Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Nanocarrier-Mediated Drug Delivery: Overcoming Solubility Issues, Improving Sustained Release, Favoring Both Cell Uptake and Tumor Delivery
2.1. In Vitro: Loading of Chemotherapeutic Drugs into Rationally-Designed Nanocarriers
2.2. In Vitro and In Vivo: Regulating Drug Release from the Nanoparticle
2.3. In Vitro and In Vivo: Maximizing Cell Uptake and Intracellular Delivery
2.4. In Vivo: Optimizing Biodistribution and Pharmacokinetics
3. Drug Delivery Systems Based on Passive and Active Targeting
3.1. Passive Targeting
3.1.1. Enhanced Permeability and Retention (EPR) Effect and Transendothelial Migration
3.1.2. Exploiting Biochemical Properties of the Tumor Microenvironment: Nanocarrier-Loaded Drugs
3.1.3. Exploiting Biochemical Properties of the Tumor Microenvironment: Prodrug-Based Nanoparticles
3.1.4. Exploiting Biochemical Properties of the Tumor Microenvironment: Multimodal Nanoparticles
3.1.5. Exploiting Specific Molecular Features of the Tumor Microenvironment
3.2. Active Targeting: Antibodies and Antibody Fragments as Targeting Moieties
3.2.1. Antibodies
3.2.2. Antibody Fragments
3.3. Active Targeting: Peptides as Targeting Moieties
3.3.1. Arginine-glycine-aspartate (RGD) Peptides
3.3.2. Rationally Designed Peptides That Target Cancer Cells
3.3.3. Affinity-Selected Peptides That Target Cancer Cells
3.3.4. Peptides That Target the Tumor Microenvironment
3.4. Active Targeting: Proteins as Targeting Moieties
3.4.1. Transferrin: Peptide Mimetics and Whole Protein
3.4.2. Ferritin
3.4.3. Lactoferrin
3.4.4. Folic Acid (Folate)
3.5. Active Targeting: Aptamers as Targeting Moieties
3.5.1. Aptamers That Target the Prostate-Specific Membrane Antigen (PSMA)
3.5.2. Aptamers That Target Nucleolin
3.5.3. Aptamers That Target Mucin 1
3.5.4. Miscellaneous Aptamers That Target Cancer Cell-Surface Proteins
3.6. Active Targeting: Sugars as Targeting Moieties
3.6.1. Hyaluronic Acid and Chondroitin Sulfate
3.6.2. Fucoidan
4. Multidrug Resistance
4.1. Reverting Multidrug Resistance by Nanoparticle-Mediated Gene Silencing or Chemical Inhibition of Efflux Pumps
4.2. Tackling Multidrug Resistance by Nanoparticle-Mediated Codelivery of Different Chemotherapeutic Drugs
4.3. Tackling Multidrug Resistance by Nanoparticle-Mediated Delivery of Natural Compounds or Their Derivatives
4.4. Tackling Multidrug Resistance by Nanoparticle-Mediated Delivery of Small-Molecule Inhibitors or Nucleic Acids
5. Nanoparticle-Based Cancer Vaccines
5.1. Strategies to Optimize Nanovaccine Formulations
5.1.1. Coencapsulation of Adjuvants and Antigens
5.1.2. Inclusion of Multiple Antigen Peptides with Different Chemical Properties
5.2. Examples of Antigens in Successful Preclinical Nanovaccines
5.2.1. Melanoma Antigens
5.2.2. Mucin Antigens
5.2.3. Miscellaneous Antigens
5.3. Multimodal Nanovaccines
5.3.1. On-Demand Activation of the Nanovaccine at the Tumor Site
5.3.2. Nanovaccines That Combine Multiple Steps and/or Functions
5.4. Nanovaccines including Antigen-Coding Nucleic Acids
5.4.1. RNA-Based Nanovaccines
5.4.2. DNA-Based Nanovaccines
5.5. Outline of Clinical Trials Involving Nanovaccines
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Markham, M.J.; Wachter, K.; Agarwal, N.; Bertagnolli, M.M.; Chang, S.M.; Dale, W.; Diefenbach, C.S.M.; Rodriguez-Galindo, C.; George, D.J.; Gilligan, T.D.; et al. Clinical cancer advances 2020: Annual report on progress against cancer from the American Society of Clinical Oncology. J. Clin. Oncol. 2020, 38, 1081. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Rosich, L. The contributions of Paul Ehrlich to pharmacology: A tribute on the occasion of the centenary of his Nobel Prize. Pharmacology 2008, 82, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Brasseur, F.; Couvreur, P.; Kante, B.; Deckers-Passau, L.; Roland, M.; Deckers, C.; Speiser, P. Actinomycin D absorbed on polymethylcyanoacrylate nanoparticles: Increased efficiency against an experimental tumor. Eur. J. Cancer 1980, 16, 1441–1445. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Sun, W.; Xie, Q.R.; Xia, W.; Gu, H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int. J. Nanomed. 2012, 7, 999–1013. [Google Scholar] [CrossRef] [Green Version]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Surapaneni, M.S.; Das, S.K.; Das, N.G. Designing Paclitaxel drug delivery systems aimed at improved patient outcomes: Current status and challenges. ISRN Pharmacol. 2012, 2012, 623139. [Google Scholar] [CrossRef] [Green Version]
- Tracey, S.R.; Smyth, P.; Barelle, C.J.; Scott, C.J. Development of next generation nanomedicine-based approaches for the treatment of cancer: We’ve barely scratched the surface. Biochem. Soc. Trans. 2021, 49, 2253–2269. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Barbalata, C.I.; Porfire, A.S.; Sesarman, A.; Rauca, V.F.; Banciu, M.; Muntean, D.; Stiufiuc, R.; Moldovan, A.; Moldovan, C.; Tomuta, I. A Screening Study for the Development of Simvastatin-Doxorubicin Liposomes, a Co-Formulation with Future Perspectives in Colon Cancer Therapy. Pharmaceutics 2021, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.Y.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J. Nanobiotechnol. 2021, 19, 411. [Google Scholar] [CrossRef]
- Maruyama, M.; Tojo, H.; Toi, K.; Ienaka, Y.; Hyodo, K.; Kikuchi, H.; Ogawara, K.I.; Higaki, K. Effect of Doxorubicin Release Rate From Polyethylene Glycol-Modified Liposome on Anti-tumor Activity in B16-BL6 Tumor-Bearing Mice. J. Pharm. Sci. 2021, 111, 293–297. [Google Scholar] [CrossRef]
- Cardoso, M.M.; Peca, I.N.; Lopes, T.; Gardner, R.; Bicho, A. Double-Walled Poly-(D,L-lactide-co-glycolide) (PLGA) and Poly(L-lactide) (PLLA) Nanoparticles for the Sustained Release of Doxorubicin. Polymers 2021, 13, 3230. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, H.; Yu, W.; Xiong, X.; Krastev, R.; Ma, X. Preparation of Cationic Amphiphilic Nanoparticles with Modified Chitosan Derivatives for Doxorubicin Delivery. Materials 2021, 14, 7010. [Google Scholar] [CrossRef]
- Kalenichenko, D.; Nifontova, G.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Designing Functionalized Polyelectrolyte Microcapsules for Cancer Treatment. Nanomaterials 2021, 11, 3055. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jia, D.; Ma, X.; Liang, M.; Xue, P.; Kang, Y.; Xu, Z. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact. Mater. 2021, 6, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Cho, H.; Choi, W.G.; Lee, S.S.; Huh, K.M.; Shim, M.S.; Park, I.S.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; et al. Beyond hydrophilic polymers in amphiphilic polymer-based self-assembled NanoCarriers: Small hydrophilic carboxylate-capped disulfide drug delivery system and its multifunct.tionality and multispatial targetability. Biomaterials 2021, 280, 121307. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.R.; Lee, S.J.; Chandrasekaran, A.P.; Tyagi, A.; Ramakrishna, S.; Kim, S.Y.; Kim, D.W.; Pack, C.G.; Oh, S.J. Smart Vitamin Micelles as Cancer Nanomedicines for Enhanced Intracellular Delivery of Doxorubicin. Int. J. Mol. Sci. 2021, 22, 1298. [Google Scholar] [CrossRef]
