Secondary Malignancy Risk Following Proton vs. X-ray Radiotherapy of Thymic Epithelial Tumors: A Comparative Modeling Study of Thoracic Organ-Specific Cancer Risk
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
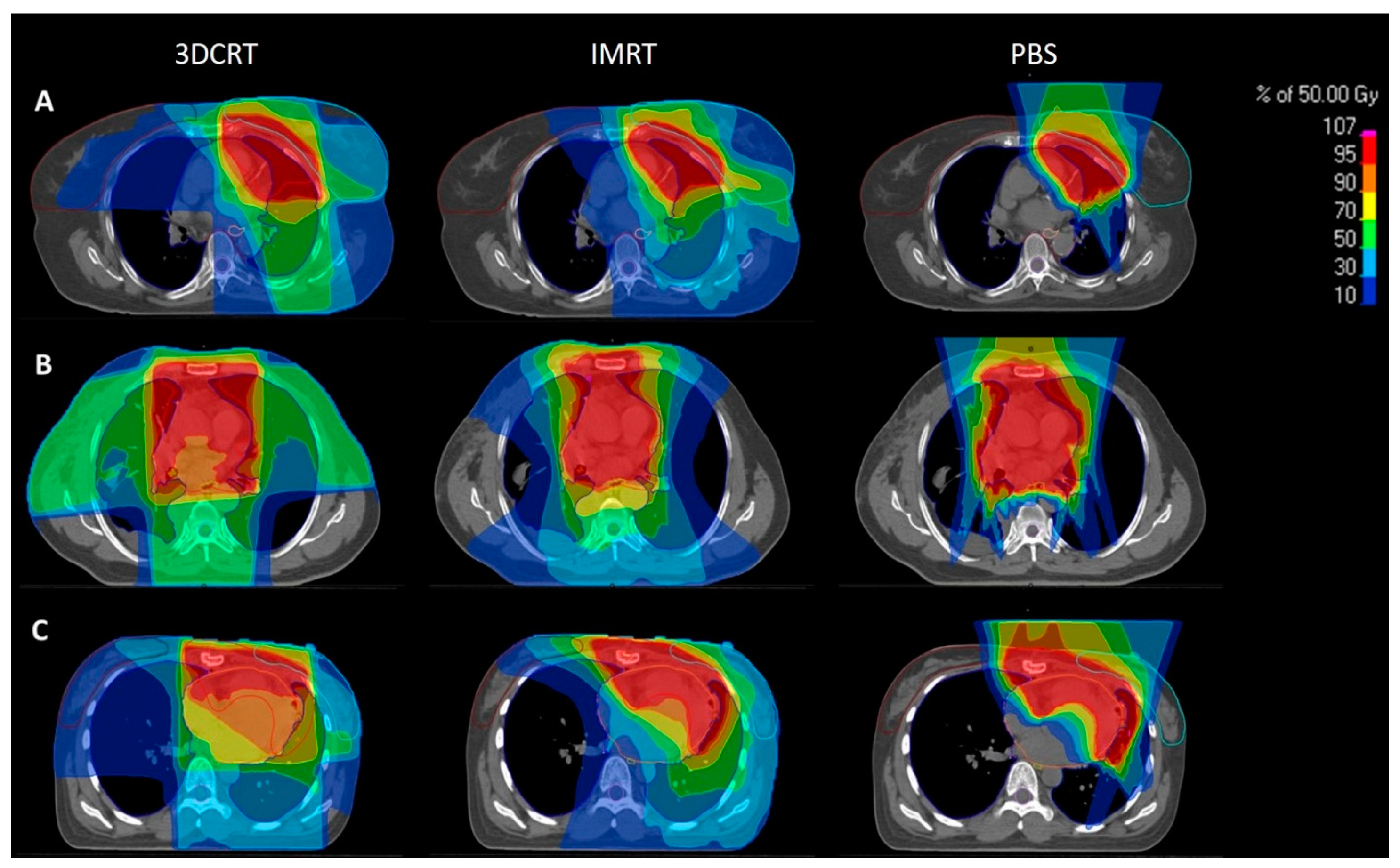
2.1. Patient Selection and Treatment Planning
2.2. Risk Estimation for Radiation-Induced Secondary Cancers
Dasu Model
2.3. Schneider Model
2.4. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Risk Estimation of Secondary Malignancies: Dasu Model
3.3. Risk Estimation of Secondary Malignancies: Schneider Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, A.; Chan, J.K.; Coindre, J.-M.; Detterbeck, F.; Girard, N.; Harris, N.L.; Jaffe, E.S.; Kurrer, M.O.; Marom, E.M.; Moreira, A.L.; et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J. Thorac. Oncol. 2015, 10, 1383–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Garg, M.; Goyal, A.; Chaudhary, N.; Soni, P.; Chandra, A.B. Changing pattern of secondary cancers among patients with malignant thymoma in the USA. Futur. Oncol. 2018, 14, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-C.; Chen, P.C.-H.; Wang, L.-S.; Chi, K.-H.; Chiang, H. Thymoma is associated with an increased risk of second malignancy. Cancer 2001, 92, 2406–2411. [Google Scholar] [CrossRef]
- Travis, L.B.; Boice, J.D., Jr.; Travis, W.D. Second primary cancers after thymoma. Int. J. Cancer 2003, 107, 868–870. [Google Scholar] [CrossRef]
- Hamaji, M.; Sozu, T.; Machida, R.; Omasa, M.; Menju, T.; Aoyama, A.; Sato, T.; Chen-Yoshikawa, T.-F.; Sonobe, M.; Date, H. Second malignancy versus recurrence after complete resection of thymoma. Asian Cardiovasc. Thorac. Ann. 2018, 26, 290–295. [Google Scholar] [CrossRef]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S.; ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v40–v55. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.W.; Palma, D.A.; Camidge, D.R.; Jones, B.; Robin, T.; Sher, D.J.; Koshy, M.; Kavanagh, B.D.; Gaspar, L.E.; Rusthoven, C.G. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2017, 12, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Willmann, J.; Rimner, A. The expanding role of radiation therapy for thymic malignancies. J. Thorac. Dis. 2018, 10 (Suppl. 21), S2555–S2564. [Google Scholar] [CrossRef]
- Vogel, J.; Berman, A.; Lin, L.; Pechet, T.T.; Levin, W.P.; Gabriel, P.; Khella, S.L.; Singhal, S.; Kucharczuk, J.K.; Simone, C. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother. Oncol. 2016, 118, 504–509. [Google Scholar] [CrossRef]
- Gomez, D.; Komaki, R.; Yu, J.; Ikushima, H.; Bezjak, A. Radiation Therapy Definitions and Reporting Guidelines for Thymic Malignancies. J. Thorac. Oncol. 2011, 6 (Suppl. 3), S1743–S1748. [Google Scholar] [CrossRef] [Green Version]
