Cardiovascular Effects of Cumulative Doses of Radioiodine in Differentiated Thyroid Cancer Patients with Type 2 Diabetes Mellitus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Statistics
3. Results
3.1. Characteristics of the Study Population
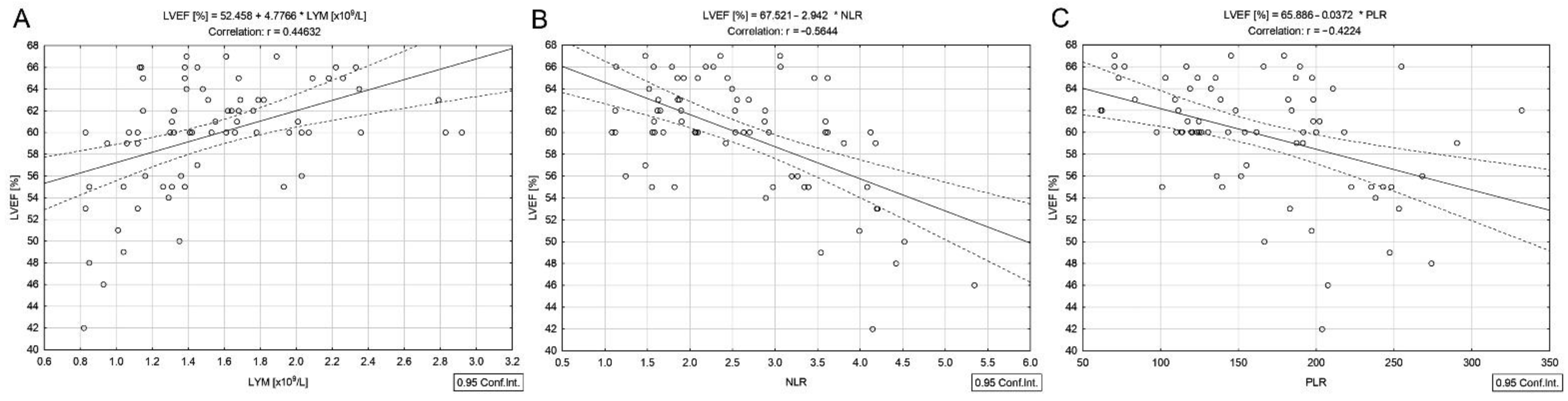
3.2. Correlations in DTC/−T2DM Group
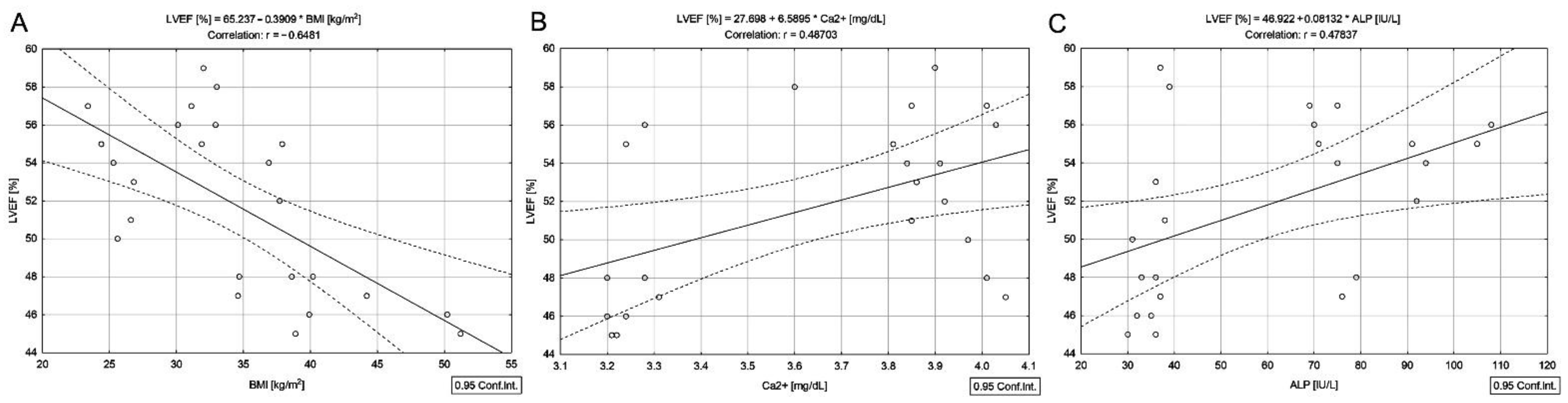
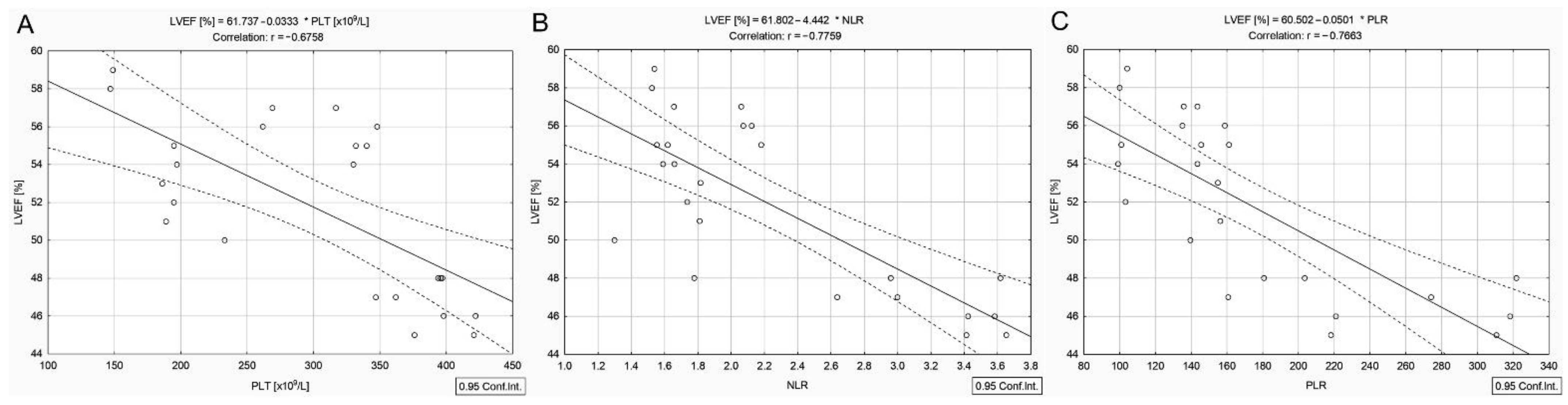
3.3. Correlations in DTC/+T2DM Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 1 February 2022).
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Association of Type 2 Diabetes With Cancer: A Meta-analysis With Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care 2020, 43, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.M.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Risk of cancer incidence and mortality associated with diabetes: A systematic review with trend analysis of 203 cohorts. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Noh, E.; Hwang, S.Y.; Kim, J.A.; Song, E.; Park, M.; Choi, K.M.; Baik, S.H.; Cho, G.J.; Yoo, H.J. Increased Risk of Type 2 Diabetes in Patients With Thyroid Cancer After Thyroidectomy: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2021, 107, e1047–e1056. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019, 10, 2035–2044. [Google Scholar] [CrossRef] [Green Version]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyr. J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- la Cour, J.L.; Hedemann-Jensen, P.; Søgaard-Hansen, J.; Nygaard, B.; Jensen, L.T. Modeling the absorbed dose to the common carotid arteries following radioiodine treatment of benign thyroid disease. Ann. Nucl. Med. 2013, 27, 862–866. [Google Scholar] [CrossRef]
- la Cour, J.L.; Andersen, U.B.; Sørensen, C.H.; Nygaard, B.; Jensen, L.T. Radioiodine Therapy Does Not Change the Atherosclerotic Burden of the Carotid Arteries. Thyroid 2016, 26, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Gheorghe, D.; Stanciu, M.; Zamfirescu, A.; Stanciu, A. TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis. Cancers 2021, 13, 3609. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Kuravi, S.J.; Harrison, P.; Rainger, G.E.; Nash, G.B. Ability of Platelet-Derived Extracellular Vesicles to Promote Neutrophil-Endothelial Cell Interactions. Inflammation 2019, 42, 290–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisdom, A.J.; Hong, C.S.; Lin, A.J.; Xiang, Y.; Cooper, D.E.; Zhang, J.; Xu, E.S.; Kuo, H.-C.; Mowery, Y.M.; Carpenter, D.J.; et al. Neutrophils promote tumor resistance to radiation therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 18584–18589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, P.; de Vries, F.; Dekkers, O.M.; Korbonits, M.; Biermasz, N.R.; Pereira, A.M. Serum Inflammation-based Scores in Endocrine Tumors. J. Clin. Endocrinol. Metab. 2021, 106, e3796–e3819. [Google Scholar] [CrossRef] [PubMed]