- Zong, W.; Shao, X.; Chai, Y.; Wang, X.; Han, S.; Chu, H.; Zhu, C.; Zhang, X. Liposomes encapsulating artificial cytosol as drug delivery system. Biophys. Chem. 2021, 281, 106728. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C.; et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, C.; Patel, P.; Staruch, R.M.; Shaikh, S.; Nofiele, J.; Wodzak Staruch, M.; Szczepanski, D.; Williams, N.S.; Laetsch, T.; Chopra, R. Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin. Int. J. Hyperth. 2019, 36, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Borys, N.; Dewhirst, M.W. Drug development of lyso-thermosensitive liposomal doxorubicin: Combining hyperthermia and thermosensitive drug delivery. Adv. Drug Deliv. Rev. 2021, 178, 113985. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, C.; Jiang, D.; Qian, C.; Yang, Z.; Wang, J.; Ye, W. Cascade Tumor Therapy Platform for Sensitized Chemotherapy and Penetration Enhanced Photothermal Therapy. Macromol. Biosci. 2021, 22, e2100429. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Gao, M.; Chen, Y.; Wu, C. Size-dependent chemosensitization of doxorubicin-loaded polymeric nanoparticles for malignant glioma chemotherapy. Bioengineered 2021, 12, 12263–12273. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.B.; Fukumura, D.; Brown, E.B.; Mazzola, L.M.; Izumi, Y.; Jain, R.K.; Torchilin, V.P.; Munn, L.L. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002, 62, 6831–6836. [Google Scholar] [PubMed]
- Xu, Z.; Yang, D.; Long, T.; Yuan, L.; Qiu, S.; Li, D.; Mu, C.; Ge, L. pH-Sensitive nanoparticles based on amphiphilic imidazole/cholesterol modified hydroxyethyl starch for tumor chemotherapy. Carbohydr. Polym. 2022, 277, 118827. [Google Scholar] [CrossRef]
- Aziz, A.; Sefidbakht, Y.; Rezaei, S.; Kouchakzadeh, H.; Uskokovic, V. Doxorubicin-Loaded, pH-Sensitive Albumin Nanoparticles for Lung Cancer Cell Targeting. J. Pharm. Sci. 2021, 111, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Poletaeva, Y.; Pyshnaya, I.; Pyshnyi, D.; Dmitrienko, E. Designing pH-Dependent Systems Based on Nanoscale Calcium Carbonate for the Delivery of an Antitumor Drug. Nanomaterials 2021, 11, 2794. [Google Scholar] [CrossRef]
- Na, Y.; Woo, J.; Choi, W.I.; Sung, D. Novel carboxylated ferrocene polymer nanocapsule with high reactive oxygen species sensitivity and on-demand drug release for effective cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111566. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Akbarialiabad, H.; Jahromi, A.M.; Maleki, R. Loading and release of cancer chemotherapy drugs utilizing simultaneous temperature and pH-responsive nanohybrid. BMC Pharmacol. Toxicol. 2021, 22, 41. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, P. Acid-triggered degradable diblock poly(doxorubicin)-polyethylene glycol polyprodrug with doxorubicin as structural unit for tumor intracellular delivery. Int. J. Pharm. 2021, 609, 121142. [Google Scholar] [CrossRef]
- Wang, B.; Hu, W.; Yan, H.; Chen, G.; Zhang, Y.; Mao, J.; Wang, L. Lung cancer chemotherapy using nanoparticles: Enhanced target ability of redox-responsive and pH-sensitive cisplatin prodrug and paclitaxel. Biomed. Pharmacother. 2021, 136, 111249. [Google Scholar] [CrossRef]
- Lu, S.; Xia, R.; Wang, J.; Pei, Q.; Xie, Z.; Jing, X. Engineering Paclitaxel Prodrug Nanoparticles via Redox-Activatable Linkage and Effective Carriers for Enhanced Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 46291–46302. [Google Scholar] [CrossRef]
- Kanwal, S.; Naveed, M.; Arshad, A.; Arshad, A.; Firdous, F.; Faisal, A.; Yameen, B. Reduction-Sensitive Dextran-Paclitaxel Polymer-Drug Conjugate: Synthesis, Self-Assembly into Nanoparticles, and In Vitro Anticancer Efficacy. Bioconjug. Chem. 2021, 32, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Liu, X.; Li, J.; Li, W.; Zhang, L.; Fu, C.; Zhang, J.; Gu, Z. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics 2021, 11, 4171–4186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, J.; Zhou, L.; Hu, X.; Wang, C.; Chai, K.; Li, R.; Feng, L.; Sun, Y.; Dong, C.; et al. Dual-Responsive and ROS-Augmented Nanoplatform for Chemo/Photodynamic/Chemodynamic Combination Therapy of Triple Negative Breast Cancer. ACS Appl. Mater. Interfaces 2021, 14, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Qi, Y.; Zhong, H.; Jiang, S.; Zhang, H.; Cai, H.; Wu, Y.; Gu, Z.; Gong, Q.; Luo, K. GSH-sensitive polymeric prodrug: Synthesis and loading with photosensitizers as nanoscale chemo-photodynamic anti-cancer nanomedicine. Acta Pharm. Sin. B 2022, 12, 424–436. [Google Scholar] [CrossRef]
- Guan, S.; Liu, X.; Fu, Y.; Li, C.; Wang, J.; Mei, Q.; Deng, G.; Zheng, W.; Wan, Z.; Lu, J. A biodegradable “Nano-donut” for magnetic resonance imaging and enhanced chemo/photothermal/chemodynamic therapy through responsive catalysis in tumor microenvironment. J. Colloid Interface Sci. 2022, 608, 344–354. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Li, Q.; Xu, Y.; Shi, Y.; Wang, Z.; Xia, M.; Li, J.; Wang, D. Synergistic chemo-photothermal cancer therapy of pH-responsive polymeric nanoparticles loaded IR825 and DTX with charge-reversal property. Colloids Surf. B Biointerfaces 2022, 209, 112164. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Chen, J.; Liu, S.; Deng, S.; Liu, C.; McCulloch, I.; Yue, W.; Cheng, D. Co-delivery of NIR-II semiconducting polymer and pH-sensitive doxorubicin-conjugated prodrug for photothermal/chemotherapy. Acta Biomater. 2022, 137, 238–251. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Yang, J.; Gong, Q.; Luo, K.; Gu, Z. Redox dual-responsive dendrimeric nanoparticles for mutually synergistic chemo-photodynamic therapy to overcome drug resistance. J. Control. Release 2021, 329, 1210–1221. [Google Scholar] [CrossRef]
- Han, Q.J.; Lan, X.T.; Wen, Y.; Zhang, C.Z.; Cleary, M.; Sayyed, Y.; Huang, G.; Tuo, X.; Yi, L.; Xi, Z.; et al. Matrix Metalloproteinase-9-Responsive Surface Charge-Reversible Nanocarrier to Enhance Endocytosis as Efficient Targeted Delivery System for Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2021, 10, e2002143. [Google Scholar] [CrossRef]
- Chen, M.; Miao, Y.; Qian, K.; Zhou, X.; Guo, L.; Qiu, Y.; Wang, R.; Gan, Y.; Zhang, X. Detachable Liposomes Combined Immunochemotherapy for Enhanced Triple-Negative Breast Cancer Treatment through Reprogramming of Tumor-Associated Macrophages. Nano Lett. 2021, 21, 6031–6041. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N.; Gao, Z.; Papahadjopoulos-Sternberg, B. Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 6039–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Targeted Delivery of Combination Therapeutics Using Monoclonal Antibody 2C5-Modified Immunoliposomes for Cancer Therapy. Pharm. Res. 2021, 38, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, R.; Pinheiro, D.P.; de Cassia Evangelista de Oliveira, F.; Galvao, G.F.; Marques, L.G.A.; Lopez, R.F.V.; Pessoa, C.; Eloy, J.O. Immunoconjugates for Cancer Targeting: A Review of Antibody-Drug Conjugates and Antibody-Functionalized Nanoparticles. Curr. Med. Chem. 2021, 28, 2485–2520. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.L.P.; Oshiro-Junior, J.A.; Garcia, C.; Turco, B.O.; da Silva Leite, J.M.; de Lima Damasceno, B.P.G.; Soares, J.C.M.; Chorilli, M. Monoclonal Antibodies Carried in Drug Delivery Nanosystems as a Strategy for Cancer Treatment. Curr. Med. Chem. 2021, 28, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.R.; Powers, K.; Aiardo, R.; Barua, D.; Barua, S. Antibody-drug nanoparticle induces synergistic treatment efficacies in HER2 positive breast cancer cells. Sci. Rep. 2021, 11, 7347. [Google Scholar] [CrossRef]