- Parikh, R.R.; Rhome, R.; Hug, E.; Tsai, H.; Cahlon, O.; Chon, B.; Goenka, A. Adjuvant Proton Beam Therapy in the Management of Thymoma: A Dosimetric Comparison and Acute Toxicities. Clin. Lung Cancer 2016, 17, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Hoppe, B.S.; Flampouri, S.; Louis, D.; Pirris, J.; Nichols, R.C.; Henderson, R.H.; Mercado, C.E. Rationale and early outcomes for the management of thymoma with proton therapy. Transl. Lung Cancer Res. 2018, 7, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Haefner, M.F.; Verma, V.; Bougatf, N.; Mielke, T.; Tonndorf-Martini, E.; König, L.; Rwigema, J.-C.M.; Nd, C.B.S.; Uhlmann, L.; Eichhorn, F.; et al. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol. 2018, 57, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- McGunigal, M.; Margolis, M.; Forsthoefel, M.; Singh, T.; Amarell, K.; Deblois, D.; Campbell, L.; Kim, C.; Liu, S.; Bergquist, P.J.; et al. Thymic malignancies treated with active scanning proton beam radiation and Monte Carlo planning: Early clinical experience. Acta Oncol. 2021, 60, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Daşu, A.; Toma-Daşu, I.; Olofsson, J.; Karlsson, M. The use of risk estimation models for the induction of secondary cancers following radiotherapy. Acta Oncol. 2005, 44, 339–347. [Google Scholar] [CrossRef]
- Schneider, U.; Zwahlen, D.; Ross, D.; Kaser-Hotz, B. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int. J. Radiat. Oncol. 2005, 61, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Mondlane, G.; Gubanski, M.; Lind, P.A.; Ureba, A.; Siegbahn, A. Comparative study of the calculated risk of radiation-induced cancer after photon- and proton-beam based radio-surgery of liver metastases. Phys. Med. 2017, 42, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Schneider, U.; Kaser-Hotz, B. A simple dose-response relationship for modeling secondary cancer incidence after radiotherapy. Z. Med. Phys. 2005, 15, 31–37. [Google Scholar] [CrossRef]
- Vogel, J.; Lin, L.; Litzky, L.A.; Berman, A.T.; Simone, C.B., II. Predicted Rate of Secondary Malignancies Following Adjuvant Proton Versus Photon Radiation Therapy for Thymoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 427–433. [Google Scholar] [CrossRef]
- Chargari, C.; Cosset, J.M. The issue of low doses in radiation therapy and impact on radiation-induced secondary malignancies. Bull. Cancer 2013, 100, 1333–1342. [Google Scholar] [CrossRef]
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Isacsson, U.; Blomquist, E.; Grusell, E.; Jung, B.; Montelius, A. Potential gains using high-energy protons for therapy of malignant tumours. Acta Oncol. 1999, 38, 137–145. [Google Scholar] [PubMed] [Green Version]
