 574 (76) | 249 (72) | |||
| Metachronous | 282 (25) | 246 (37) | 36 (8) | 185 (24) | 97 (28) | |||
| Number of liver metastases | <0.001 | <0.001 | ||||||
| Median (IQI) | 2 (1–4) | 1 (1–3) | 3 (2–6) | 2 (1–4) | 2 (1–3) | |||
| Missing | 42 | 19 | 23 | 33 | 9 | |||
| CEA level | <0.001 | 0.90 | ||||||
| Median (IQI) | 9 (3.4–36) | 6.3 (3.0–21) | 14 (4.4–74) | 18 (4.0–413) | 17 (4.7–168) | |||
| Unknown | 231 | 180 | 51 | 160 | 71 | |||
| Size largest liver metastasis, mm | 0.002 | 0.30 | ||||||
| Median (IQI) | 25 (16–36) | 23 (16–35) | 27 (16–45) | 25 (15–45) | 26 (18–42) | |||
| Missing | 86 | 45 | 41 | 58 | 28 | |||
| Type of surgery | <0.001 | 0.34 | ||||||
| Wedge/segment resection only | 589 (53) | 416 (63) | 173 (39) | 400 (53) | 189 (55) | |||
| Local ablative therapy only | 95 (9) | 63 (9) | 32 (7) | 59 (8) | 36 (10) | |||
| Wedge/segment and local ablative therapy | 189 (17) | 90 (14) | 99 (22) | 134 (18) | 55 (16) | |||
| Hemihepatectomy with/without ablation/wedge (major resection) | 232 (21) | 89 (14) | 143 (32) | 166 (22) | 66 (19) | |||
| One- or two-stage | <0.001 | 0.84 | ||||||
| 1-stage | 1042 (94) | 643 (98) | 399 (89) | 715 (94) | 327 (95) | |||
| 2-stage | 63 (6) | 15 (2) | 48 (11) | 44 (6) | 19 (6) | |||
| R-status | 0.07 | |||||||
| R0 | 866 (78) | 521 (79) | 345 (77) | 0.37 | ||||
| R1 | 143 (13) | 74 (11) | 69 (15) | 598 (79) | 268 (78) | |||
| Unknown because RFA/MWA | 96 (9) | 63 (10) | 33 (7) | 101 (13) | 42 (12) | |||
| Tumor mutational status | 0.36 | 0.93 | ||||||
| RAS mutation | 362 (51) | 221 (53) | 141 (48) | 247 (50) | 115 (52) | |||
| BRAFV600E mutation | 19 (3) | 10 (2) | 9 (3) | 13 (3) | 6 (3) | |||
| RAS and BRAFV600E wildtype | 335 (47) | 188 (45) | 147 (50) | 233 (47) | 102 (46) | |||
| Missing (RAS and/or BRAF status) | 389 (-) | 239 (-) | 150 (-) | 266 (-) | 123 (-) | |||
| GAME | Fong | NCR | |
|---|---|---|---|
| Number of patients (design/validation) | 502/747 | 1001/- | -/1105 |
| Country (design/validation) | USA/USA | USA/- | -/Dutch |
| Study design | Single center | Single center | Nation-wide multicenter |
| Patients with liver-only metastases, % | 90 | 100 | 100 |
| Handling of missing data | Patients excluded with KRAS status missing | NR | No patients excluded based on missing data |
| Available mutation status | KRAS codon 12, 13, and 61 | - | RAS/BRAF |
| Primary endpoint | OS | OS | OS |
| Preoperative systemic therapy, % | 67 | NR | 55 |
| Adjuvant systemic therapy, % | 71 | NR | 6 |
| DFI < 12 months, % | 74 | 49 | 84 |
| Factors included in CRS, (points) | Nodal status (1) CEA > 20 (1) TBS < 9 (1) TBS ≤ 9 (2) KRAS mutation (1) Extrahepatic disease (2) | Nodal status (1) CEA > 200 (1) DFI < 1year (1) >1 Liver tumor (1) Largest tumor > 5 cm (1) | - |
| GAME Score | Survival Estimates GAME Risk Categories | Fong Score | Survival Estimates Fong Risk Categories | |||||
|---|---|---|---|---|---|---|---|---|
| C-Index [95% CI] | Low (%) | Moderate (%) | High (%) | C-Index [95% CI] | Low (%) | Moderate (%) | High (%) | |
| OS | ||||||||
| 1-year | 0.583 [0.531–0.636] | 94 | 88 | 86 | 0.570 [0.521–0.619] | 95 | 89 | 87 |
| 3-year | 0.600 [0.573–0.627] | 77 | 57 | 47 | 0.578 [0.552–0.604] | 74 | 60 | 50 |
| 5-year | 0.597 [0.573–0.621] | 50 | 42 | 21 | 0.577 [0.554–0.601] | 57 | 40 | 31 |
| DFS | ||||||||
| 1-year | 0.585 [0.561–0.608] | 57 | 39 | 27 | 0.586 [0.564–0.608] | 60 | 39 | 34 |
| 3-year | 0.579 [0.557–0.600] | 30 | 21 | 14 | 0.581 [0.561–0.602] | 32 | 20 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolhuis, K.; Wensink, G.E.; Elferink, M.A.G.; Bond, M.J.G.; Dijksterhuis, W.P.M.; Fijneman, R.J.A.; Kranenburg, O.W.; Rinkes, I.H.M.B.; Koopman, M.; Swijnenburg, R.-J.; et al. External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort. Cancers 2022, 14, 2356. https://doi.org/10.3390/cancers14102356
Bolhuis K, Wensink GE, Elferink MAG, Bond MJG, Dijksterhuis WPM, Fijneman RJA, Kranenburg OW, Rinkes IHMB, Koopman M, Swijnenburg R-J, et al. External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort. Cancers. 2022; 14(10):2356. https://doi.org/10.3390/cancers14102356
Chicago/Turabian StyleBolhuis, Karen, G. Emerens Wensink, Marloes A. G. Elferink, Marinde J. G. Bond, Willemieke P. M. Dijksterhuis, Remond J. A. Fijneman, Onno W. Kranenburg, Inne H. M. Borel Rinkes, Miriam Koopman, Rutger-Jan Swijnenburg, and et al. 2022. "External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort" Cancers 14, no. 10: 2356. https://doi.org/10.3390/cancers14102356
APA StyleBolhuis, K., Wensink, G. E., Elferink, M. A. G., Bond, M. J. G., Dijksterhuis, W. P. M., Fijneman, R. J. A., Kranenburg, O. W., Rinkes, I. H. M. B., Koopman, M., Swijnenburg, R.-J., Vink, G. R., Hagendoorn, J., Punt, C. J. A., Elias, S. G., & Roodhart, J. M. L. (2022). External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort. Cancers, 14(10), 2356. https://doi.org/10.3390/cancers14102356