- Wang, Y.; Pasternak, M.; Sathiyamoorthy, K.; Kolios, M.C. Anti-HER2 PLGA-PEG polymer nanoparticle containing gold nanorods and paclitaxel for laser-activated breast cancer detection and therapy. Biomed. Opt. Express 2021, 12, 2171–2185. [Google Scholar] [CrossRef]
- Escareno, N.; Hassan, N.; Kogan, M.J.; Juarez, J.; Topete, A.; Daneri-Navarro, A. Microfluidics-assisted conjugation of chitosan-coated polymeric nanoparticles with antibodies: Significance in drug release, uptake, and cytotoxicity in breast cancer cells. J. Colloid Interface Sci. 2021, 591, 440–450. [Google Scholar] [CrossRef]
- Oliveira, C.; Goncalves, C.S.; Martins, E.P.; Neves, N.M.; Reis, R.L.; Costa, B.M.; Silva, T.H.; Martins, A. Fucoidan/chitosan nanoparticles functionalized with anti-ErbB-2 target breast cancer cells and impair tumor growth in vivo. Int. J. Pharm. 2021, 600, 120548. [Google Scholar] [CrossRef]
- El Hallal, R.; Lyu, N.; Wang, Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules 2021, 26, 567. [Google Scholar] [CrossRef]
- Bhattacharya, S. Anti-EGFR-mAb and 5-Fluorouracil Conjugated Polymeric Nanoparticles for Colorectal Cancer. Recent Pat. Anticancer Drug Discov. 2021, 16, 84–100. [Google Scholar] [CrossRef]
- Castro, A.; Berois, N.; Malanga, A.; Ortega, C.; Oppezzo, P.; Pristch, O.; Mombru, A.W.; Osinaga, E.; Pardo, H. Docetaxel in chitosan-based nanocapsules conjugated with an anti-Tn antigen mouse/human chimeric antibody as a promising targeting strategy of lung tumors. Int. J. Biol. Macromol. 2021, 182, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Marchal, S.A.; Grinan-Lison, C.; Entrena, J.M.; Ruiz-Alcala, G.; Tristan-Manzano, M.; Martin, F.; Perez-Victoria, I.; Peula-Garcia, J.M.; Marchal, J.A. Anti-CD44-Conjugated Olive Oil Liquid Nanocapsules for Targeting Pancreatic Cancer Stem Cells. Biomacromolecules 2021, 22, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, M.; Gowrikumar, S.; Dhawan, P. Role of CD44 isoforms in epithelial-mesenchymal plasticity and metastasis. Clin. Exp. Metastasis 2022. [Google Scholar] [CrossRef] [PubMed]
- Pindiprolu, S.; Krishnamurthy, P.T.; Dev, C.; Chintamaneni, P.K. DR5 antibody conjugated lipid-based nanocarriers of gamma-secretase inhibitor for the treatment of triple negative breast cancer. Chem. Phys. Lipids 2021, 235, 105033. [Google Scholar] [CrossRef] [PubMed]
- McCaw, T.R.; Inga, E.; Chen, H.; Jaskula-Sztul, R.; Dudeja, V.; Bibb, J.A.; Ren, B.; Rose, J.B. Gamma Secretase Inhibitors in Cancer: A Current Perspective on Clinical Performance. Oncologist 2021, 26, e608–e621. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Zhang, W.; Radmanesh, M.; Cathcart, N.; Maleki, A.; Kitaev, V. Plasmonic photothermal release of docetaxel by gold nanoparticles incorporated onto halloysite nanotubes with conjugated 2D8-E3 antibodies for selective cancer therapy. J. Nanobiotechnol. 2021, 19, 239. [Google Scholar] [CrossRef]
- Tian, H.; Huang, Y.; He, J.; Zhang, M.; Ni, P. CD147 Monoclonal Antibody Targeted Reduction-Responsive Camptothecin Polyphosphoester Nanomedicine for Drug Delivery in Hepatocellular Carcinoma Cells. ACS Appl. Bio Mater. 2021, 4, 4422–4431. [Google Scholar] [CrossRef]
- Bates, A.; Power, C.A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Heath, T.D.; Fraley, R.T.; Papahdjopoulos, D. Antibody targeting of liposomes: Cell specificity obtained by conjugation of F(ab’)2 to vesicle surface. Science 1980, 210, 539–541. [Google Scholar] [CrossRef]
- Ni, L.; Li, Y.X. Anti-Human Epidermal Growth Factor Receptor 2 Single-Chain Fv Fragment-Decorated DM1 Nanoparticles for Specific Targeting of Human Epidermal Growth Factor Receptor 2-Positive Breast Tumor Cells. J. Biomed. Nanotechnol. 2021, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhou, L.; Tang, W.; Ma, T.; Li, H.; Wang, X.; Chen, C.; Wang, P. Tumor targeting antibody-conjugated nanocarrier with pH/thermo dual-responsive macromolecular film layer for enhanced cancer chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111361. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, B.N.; Pereira, R.F.; Barrias, C.C.; Fischbach, C.; Oliveira, C.; Granja, P.L. Engineering Modular Half-Antibody Conjugated Nanoparticles for Targeting CD44v6-Expressing Cancer Cells. Nanomaterials 2021, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rafael, D.; Vilar-Hernandez, M.; Montero, S.; Martinez-Trucharte, F.; Seras-Franzoso, J.; Diaz-Riascos, Z.V.; Boullosa, A.; Garcia-Aranda, N.; Camara-Sanchez, P.; et al. Polymeric micelles targeted against CD44v6 receptor increase niclosamide efficacy against colorectal cancer stem cells and reduce circulating tumor cells in vivo. J. Control. Release 2021, 331, 198–212. [Google Scholar] [CrossRef]
- Lin, W.W.; Cheng, Y.A.; Li, C.C.; Ho, K.W.; Chen, H.J.; Chen, I.U.; Huang, B.C.; Liu, H.J.; Lu, Y.C.; Cheng, C.M.; et al. Enhancement of tumor tropism of mPEGylated nanoparticles by anti-mPEG bispecific antibody for ovarian cancer therapy. Sci. Rep. 2021, 11, 7598. [Google Scholar] [CrossRef]
- Ho, K.W.; Chen, I.U.; Cheng, Y.A.; Liao, T.Y.; Liu, E.S.; Chen, H.J.; Lu, Y.C.; Su, Y.C.; Roffler, S.R.; Huang, B.C.; et al. Double attack strategy for leukemia using a pre-targeting bispecific antibody (CD20 Ab-mPEG scFv) and actively attracting PEGylated liposomal doxorubicin to enhance anti-tumor activity. J. Nanobiotechnol. 2021, 19, 16. [Google Scholar] [CrossRef]
- Cheng, W.J.; Lin, S.Y.; Chen, M.; Chen, L.C.; Ho, H.O.; Chuang, K.H.; Sheu, M.T. Active Tumoral/Tumor Environmental Dual-Targeting by Non-Covalently Arming with Trispecific Antibodies or Dual-Bispecific Antibodies on Docetaxel-Loaded mPEGylated Nanocarriers to Enhance Chemotherapeutic Efficacy and Minimize Systemic Toxicity. Int. J. Nanomed. 2021, 16, 4017–4030. [Google Scholar] [CrossRef]
- Liang, X.; Li, F.; Chen, J.; Li, J.; Wu, H.; Li, S.; Song, J.; Liu, Q. Large-scale comparative review and assessment of computational methods for anti-cancer peptide identification. Brief. Bioinform. 2021, 22, bbaa312. [Google Scholar] [CrossRef]
- Aloisio, A.; Nistico, N.; Mimmi, S.; Maisano, D.; Vecchio, E.; Fiume, G.; Iaccino, E.; Quinto, I. Phage-Displayed Peptides for Targeting Tyrosine Kinase Membrane Receptors in Cancer Therapy. Viruses 2021, 13, 649. [Google Scholar] [CrossRef]
- Foglizzo, V.; Marchio, S. Bacteriophages as Therapeutic and Diagnostic Vehicles in Cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef]