- Adeberg, S.; Harrabi, S.; Bougatf, N.; Bernhardt, D.; Rieber, J.; Koerber, S.A.; Syed, M.; Sprave, T.; Mohr, A.; Abdollahi, A.; et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in ana-plastic astrocytoma and glioblastoma: A dosimetric comparison. Strahlenther. Onkol. 2016, 192, 770–779. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int. J. Cancer 2003, 105, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Gadalla, S.M.; Rajan, A.; Pfeiffer, R.; Kristinsson, S.Y.; Björkholm, M.; Landgren, O.; Giaccone, G. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int. J. Cancer 2010, 128, 2688–2694. [Google Scholar] [CrossRef] [PubMed]


| Organ | α1 (Gy−1) Fatal Risk | α1 (Gy−1) Total Risk |
|---|---|---|
| Lung | 0.0101 | 0.0144 |
| Breast | 0.0028 | 0.0144 |
| Esophagus | 0.0014 | 0.0015 |
| Thyroid | 0.0028 | 0.0144 |
| Organ | (per 10,000 Patients/year/Gy) | (Gy−1) |
|---|---|---|
| Lung | 1.68 | 0.129 |
| Breast | 0.78 | 0.08 |
| Esophagus | 0.61 | 0.274 |
| Thyroid | 0.75 | 0.033 |
| Patient No. | Sex | Age | Masaoka Stage | WHO Type | R-Status | Max. Tumor Size [mm] | RT Total Dose [Gy] | Fractions |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 77 | IIa | B1/B2 | R0 | 40 | 50 | 25 |
| 2 | F | 23 | I | B2 | R0 | 13 | 50 | 25 |
| 3 | F | 58 | IIa | B2 | R0 | 37 | 50 | 25 |
| 4 | M | 42 | IIa | AB | R1 | 37 | 54 | 27 |
| 5 | M | 71 | IIa | B3 | R0 | 30 | 50 | 25 |
| 6 | M | 56 | III | AB | R0 | 80 | 50 | 25 |
| 7 | F | 53 | IIa | B2 | R0 | 17 | 50 | 25 |
| 8 | F | 44 | IIa | B2/B3 | R0 | 42 | 54 | 27 |
| 9 | M | 47 | IVa | B3 | R2 | 53 | 66 | 33 |
| 10 | M | 62 | IVa | B1/B2 | R2 | 99 | 66 | 33 |
| 11 | F | 69 | I | B3 | R0 | 65 | 54 | 30 |
| 12 | M | 70 | II | B2 | R1 | 59 | 54 | 30 |
| 13 | F | 78 | IIA | C | R0 | 44 | 54 | 30 |
| 14 | M | 65 | IVB | N/A | N/A | 49 | 45 | 25 |
| 15 | F | 73 | IVB | B2 | R0 | 139 | 54 | 30 |
| 16 | F | 31 | III | B2 | R1 | 90 | 54 | 30 |
| 17 | M | 17 | I | B2 | R1 | 110 | 54 | 30 |
| Dasu Total | 3DCRT (%) | IMRT (%) | PBS (%) | PBS vs. 3DCRT | PBS vs. IMRT | IMRT vs. 3DCRT |
|---|---|---|---|---|---|---|
| Lung total | 1.95 (1.01–2.36) | 2.13 (1.57–2.59) | 0.84 (0.38–1.47) | p < 0.001 | p < 0.001 | p = 0.055 |
| Breast left | 16.38 (2.83–34.73) | 11.32 (3.35–23.88) | 5.18 (1.27–17.37) | p = 0.001 | p < 0.001 | p = 0.019 |
| Breast right | 17.94 (0.15–34.01) | 11.59 (1.79–2.38) | 3.21 (0–14.56) | p < 0.001 | p < 0.001 | p = 0.019 |
| Esophagus | 0.96 (0.58–1.5) | 0.96 (0.61–1.5) | 0.57 (0.03–1.03) | p = 0.001 | p < 0.001 | p = 0.868 |
| Thyroid | 3.06 (0–49.15) | 2.51 (0–59.18) | 2.03 (0–58.96) | p = 0.379 | p = 0.796 | p = 0.918 |
| Dasu Fatal | 3DCRT (%) | IMRT (%) | PBS (%) | PBS vs. 3DCRT | PBS vs. IMRT | IMRT vs. 3DCRT |
| Lung fatal | 1.37 (0.71–1.65) | 1.49 (1.1–1.82) | 0.59 (0.27–1.03) | p < 0.001 | p < 0.001 | p = 0.055 |