- D’Emic, N.; Engelman, A.; Molitoris, J.; Hanlon, A.; Sharma, N.K.; Moeslein, F.M.; Chuong, M.D. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J. Gastrointest. Oncol. 2016, 7, 269–277. [Google Scholar] [CrossRef]
- Roman, G.; Pantea Stoian, A. Cardiovascular risk/disease in type 2 diabetes mellitus. In Type 2 Diabetes: From Pathophysiology to Cyber Systems; Stoian, A.P., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; Wexler, J.A.; et al. Radiation Safety in the Treatment of Patients with Thyroid Diseases by Radioiodine 131I: Practice Recommendations of the American Thyroid Association, The American Thyroid Associ-ation Taskforce on Radioiodine Safety. Thyroid 2011, 21, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Bedel, C.; Korkut, M.; Armağan, H.H. NLR, d-NLR and PLR can be affected by many factors. Int. Immunopharmacol. 2020, 90, 107154. [Google Scholar] [CrossRef]
- Jacobse, J.N.; Steggink, L.; Sonke, G.S.; Schaapveld, M.; Hummel, Y.M.; Steenbruggen, T.G.; Lefrandt, J.D.; Nuver, J.; Crijns, A.P.; Aleman, B.M.; et al. Myocardial dysfunction in long-term breast cancer survivors treated at ages 40–50 years. Eur. J. Heart Fail. 2019, 22, 338–346. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Zamfir-Chiru-Anton, A.; Stanciu, M.M.; Pantea-Stoian, A.; Nitipir, C.; Gheorghe, D.C. Serum melatonin is inversely associated with matrix metalloproteinase-9 in oral squamous cell carcinoma. Oncol. Lett. 2020, 19, 3011–3020. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; Updated 29 June 2021; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prinsen, H.T.; Hesselink, E.N.K.; Brouwers, A.H.; Plukker, J.T.M.; Sluiter, W.J.; van der Horst-Schrivers, A.N.A.; van Imhoff, G.W.; Links, T.P. Bone Marrow Function After131I Therapy in Patients With Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2015, 100, 3911–3917. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Meng, Z.; Zhang, G.; Jia, Q.; Tan, J.; Zheng, W.; Wang, R.; Li, X.; Liu, N.; Zhou, P.; et al. Influence of the first radioactive iodine ablation on peripheral complete blood count in patients with differentiated thyroid cancer. Medicine 2016, 95, e4451. [Google Scholar] [CrossRef]
- Rui, Z.; Wu, R.; Zheng, W.; Wang, X.; Meng, Z.; Tan, J. Effect of ¹³¹I Therapy on Complete Blood Count in Patients with Differentiated Thyroid Cancer. Med. Sci. Monit. 2021, 27, e929590. [Google Scholar] [CrossRef] [PubMed]
- Shlomai, G.; Neel, B.; Leroith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Qiu, B.; Wang, H.; Jiang, P.; Sukocheva, O.; Fan, R.; Xue, L.; Wang, J. Stereotactic Ablative Brachytherapy: Recent Advances in Optimization of Radiobiological Cancer Therapy. Cancers 2021, 13, 3493. [Google Scholar] [CrossRef]