- Goracci, M.; Pignochino, Y.; Marchio, S. Phage Display-Based Nanotechnology Applications in Cancer Immunotherapy. Molecules 2020, 25, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingasamy, P.; Teesalu, T. Homing Peptides for Cancer Therapy. Adv. Exp. Med. Biol. 2021, 1295, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qi, H.; Zhu, J.; Sun, W.X.; Zhang, B.; Tang, C.Y.; Cheng, Q. Vascular-homing peptides for cancer therapy. Biomed. Pharmacother. 2017, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Seyyednia, E.; Oroojalian, F.; Baradaran, B.; Mojarrad, J.S.; Mokhtarzadeh, A.; Valizadeh, H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J. Control. Release 2021, 338, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release 2021, 329, 624–644. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Muluh, T.A.; Xiong, K.; Wang, B.; Lu, Y.; Wu, Z.; Liu, Y.; Shi, H.; Xiao, S.; et al. Erythrocyte membrane-camouflaged gefitinib/albumin nanoparticles for tumor imaging and targeted therapy against lung cancer. Int. J. Biol. Macromol. 2021, 193, 228–237. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Liang, Y.; Li, Y.; Wang, M.; Zhao, F.; Wang, X.; Sun, Y.; Chen, W. Nuclear-targeted nanocarriers based on pH-sensitive amphiphiles for enhanced GNA002 delivery and chemotherapy. Nanoscale 2021, 13, 4774–4784. [Google Scholar] [CrossRef]
- Bari, E.; Serra, M.; Paolillo, M.; Bernardi, E.; Tengattini, S.; Piccinini, F.; Lanni, C.; Sorlini, M.; Bisbano, G.; Calleri, E.; et al. Silk Fibroin Nanoparticle Functionalization with Arg-Gly-Asp Cyclopentapeptide Promotes Active Targeting for Tumor Site-Specific Delivery. Cancers 2021, 13, 1185. [Google Scholar] [CrossRef]
- Yuba, E.; Takashima, M.; Hayashi, T.; Kokuryo, D.; Aoki, I.; Harada, A.; Aoshima, S.; Krishnan, U.M.; Kono, K. Multifunctional Traceable Liposomes with Temperature-Triggered Drug Release and Neovasculature-Targeting Properties for Improved Cancer Chemotherapy. Mol. Pharm. 2021, 18, 3342–3351. [Google Scholar] [CrossRef]
- Zhang, L.P.; Li, X.; Zhao, H.; Kang, L.; Liu, S.; Liu, T.; Zhao, Y. Ultra-high photoactive thiadiazolo[3,4-g]quinoxaline nanoparticles with active-targeting capability for deep photodynamic therapy. J. Mater. Chem. B 2021, 9, 8330–8340. [Google Scholar] [CrossRef]
- Han, R.; Liu, Q.; Lu, Y.; Peng, J.; Pan, M.; Wang, G.; Chen, W.; Xiao, Y.; Yang, C.; Qian, Z. Tumor microenvironment-responsive Ag2S-PAsp(DOX)-cRGD nanoparticles-mediated photochemotherapy enhances the immune response to tumor therapy. Biomaterials 2022, 281, 121328. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wang, Y.; Kang, X.; Xu, F.; Han, Z.; Zhang, C.; Wang, Z.Y.; Liu, J.Q.; Zhao, X.; Chen, X.; et al. A multifunctional AIE gold cluster-based theranostic system: Tumor-targeted imaging and Fenton reaction-assisted enhanced radiotherapy. J. Nanobiotechnol. 2021, 19, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, K.; Fu, X.; Sun, H.; Wang, H.; Yu, D.; Li, Z.; Ma, Y.; Liu, X.; Huang, B.; et al. Versatile metal-phenolic network nanoparticles for multitargeted combination therapy and magnetic resonance tracing in glioblastoma. Biomaterials 2021, 278, 121163. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Jiao, J.; Xu, C.; Dong, Y.; Li, D.; He, D.; Zhao, D.; Yu, J.; Sun, Y.; Zhang, W.; et al. The targetable nanoparticle BAF312@cRGD-CaP-NP represses tumor growth and angiogenesis by downregulating the S1PR1/P-STAT3/VEGFA axis in triple-negative breast cancer. J. Nanobiotechnol. 2021, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, A.; Gao, Y.; Zhang, Y.; Duan, X.; Men, K. Treatment of Melanoma by Nano-conjugate-Delivered Wee1 siRNA. Mol. Pharm. 2021, 18, 3387–3400. [Google Scholar] [CrossRef]
- Yang, S.; Wang, D.; Zhang, X.; Sun, Y.; Zheng, B. cRGD peptide-conjugated polyethylenimine-based lipid nanoparticle for intracellular delivery of siRNA in hepatocarcinoma therapy. Drug Deliv. 2021, 28, 995–1006. [Google Scholar] [CrossRef]
- Yang, H.; Liu, T.; Xu, Y.; Su, G.; Liu, T.; Yu, Y.; Xu, B. Protein corona precoating on redox-responsive chitosan-based nano-carriers for improving the therapeutic effect of nucleic acid drugs. Carbohydr. Polym. 2021, 265, 118071. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, M.; Li, R.; Hu, J.; Yu, L.; Qian, X. iRGD Co-Administration with Paclitaxel-Loaded PLGA Nanoparticles Enhance Targeting and Antitumor Effect in Colorectal Cancer Treatment. Anticancer Agents Med. Chem. 2021, 21, 910–918. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Qi, X.; Meng, X.; Xu, K. Co-Administration of iRGD with Sorafenib-Loaded Iron-Based Metal-Organic Framework as a Targeted Ferroptosis Agent for Liver Cancer Therapy. Int. J. Nanomed. 2021, 16, 1037–1050. [Google Scholar] [CrossRef]
- Wan, W.J.; Huang, G.; Wang, Y.; Tang, Y.; Li, H.; Jia, C.H.; Liu, Y.; You, B.G.; Zhang, X.N. Coadministration of iRGD peptide with ROS-sensitive nanoparticles co-delivering siFGL1 and siPD-L1 enhanced tumor immunotherapy. Acta Biomater. 2021, 136, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lai, W.; Wang, Q.; Tang, Q.; Hu, C.; Zhou, M.; Wang, F.; Xie, D.; Zhang, Q.; Liu, W.; et al. Effective Triple-Negative Breast Cancer Targeted Treatment Using iRGD-Modified RBC Membrane-Camouflaged Nanoparticles. Int. J. Nanomed. 2021, 16, 7497–7515. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, W.; Zhao, D.; Li, J.; Li, Q.; Liu, Y.; Hao, L.; Lin, Y. Enhanced Penetrability of a Tetrahedral Framework Nucleic Acid by Modification with iRGD for DOX-Targeted Delivery to Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 25825–25835. [Google Scholar] [CrossRef] [PubMed]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted polymeric nanoparticles for drug delivery to hypoxic, triple-negative breast tumors. ACS Appl. Bio Mater. 2021, 4, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Y.; Liang, W.; Li, L.; Du, J.; Pan, C.; Zhang, C. ROS-responsive dimeric prodrug-based nanomedicine targeted therapy for gastric cancer. Drug Deliv. 2021, 28, 1204–1213. [Google Scholar] [CrossRef]
- Sui, M.; Xiong, M.; Li, Y.; Zhou, Q.; Shen, X.; Jia, D.; Gou, M.; Sun, Q. Cancer Therapy with Nanoparticle-Medicated Intracellular Expression of Peptide CRM1-Inhibitor. Int. J. Nanomed. 2021, 16, 2833–2847. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Wu, M.; Shen, W.; Ren, J.; Mei, Z.; Ran, H.; Wang, Z.; Tian, Y.; Gao, J.; et al. iRGD Peptide-Mediated Liposomal Nanoparticles with Photoacoustic/Ultrasound Dual-Modality Imaging for Precision Theranostics Against Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 6455–6475. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Z.; Yuan, Q.; Zhang, C.; Tang, Y. ROS-Responsive and active targeted drug delivery based on conjugated polymer nanoparticles for synergistic chemo-/photodynamic therapy. J. Mater. Chem. B 2021, 9, 2240–2248. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhang, K.; Zheng, H.; Wei, Y.; Zheng, H.; Zhu, J.; Wu, F.; et al. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem. Biophys. Res. Commun. 2021, 534, 902–907. [Google Scholar] [CrossRef]