| Breast left | 3.19 (0.55–6.75) | 2.20 (0.65–4.64) | 1.01 (0.25–3.38) | p = 0.001 | p < 0.001 | p = 0.019 |
| Breast right | 3.49 (0.03–6.61) | 2.25 (0.35–4.35) | 0.62 (0–2.83) | p < 0.001 | p < 0.001 | p = 0.019 |
| Esophagus | 0.89 (0.55-1.40) | 0.90 (0.57-1.4) | 0.53 (0.03–0.96) | p = 0.001 | p < 0.001 | p = 0.868 |
| Thyroid | 0.59 (0–9.56) | 0.49 (0–11.51) | 0.4 (0–11.46) | p = 0.379 | p = 0.796 | p = 0.918 |
| Cancer Incidence Rates | 3DCRT | IMRT | PBS | PBS vs. 3DCRT | PBS vs. IMRT | IMRT vs. 3DCRT |
|---|---|---|---|---|---|---|
| Lung total | 2.74 (1.52–3.36) | 2.88 (2.05–3.24) | 1.49 (0.69–2.03) | p < 0.001 | p < 0.001 | p = 0.619 |
| Breast left | 2.15 (0.47–3.13) | 1.68 (0.59–2.62) | 0.81 (0.22–2.05) | p = 0.001 | p < 0.001 | p = 0.035 |
| Breast right | 2.26 (0.03–3.11) | 1.72 (0.34–2.26) | 0.55 (0–1.75) | p < 0.001 | p < 0.001 | p = 0.044 |
| Esophagus | 1.56 (1.11–2.26) | 1.54 (0.99–2.23) | 1.04 (0.05–1.96) | p = 0.001 | p < 0.001 | p = 0.463 |
| Thyroid | 0.83 (0.22–6.27) | 0.61 (0.15–6.86) | 0.79 (0–6.85) | p = 0.679 | p = 0.959 | p = 0.717 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, L.; Hörner-Rieber, J.; Forsthoefel, M.; Haering, P.; Meixner, E.; Eichkorn, T.; Krämer, A.; Mielke, T.; Tonndorf-Martini, E.; Haefner, M.F.; et al. Secondary Malignancy Risk Following Proton vs. X-ray Radiotherapy of Thymic Epithelial Tumors: A Comparative Modeling Study of Thoracic Organ-Specific Cancer Risk. Cancers 2022, 14, 2409. https://doi.org/10.3390/cancers14102409
König L, Hörner-Rieber J, Forsthoefel M, Haering P, Meixner E, Eichkorn T, Krämer A, Mielke T, Tonndorf-Martini E, Haefner MF, et al. Secondary Malignancy Risk Following Proton vs. X-ray Radiotherapy of Thymic Epithelial Tumors: A Comparative Modeling Study of Thoracic Organ-Specific Cancer Risk. Cancers. 2022; 14(10):2409. https://doi.org/10.3390/cancers14102409
Chicago/Turabian StyleKönig, Laila, Juliane Hörner-Rieber, Matthew Forsthoefel, Peter Haering, Eva Meixner, Tanja Eichkorn, Anna Krämer, Thomas Mielke, Eric Tonndorf-Martini, Matthias F. Haefner, and et al. 2022. "Secondary Malignancy Risk Following Proton vs. X-ray Radiotherapy of Thymic Epithelial Tumors: A Comparative Modeling Study of Thoracic Organ-Specific Cancer Risk" Cancers 14, no. 10: 2409. https://doi.org/10.3390/cancers14102409
APA StyleKönig, L., Hörner-Rieber, J., Forsthoefel, M., Haering, P., Meixner, E., Eichkorn, T., Krämer, A., Mielke, T., Tonndorf-Martini, E., Haefner, M. F., Debus, J., & Lischalk, J. W. (2022). Secondary Malignancy Risk Following Proton vs. X-ray Radiotherapy of Thymic Epithelial Tumors: A Comparative Modeling Study of Thoracic Organ-Specific Cancer Risk. Cancers, 14(10), 2409. https://doi.org/10.3390/cancers14102409