- Kim, K.J.; Song, J.E.; Kim, J.Y.; Bae, J.H.; Kim, N.H.; Yoo, H.J.; Kim, H.Y.; Seo, J.A.; Kim, N.H.; Lee, J.; et al. Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: A nationwide cohort study. Ann. Transl. Med. 2020, 8, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-H.; Chung, C.-H.; Chien, W.-C.; Shen, D.H.-Y.; Lin, L.-F.; Chiu, C.-H.; Cheng, C.-Y.; Sun, C.-A.; Chang, P.-Y. Radioactive Iodine Treatment and the Risk of Long-Term Cardiovascular Morbidity and Mortality in Thyroid Cancer Patients: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 4032. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.I.; Kim, D.; Ahn, S.G.; Bae, S.J.; Cha, C.; Park, S.; Park, S.; Kim, S.I.; Lee, H.S.; Park, J.Y.; et al. Radiotherapy-Induced High Neutrophil-to-Lymphocyte Ratio is a Negative Prognostic Factor in Patients with Breast Cancer. Cancers 2020, 12, 1896. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, A.E. Cytokines in heart failure. Adv. Clin. Chem. 2019, 93, 63–113. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Offersen, B.V.; Nielsen, H.M.; Vaage-Nilsen, M.; Yusuf, S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol. 2017, 40, 255–261. [Google Scholar] [CrossRef]
- Ceriello, A.; Catrinoiu, D.; Chandramouli, C.; Cosentino, F.; Dombrowsky, A.C.; Itzhak, B.; Lalic, N.M.; Prattichizzo, F.; Schnell, O.; Seferović, P.M.; et al. Heart failure in type 2 diabetes: Current perspectives on screening, diagnosis and management. Cardiovasc. Diabetol. 2021, 20, 218. [Google Scholar] [CrossRef]
- Rodriguez, B.A.T.; Johnson, A.D. Platelet Measurements and Type 2 Diabetes: Investigations in Two Population-Based Cohorts. Front. Cardiovasc. Med. 2020, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Randeria, S.; Thomson, G.J.A.; Nell, T.A.; Roberts, T.; Pretorius, E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc. Diabetol. 2019, 18, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johny, E.; Bhaskar, P.; Alam, J.; Kuladhipati, I.; Das, R.; Adela, R. Platelet Mediated Inflammation in Coronary Artery Disease with Type 2 Diabetes Patients. J. Inflamm. Res. 2021, 14, 5131–5147. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.A.F.; Costa, T.; Rema, J.; Pinto, C.; Magalhães, M.; Esperança, A.; Sousa, L. Hypocalcemia in cancer patients: An exploratory study. Porto Biomed. J. 2019, 4, e45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, H.; Hao, X.; Yang, J.; Chen, Q.; Lu, L.; Zhang, R. Low serum calcium is associated with left ventricular systolic dysfunction in a Chinese population with coronary artery disease. Sci. Rep. 2016, 6, 22283. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, N.; Dai, W.; Jiang, L.; Li, Y.; Li, S.; Wen, Z. Association of serum calcium and heart failure with preserved ejection fraction in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Pakhetra, R.; Garg, M. Evaluation of bone mineral density in type 2 diabetes mellitus patients before and after treatment. Med. J. Armed Forces India 2012, 68, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Kats, S.; Brands, R.; Seinen, W.; de Jager, W.; Bekker, M.W.A.; Hamad, M.A.S.; Tan, M.E.S.H.; Schonberger, J.P.A.M. Anti-Inflammatory Effects of Alkaline Phosphatase in Coronary Artery Bypass Surgery with Cardiopulmonary Bypass. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Presbitero, A.; Mancini, E.; Brands, R.; Krzhizhanovskaya, V.V.; Sloot, P.M.A. Supplemented Alkaline Phosphatase Supports the Immune Response in Patients Undergoing Cardiac Surgery: Clinical and Computational Evidence. Front. Immunol. 2018, 9, 2342. [Google Scholar] [CrossRef]


| Variables | DTC/−T2DM | DTC/+T2DM | p-Value |
|---|---|---|---|
| n = 72 | n = 24 | ||
| Age (years) a | 57.9 ± 8.7 | 61.1 ± 7.2 | 0.147 |
| BMI (kg/m2) b | 29.0 (26.2–33.4) | 33.8 (28.4–38.7) | 0.006 |
| LVEF (%) b | 60.0 (56.5–63.5) | 52.5 (47.5–55.5) | <0.001 |
| Cumulative 131I dose (mCi) b Levothyroxine dose (mcg/day) | 208.5 (152.8–577.0) 107.4 (86.2–149.5) | 494.0 (176.0–817.0) 107.2 (83.4–143.8) | 0.041 0.231 |
| Lymphocytes (×109/L) b | 1.4 (1.1–1.8) | 1.9 (1.4–2.1) | 0.015 |
| Neutrophils (×109/L) b | 3.7 (3.0–4.4) | 3.6 (3.1–4.5) | 0.731 |
| Platelets (×109/L) b | 230.0 (193.0–279.5) | 331.0 (196.0–385.0) | 0.002 |
| NLR b | 2.5 (1.8–3.4) | 1.9 (1.6–2.9) | 0.150 |
| PLR b | 152.9 (119.3–199.1) | 155.6 (135.4–210.8) | 0.629 |
| Total Cholesterol (mg/dL) b | 277.0 (215.5–350.0) | 272.0 (149.5–332.5) | 0.265 |
| Lipids (mg/dL) b | 851.5 (688.0–1057.0) | 852.0 (682.0–1045.5) | 0.479 |
| Triglycerides (mg/dL) b | 111.0 (94.0–170.0) | 154.5 (102.5–272.0) | 0.049 |
| Ca2+ (mg/dL) b | 3.8 (3.6–3.9) | 3.8 (3.2–3.9) | 0.087 |
| ALP (IU/L) b | 66.5 (54.0–82.0) | 54.0 (36.0–77.5) | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, A.E.; Stanciu, M.M.; Zamfirescu, A.; Gheorghe, D.C. Cardiovascular Effects of Cumulative Doses of Radioiodine in Differentiated Thyroid Cancer Patients with Type 2 Diabetes Mellitus. Cancers 2022, 14, 2359. https://doi.org/10.3390/cancers14102359
Stanciu AE, Stanciu MM, Zamfirescu A, Gheorghe DC. Cardiovascular Effects of Cumulative Doses of Radioiodine in Differentiated Thyroid Cancer Patients with Type 2 Diabetes Mellitus. Cancers. 2022; 14(10):2359. https://doi.org/10.3390/cancers14102359
Chicago/Turabian StyleStanciu, Adina Elena, Marcel Marian Stanciu, Anca Zamfirescu, and Dan Cristian Gheorghe. 2022. "Cardiovascular Effects of Cumulative Doses of Radioiodine in Differentiated Thyroid Cancer Patients with Type 2 Diabetes Mellitus" Cancers 14, no. 10: 2359. https://doi.org/10.3390/cancers14102359