- Costagliola di Polidoro, A.; Zambito, G.; Haeck, J.; Mezzanotte, L.; Lamfers, M.; Netti, P.A.; Torino, E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-cHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, N.; Mehrabian, A.; Amiri-Darban, S.; Moosavian, S.A.; Jaafari, M.R. Preparation and characterization of PEGylated liposomal Doxorubicin targeted with leptin-derived peptide and evaluation of their anti-tumor effects, in vitro and in vivo in mice bearing C26 colon carcinoma. Colloids Surf. B Biointerfaces 2021, 200, 111589. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, L.; Li, X.; Hu, A.; Wang, H.; Zhou, H.; Tian, B.; Dong, J. Rationally integrating peptide-induced targeting and multimodal therapies in a dual-shell theranostic platform for orthotopic metastatic spinal tumors. Biomaterials 2021, 275, 120917. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, Y.; Won, W.R.; Lim, C.; Kim, J.C.; Kang, K.; Husni, P.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of AE147 Peptide-Conjugated Nanocarriers for Targeting uPAR-Overexpressing Cancer Cells. Int. J. Nanomed. 2021, 16, 5437–5449. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, Q.; Suby, N.; Xiao, W.; Agrawal, R.; Vu, M.; Zhang, H.; Luo, Y.; Li, Y.; Lam, K.S. LHRH-Targeted Redox-Responsive Crosslinked Micelles Impart Selective Drug Delivery and Effective Chemotherapy in Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2021, 10, e2001196. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, M.K.; Yang, S.; Moon, Y.; Song, S.; Choi, J.; Kim, J.; Kim, K. Combination of cancer-specific prodrug nanoparticle with Bcl-2 inhibitor to overcome acquired drug resistance. J. Control. Release 2021, 330, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Duan, Z.; Li, X.; Gu, L.; Ren, L.; Zhu, H.; Tian, X.; Chen, R.; Zhang, H.; Gong, Q.; et al. Branched polymer-based redox/enzyme-activatable photodynamic nanoagent to trigger STING-dependent immune responses for enhanced therapeutic effect. Adv. Funct. Mater. 2021, 32, 2110408. [Google Scholar] [CrossRef]
- Mashreghi, M.; Faal Maleki, M.; Karimi, M.; Kalalinia, F.; Badiee, A.; Jaafari, M.R. Improving anti-tumour efficacy of PEGylated liposomal doxorubicin by dual targeting of tumour cells and tumour endothelial cells using anti-p32 CGKRK peptide. J. Drug Target. 2021, 29, 617–630. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Zhang, Y.; Zhou, S.; Cai, H.H.; Li, T.; Jin, H.; Cai, J.; Zhou, H.; Pi, J. GE11 Peptide Conjugated Liposomes for EGFR-Targeted and Chemophotothermal Combined Anticancer Therapy. Bioinorg. Chem. Appl. 2021, 2021, 5534870. [Google Scholar] [CrossRef]
- Dai, G.; Chu, J.C.H.; Chan, C.K.W.; Choi, C.H.J.; Ng, D.K.P. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021, 13, 15899–15915. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Cheng, L.; Yuan, J.; Zhong, Z. SP94 peptide mediating highly specific and efficacious delivery of polymersomal doxorubicin hydrochloride to hepatocellular carcinoma in vivo. Colloids Surf. B Biointerfaces 2021, 197, 111399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, X.; Zhou, Y.; Zou, C.; Wang, L.; Wang, W.; Zhang, D.; Xu, H.; Li, J.; Li, F.; et al. TMTP1-Modified, Tumor Microenvironment Responsive Nanoparticles Co-Deliver Cisplatin and Paclitaxel Prodrugs for Effective Cervical Cancer Therapy. Int. J. Nanomed. 2021, 16, 4087–4104. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- d’Avanzo, N.; Torrieri, G.; Figueiredo, P.; Celia, C.; Paolino, D.; Correia, A.; Moslova, K.; Teesalu, T.; Fresta, M.; Santos, H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021, 597, 120346. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Hong, B.V.; Mackenzie, G.G.; Amiji, M.M. Hyaluronic acid nanoparticle-encapsulated microRNA-125b repolarizes tumor-associated macrophages in pancreatic cancer. Nanomedicine 2021, 16, 2291–2303. [Google Scholar] [CrossRef]
- Hao, T.; Fu, Y.; Yang, Y.; Yang, S.; Liu, J.; Tang, J.; Ridwan, K.A.; Teng, Y.; Liu, Z.; Li, J.; et al. Tumor vasculature-targeting PEGylated peptide-drug conjugate prodrug nanoparticles improve chemotherapy and prevent tumor metastasis. Eur. J. Med. Chem. 2021, 219, 113430. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Teng, B.; Wang, Z.; Li, F.; Zhao, Y.; Guo, Y.; Zeng, Q. Peptide-Targeted High-Density Lipoprotein Nanoparticles for Combinatorial Treatment against Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 35248–35265. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Mojarad-Jabali, S.; Farshbaf, M.; Hemmati, S.; Sarfraz, M.; Motasadizadeh, H.; Shahbazi Mojarrad, J.; Atyabi, F.; Zakeri-Milani, P.; Valizadeh, H. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int. J. Pharm. 2021, 613, 121395. [Google Scholar] [CrossRef]
- He, G.Z.; Lin, W.J. Peptide-Functionalized Nanoparticles-Encapsulated Cyclin-Dependent Kinases Inhibitor Seliciclib in Transferrin Receptor Overexpressed Cancer Cells. Nanomaterials 2021, 11, 772. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef] [PubMed]
- Arumov, A.; Liyanage, P.Y.; Trabolsi, A.; Roberts, E.R.; Li, L.; Ferreira, B.; Gao, Z.; Ban, Y.; Newsam, A.D.; Taggart, M.W.; et al. Optimized Doxorubicin Chemotherapy for Diffuse Large B-cell Lymphoma Exploits Nanocarrier Delivery to Transferrin Receptors. Cancer Res. 2021, 81, 763–775. [Google Scholar] [CrossRef]
- Jin, X.; Yu, J.; Yin, M.; Sinha, A.; Jin, G. Combined Ultrasound Treatment with Transferrin-Coupled Nanoparticles Improves Active Targeting of 4T1 Mammary Carcinoma Cells. Technol. Cancer Res. Treat. 2021, 20, 15330338211062325. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Jin, S.; Liang, X.J.; Ma, X. Enhanced anti-tumor activity of a drug through pH-triggered release and dual targeting by calcium phosphate-covered mesoporous silica vehicles. J. Mater. Chem. B 2022, 10, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, J.; Tian, Z.; Chen, J.; Gou, G.; Niu, Y.; Li, L.; Yang, J. Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles Modified with Transferrin for Antitumor: Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles. AAPS PharmSciTech 2021, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufes, C. Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles. Int. J. Nanomed. 2021, 16, 2615–2631. [Google Scholar] [CrossRef]
- Yang, A.; Sun, Z.; Liu, R.; Liu, X.; Zhang, Y.; Zhou, Y.; Qiu, Y.; Zhang, X. Transferrin-Conjugated Erianin-Loaded Liposomes Suppress the Growth of Liver Cancer by Modulating Oxidative Stress. Front. Oncol. 2021, 11, 727605. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Ghosh, A.; Basu, A.; Pranati, P.A.; Gupta, P.; Das, S.; Sarker, S.; Bhattacharjee, M.; Bhattacharya, S.; Ghosh, S.; et al. Delivery of gefitinib in synergism with thymoquinone via transferrin-conjugated nanoparticle sensitizes gefitinib-resistant non-small cell lung carcinoma to control metastasis and stemness. Biomater. Sci. 2021, 9, 8285–8312. [Google Scholar] [CrossRef]
- Song, N.; Zhang, J.; Zhai, J.; Hong, J.; Yuan, C.; Liang, M. Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. Acc. Chem. Res. 2021, 54, 3313–3325. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, Y.; Li, X.; Wang, F.; Zhang, Y. ERK-Peptide-Inhibitor-Modified Ferritin Enhanced the Therapeutic Effects of Paclitaxel in Cancer Cells and Spheroids. Mol. Pharm. 2021, 18, 3365–3377. [Google Scholar] [CrossRef]
- Huang, C.W.; Chuang, C.P.; Chen, Y.J.; Wang, H.Y.; Lin, J.J.; Huang, C.Y.; Wei, K.C.; Huang, F.T. Integrin alpha2beta1-targeting ferritin nanocarrier traverses the blood-brain barrier for effective glioma chemotherapy. J. Nanobiotechnol. 2021, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, R.; Dong, Y.; You, C.; Huang, S.; Li, X.; Wang, F.; Zhang, Y. tLyP-1 Peptide Functionalized Human H Chain Ferritin for Targeted Delivery of Paclitaxel. Int. J. Nanomed. 2021, 16, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneem, M.A.; Abd Elwakil, M.M.; Khattab, S.N.; Helmy, M.W.; Bekhit, A.A.; Abdulkader, M.A.; Zaky, A.; Teleb, M.; Elkhodairy, K.A.; Albericio, F.; et al. Lactoferrin-dual drug nanoconjugate: Synergistic anti-tumor efficacy of docetaxel and the NF-kappaB inhibitor celastrol. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111422. [Google Scholar] [CrossRef] [PubMed]
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Ahmed-Cox, A.; Meka, A.K.; Mansfeld, F.M.; Forgham, H.; Ignacio, R.M.C.; Cao, Y.; McCarroll, J.A.; Mazzieri, R.; Kavallaris, M.; et al. Facile synthesis of lactoferrin conjugated ultra small large pore silica nanoparticles for the treatment of glioblastoma. Nanoscale 2021, 13, 16909–16922. [Google Scholar] [CrossRef]
- Nisha, R.; Kumar, P.; Kumar, U.; Mishra, N.; Maurya, P.; Singh, S.; Singh, P.; Guleria, A.; Saha, S.; Saraf, S.A. Fabrication of Imatinib Mesylate-Loaded Lactoferrin-Modified PEGylated Liquid Crystalline Nanoparticles for Mitochondrial-Dependent Apoptosis in Hepatocellular Carcinoma. Mol. Pharm. 2021, 18, 1102–1120. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/phenylboronic acid-functionalized hyaluronic acid nanogels loading doxorubicin hydrochloride for targeting glioma. Carbohydr. Polym. 2021, 253, 117194. [Google Scholar] [CrossRef]
- Gui, G.; Fan, Z.; Ning, Y.; Yuan, C.; Zhang, B.; Xu, Q. Optimization, Characterization and in vivo Evaluation of Paclitaxel-Loaded Folate-Conjugated Superparamagnetic Iron Oxide Nanoparticles. Int. J. Nanomed. 2021, 16, 2283–2295. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, T.; Xu, Y.; Huo, P.; Xu, X.; Zhang, Z.; Tian, Q.; Zhang, N. Co-administration of paclitaxel and 2-methoxyestradiol using folate-conjugated human serum albumin nanoparticles for improving drug resistance and antitumor efficacy. Pharm. Dev. Technol. 2021, 26, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Du, J.; Wang, J.; An, N.; Zhou, K.; Hu, X.; Dong, Z.; Liu, Y. Folic Acid and Poly(ethylene glycol) Decorated Paclitaxel Nanocrystals Exhibit Enhanced Stability and Breast Cancer-Targeting Capability. ACS Appl. Mater. Interfaces 2021, 13, 14577–14586. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2021, 156, 105576. [Google Scholar] [CrossRef]
- Lin, C.; Yang, X.; Li, H.; Zou, Y.; Mohammad, I.S.; Rong, H.; Rao, Y.; Song, J.; Leung, S.S.Y.; Hu, H. Self-assembled nanomedicine combining a berberine derivative and doxorubicin for enhanced antitumor and antimetastatic efficacy via mitochondrial pathways. Nanoscale 2021, 13, 6605–6623. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Su, Y.; Wang, B.; Chen, Q.; Guo, H.; Kong, M.; Chen, D. Novel polysaccharide building hybrid nanoparticles: Remodeling TAMs to target ERalpha-positive breast cancer. J. Drug Target. 2021, 30, 450–462. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr. Polym. 2022, 276, 118747. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Daniels, D.A.; Chen, H.; Hicke, B.J.; Swiderek, K.M.; Gold, L. A tenascin-C aptamer identified by tumor cell SELEX: Systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA 2003, 100, 15416–15421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, G.; Lee, C.S.; Yoon, Y.M.; Lim, J.H.; Kim, T.H.; Lee, S.H. PrP(C) Aptamer Conjugated-Gold Nanoparticles for Targeted Delivery of Doxorubicin to Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1976. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; Lavan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Dong, Y.; Zhang, Y.; Fu, H.; Chen, C.; Wang, L.; Yang, X.; Shen, M.; Yu, J.; Chen, M.; et al. Sequential Release of Pooled siRNAs and Paclitaxel by Aptamer-Functionalized Shell-Core Nanoparticles to Overcome Paclitaxel Resistance of Prostate Cancer. ACS Appl. Mater. Interfaces 2021, 13, 13990–14003. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Wei, D.; Zhang, J.; Shen, T.; Zhao, Y.; Liang, J.; Ma, W.; Zhang, L.; Liu, Q.; Zheng, Y. Aptamer-conjugated mesoporous polydopamine for docetaxel targeted delivery and synergistic photothermal therapy of prostate cancer. Cell Prolif. 2021, 54, e13130. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, S.; Hu, Y.; Yang, F.; Huo, Q.; Xie, N. Tumour-Targeted and Redox-Responsive Mesoporous Silica Nanoparticles for Controlled Release of Doxorubicin and an siRNA Against Metastatic Breast Cancer. Int. J. Nanomed. 2021, 16, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Matin, M.M.; Danesh, N.M.; Bahrami, A.R.; Abnous, K.; Taghdisi, S.M. Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR-21. Carbohydr. Polym. 2021, 266, 118111. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Y.; Sun, Q.; Cheng, J.; Yue, C.; Liu, Y.; Song, J.; Jin, W.; Ding, X.; de la Fuente, J.M.; et al. Au-siRNA@ aptamer nanocages as a high-efficiency drug and gene delivery system for targeted lung cancer therapy. J. Nanobiotechnol. 2021, 19, 54. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Li, Y.; Xie, X.; Wei, X.; Yang, G.; Zhang, H.; Li, N.; Li, T.; Qin, X.; et al. Aptamer-Dendrimer Functionalized Magnetic Nano-Octahedrons: Theranostic Drug/Gene Delivery Platform for Near-Infrared/Magnetic Resonance Imaging-Guided Magnetochemotherapy. ACS Nano 2021, 15, 16683–16696. [Google Scholar] [CrossRef]
- Zahiri, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Fabrication of versatile targeted lipopolymersomes for improved camptothecin efficacy against colon adenocarcinoma in vitro and in vivo. Expert. Opin. Drug Deliv. 2021, 18, 1309–1322. [Google Scholar] [CrossRef]
- Sanati, S.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Babaei, M.; Ramezani, M.; Alibolandi, M. Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma. Gene Ther. 2021, 29, 55–68. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.H. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int. J. Biol Macromol. 2021, 195, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil-An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111591. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Nunes, J.; Agonia, A.S.; Rosado, T.; Gallardo, E.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Fonseca-Moutinho, J.; Campello, M.P.C.; Paiva, A.; et al. Aptamer-Functionalized Gold Nanoparticles for Drug Delivery to Gynecological Carcinoma Cells. Cancers 2021, 13, 4038. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, E.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M. Targeted delivery and controlled release of doxorubicin to cancer cells by smart ATP-responsive Y-shaped DNA structure-capped mesoporous silica nanoparticles. J. Mater. Chem. B 2021, 9, 1351–1363. [Google Scholar] [CrossRef]
- Nosrati, R.; Abnous, K.; Alibolandi, M.; Mosafer, J.; Dehghani, S.; Taghdisi, S.M.; Ramezani, M. Targeted SPION siderophore conjugate loaded with doxorubicin as a theranostic agent for imaging and treatment of colon carcinoma. Sci. Rep. 2021, 11, 13065. [Google Scholar] [CrossRef]
- Ranjbar-Navazi, Z.; Fathi, M.; Abdolahinia, E.D.; Omidi, Y.; Davaran, S. MUC-1 aptamer conjugated InP/ZnS quantum dots/nanohydrogel fluorescent composite for mitochondria-mediated apoptosis in MCF-7 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111469. [Google Scholar] [CrossRef]
- Nabi, P.N.; Vahidfar, N.; Tohidkia, M.R.; Hamidi, A.A.; Omidi, Y.; Aghanejad, A. Mucin-1 conjugated polyamidoamine-based nanoparticles for image-guided delivery of gefitinib to breast cancer. Int. J. Biol. Macromol. 2021, 174, 185–197. [Google Scholar] [CrossRef]
- Mashreghi, M.; Zamani, P.; Karimi, M.; Mehrabian, A.; Arabsalmani, M.; Zarqi, J.; Moosavian, S.A.; Jaafari, M.R. Anti-epithelial cell adhesion molecule RNA aptamer-conjugated liposomal doxorubicin as an efficient targeted therapy in mice bearing colon carcinoma tumor model. Biotechnol. Prog. 2021, 37, e3116. [Google Scholar] [CrossRef]
- Khodadadi, E.; Mahjoub, S.; Arabi, M.S.; Najafzadehvarzi, H.; Nasirian, V. Fabrication and evaluation of aptamer-conjugated paclitaxel-loaded magnetic nanoparticles for targeted therapy on breast cancer cells. Mol. Biol. Rep. 2021, 48, 2105–2116. [Google Scholar] [CrossRef]
- Smiley, S.B.; Yun, Y.; Ayyagari, P.; Shannon, H.E.; Pollok, K.E.; Vannier, M.W.; Das, S.K.; Veronesi, M.C. Development of CD133 Targeting Multi-Drug Polymer Micellar Nanoparticles for Glioblastoma—In Vitro Evaluation in Glioblastoma Stem Cells. Pharm. Res. 2021, 38, 1067–1079. [Google Scholar] [CrossRef]
- Behrooz, A.B.; Vazifehmand, R.; Tajudin, A.A.; Masarudin, M.J.; Sekawi, Z.; Masomian, M.; Syahir, A. Tailoring drug co-delivery nanosystem for mitigating U-87 stem cells drug resistance. Drug Deliv. Transl. Res. 2021, 12, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, L.; Li, X.; Li, Z.; Wang, J.; Chen, H.; Li, X.; Gao, Y. Co-delivery of gefitinib and hematoporphyrin by aptamer-modified fluorinated dendrimer for hypoxia alleviation and enhanced synergistic chemo-photodynamic therapy of NSCLC. Eur. J. Pharm. Sci. 2021, 167, 106004. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Tortorella, S.; d’Argenio, A.; Carbone, C.; Camorani, S.; Locatelli, E.; Auletta, L.; Sorrentino, D.; Fedele, M.; Zannetti, A.; et al. Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J. Exp. Clin. Cancer Res. 2021, 40, 239. [Google Scholar] [CrossRef] [PubMed]
- Lohiya, G.; Katti, D.S. Carboxylated chitosan-mediated improved efficacy of mesoporous silica nanoparticle-based targeted drug delivery system for breast cancer therapy. Carbohydr. Polym. 2022, 277, 118822. [Google Scholar] [CrossRef]
- Resnick, N.M.; Clarke, M.R.; Siegfried, J.M.; Landreneau, R.; Asman, D.C.; Ge, L.; Kierstead, L.S.; Dougherty, G.D.; Cooper, D.L. Expression of the Cell Adhesion Molecule CD44 in Human Lung Tumors and Cell Lines. Mol. Diagn. 1998, 3, 93–103. [Google Scholar] [CrossRef]
- Solis, M.A.; Chen, Y.H.; Wong, T.Y.; Bittencourt, V.Z.; Lin, Y.C.; Huang, L.L. Hyaluronan regulates cell behavior: A potential niche matrix for stem cells. Biochem. Res. Int. 2012, 2012, 346972. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-coated nanoparticles for active tumor targeting: Influence of polysaccharide molecular weight on cell uptake. Colloids Surf. B Biointerfaces 2021, 210, 112240. [Google Scholar] [CrossRef]
- Du, W.; Yang, X.; He, S.; Wang, J.; Guo, Y.; Kou, B.; Jiang, Y.; Bian, P.; Li, B.; Yin, L. Novel hyaluronic acid oligosaccharide-loaded and CD44v6-targeting oxaliplatin nanoparticles for the treatment of colorectal cancer. Drug Deliv. 2021, 28, 920–929. [Google Scholar] [CrossRef]
- Xia, D.; Wang, F.; Pan, S.; Yuan, S.; Liu, Y.; Xu, Y. Redox/pH-Responsive Biodegradable Thiol-Hyaluronic Acid/Chitosan Charge-Reversal Nanocarriers for Triggered Drug Release. Polymers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Yang, X.; Shang, P.; Ji, J.; Malichewe, C.; Yao, Z.; Liao, J.; Du, D.; Sun, C.; Wang, L.; Tang, Y.J.; et al. Hyaluronic Acid-Modified Nanoparticles Self-Assembled from Linoleic Acid-Conjugated Chitosan for the Codelivery of miR34a and Doxorubicin in Resistant Breast Cancer. Mol. Pharm. 2021, 19, 2–17. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Nicoletta, F.P.; Leggio, A.; Iemma, F. Dual-Targeted Hyaluronic Acid/Albumin Micelle-Like Nanoparticles for the Vectorization of Doxorubicin. Pharmaceutics 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Xu, F.; Yao, T.; Zhu, J.; Yu, H.; Wang, G.; Li, J. TPGS and chondroitin sulfate dual-modified lipid-albumin nanosystem for targeted delivery of chemotherapeutic agent against multidrug-resistant cancer. Int. J. Biol. Macromol. 2021, 183, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, Y.; Ji, W.; Mei, H.; Wu, T.; He, Z.; Wang, K.; Shi, C. Hyaluronic acid-coated and Olaparib-loaded PEI-PLGA nanoparticles for the targeted therapy of triple negative breast cancer. J. Microencapsul. 2021, 39, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, X.; Wang, H.; Fan, R.; Hu, Y.; Hu, X.; Li, J. Dasatinib self-assembled nanoparticles decorated with hyaluronic acid for targeted treatment of tumors to overcome multidrug resistance. Drug Deliv. 2021, 28, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shang, E.; Ju, A.; Li, Y.; Wu, Q.; Li, Q.; Yang, Y.; Guo, Y.; Yang, D.; Lv, S. Tumor-targeted hyaluronic acid-mPEG modified nanostructured lipid carriers for cantharidin delivery: An in vivo and in vitro study. Fitoterapia 2021, 155, 105033. [Google Scholar] [CrossRef]
- Qu, Z.; Ren, Y.; Shen, H.; Wang, H.; Shi, L.; Tong, D. Combination Therapy of Metastatic Castration-Recurrent Prostate Cancer: Hyaluronic Acid Decorated, Cabazitaxel-Prodrug and Orlistat Co-Loaded Nano-System. Drug Des. Devel. Ther. 2021, 15, 3605–3616. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, D.; Gou, S.; Canup, B.S.B.; Zhang, G.; Dai, F.; Li, C.; Xiao, B. Quadruple-responsive nanoparticle-mediated targeted combination chemotherapy for metastatic breast cancer. Nanoscale 2021, 13, 5765–5779. [Google Scholar] [CrossRef]
- Al-Jubori, A.A.; Sulaiman, G.M.; Tawfeeq, A.T.; Mohammed, H.A.; Khan, R.A.; Mohammed, S.A.A. Layer-by-Layer Nanoparticles of Tamoxifen and Resveratrol for Dual Drug Delivery System and Potential Triple-Negative Breast Cancer Treatment. Pharmaceutics 2021, 13, 1098. [Google Scholar] [CrossRef]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Self-targeted polymersomal co-formulation of doxorubicin, camptothecin and FOXM1 aptamer for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 335, 369–388. [Google Scholar] [CrossRef]
- Bala Tannan, N.; Manzari, M.T.; Herviou, L.; Da Silva Ferreira, M.; Hagen, C.; Kiguchi, H.; Manova-Todorova, K.; Seshan, V.; de Stanchina, E.; Heller, D.A.; et al. Tumor-targeted nanoparticles improve the therapeutic index of BCL2 and MCL1 dual inhibition. Blood 2021, 137, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R., Jr.; Neufeld, M.J.; Sun, C. Preclinical data on co-delivery of temozolomide and talazoparib by fucodain-coated nanoscale metal organic frameworks for colorectal cancer chemoradiation. Data Brief. 2021, 38, 107394. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R., Jr.; Neufeld, M.J.; Sun, C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021, 500, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, C.; Yan, X.; Sung, J.; Garg, P.; Merlin, D. Highly Biocompatible Functionalized Layer-by-Layer Ginger Lipid Nano Vectors Targeting P-selectin for Delivery of Doxorubicin to Treat Colon Cancer. Adv. Ther. 2019, 2, 1900129. [Google Scholar] [CrossRef]
- Li, J.; Burgess, D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm. Sin. B 2020, 10, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Q.; Dong, X.; Xu, M.; Yang, J.; Yi, X.; Chen, B.; Dong, X.; Wang, Y.; Lou, X.; et al. Biocompatible AIEgen/p-glycoprotein siRNA@reduction-sensitive paclitaxel polymeric prodrug nanoparticles for overcoming chemotherapy resistance in ovarian cancer. Theranostics 2021, 11, 3710–3724. [Google Scholar] [CrossRef]
- Jia, L.; Li, Z.; Zheng, D.; Li, Z.; Zhao, Z. A targeted and redox/pH-responsive chitosan oligosaccharide derivatives based nanohybrids for overcoming multidrug resistance of breast cancer cells. Carbohydr. Polym. 2021, 251, 117008. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Chen, S.; Zhang, S.; Cui, C. Cetuximab-Modified Human Serum Albumin Nanoparticles Co-Loaded with Doxorubicin and MDR1 siRNA for the Treatment of Drug-Resistant Breast Tumors. Int. J. Nanomed. 2021, 16, 7051–7069. [Google Scholar] [CrossRef]
- Wang, H.; Ellipilli, S.; Lee, W.J.; Li, X.; Vieweger, M.; Ho, Y.S.; Guo, P. Multivalent rubber-like RNA nanoparticles for targeted co-delivery of paclitaxel and MiRNA to silence the drug efflux transporter and liver cancer drug resistance. J. Control. Release 2021, 330, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, T.; Cui, M.; Li, N.; Li, Q.; Zhu, L.; Duan, S.; Wang, Y.; Guo, Y. A novel targeted co-delivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway. J. Nanobiotechnol. 2021, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Yu, X.; Liu, X.; Cheng, Y.; Zhou, C.; Li, M.; Shi, L.; Deng, Y.; Liu, H.; et al. Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA. Acta Biomater. 2021, 135, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Hu, X.; Zhang, J. Tumor Targeted Polymer Nanoparticles Co-loaded with Docetaxel and siCCAT2 for Combination Therapy of Lung Cancer. J. Drug Target. 2021, 30, 534–543. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Wang, S.; Chang, H.; Zhang, T.; Qu, J. LncRNA CCAT2 promotes tumorigenesis by over-expressed Pokemon in non-small cell lung cancer. Biomed. Pharmacother. 2017, 87, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Bin, J.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon nano-onion-mediated dual targeting of P-selectin and P-glycoprotein to overcome cancer drug resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Gote, V.; Sharma, A.D.; Pal, D. Hyaluronic Acid-Targeted Stimuli-Sensitive Nanomicelles Co-Encapsulating Paclitaxel and Ritonavir to Overcome Multi-Drug Resistance in Metastatic Breast Cancer and Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1257. [Google Scholar] [CrossRef]
- Roque, M.C.; da Silva, C.D.; Lempek, M.R.; Cassali, G.D.; de Barros, A.L.B.; Melo, M.M.; Oliveira, M.C. Preclinical toxicological study of long-circulating and fusogenic liposomes co-encapsulating paclitaxel and doxorubicin in synergic ratio. Biomed. Pharmacother. 2021, 144, 112307. [Google Scholar] [CrossRef]
- Logan, K.A.; Nesbitt, H.; Callan, B.; Gao, J.; McKaig, T.; Taylor, M.; Love, M.; McHale, A.P.; Callan, J.F. Synthesis of a gemcitabine-modified phospholipid and its subsequent incorporation into a single microbubble formulation loaded with paclitaxel for the treatment of pancreatic cancer using ultrasound-targeted microbubble destruction. Eur. J. Pharm. Biopharm. 2021, 165, 374–382. [Google Scholar] [CrossRef]
- Atchison, N.A.; Fan, W.; Papas, K.K.; Hering, B.J.; Tsapatsis, M.; Kokkoli, E. Binding of the fibronectin-mimetic peptide, PR_b, to alpha5beta1 on pig islet cells increases fibronectin production and facilitates internalization of PR_b functionalized liposomes. Langmuir 2010, 26, 14081–14088. [Google Scholar] [CrossRef] [Green Version]
- Shabana, A.M.; Kambhampati, S.P.; Hsia, R.C.; Kannan, R.M.; Kokkoli, E. Thermosensitive and biodegradable hydrogel encapsulating targeted nanoparticles for the sustained co-delivery of gemcitabine and paclitaxel to pancreatic cancer cells. Int. J. Pharm. 2021, 593, 120139. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, C.; Fu, Q.; Lv, H. Combined chemotherapy for triple negative breast cancer treatment by paclitaxel and niclosamide nanocrystals loaded thermosensitive hydrogel. Eur. J. Pharm. Sci. 2021, 167, 105992. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.M.; Yadava, S.K.; Singh, R.; Giri, J. Lipid nanocapsules co-encapsulating paclitaxel and salinomycin for eradicating breast cancer and cancer stem cells. Colloids Surf. B Biointerfaces 2021, 204, 111775. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Hossain, M.A.; Holmes, B.; Yan, S.; Mukherjee, P.; Vivero-Escoto, J.L. Advanced Nanoengineering Approach for Target-Specific, Spatiotemporal, and Ratiometric Delivery of Gemcitabine-Cisplatin Combination for Improved Therapeutic Outcome in Pancreatic Cancer. Small 2021, 18, e2104449. [Google Scholar] [CrossRef]
- Kim, K.R.; You, S.J.; Kim, H.J.; Yang, D.H.; Chun, H.J.; Lee, D.; Khang, G. Theranostic potential of biodegradable polymeric nanoparticles with paclitaxel and curcumin against breast carcinoma. Biomater. Sci. 2021, 9, 3750–3761. [Google Scholar] [CrossRef]
- Hussain, T.; Paranthaman, S.; Rizvi, S.M.D.; Moin, A.; Gowda, D.V.; Subaiea, G.M.; Ansari, M.; Alanazi, A.S. Fabrication and Characterization of Paclitaxel and Resveratrol Loaded Soluplus Polymeric Nanoparticles for Improved BBB Penetration for Glioma Management. Polymers 2021, 13, 3210. [Google Scholar] [CrossRef]
- Maleki, H.; Hosseini Najafabadi, M.R.; Webster, T.J.; Hadjighassem, M.R.; Sadroddiny, E.; Ghanbari, H.; Khosravani, M.; Adabi, M. Effect of Paclitaxel/etoposide co-loaded polymeric nanoparticles on tumor size and survival rate in a rat model of glioblastoma. Int. J. Pharm. 2021, 604, 120722. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A.; Singh, T.; et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef] [PubMed]
- El-Sahli, S.; Hua, K.; Sulaiman, A.; Chambers, J.; Li, L.; Farah, E.; McGarry, S.; Liu, D.; Zheng, P.; Lee, S.H.; et al. A triple-drug nanotherapy to target breast cancer cells, cancer stem cells, and tumor vasculature. Cell Death Dis. 2021, 12, 8. [Google Scholar] [CrossRef]
- Berko, Y.A.; Funmilola, A.F.; Akala, E.O. Fabrication of Paclitaxel and 17AAG-loaded Poly-epsilon-Caprolactone Nanoparticles for Breast Cancer Treatment. J. Pharm Drug Deliv. Res. 2021, 10, 196. [Google Scholar]
- Chemmalar, S.; Intan-Shameha, A.R.; Abdullah, C.A.C.; Ab Razak, N.A.; Yusof, L.M.; Ajat, M.; Gowthaman, N.S.K.; Bakar, M.Z.A. Synthesis and Characterization of Gefitinib and Paclitaxel Mono and Dual Drug-Loaded Blood Cockle Shells (Anadara granosa)-Derived Aragonite CaCO3 Nanoparticles. Nanomaterials 2021, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.T.; Ho, H.O.; Lin, S.Y.; Liu, D.Z.; Chen, L.C.; Sheu, M.T. Carfilzomib and Paclitaxel Co-Loaded Protein Nanoparticles an Effective Therapy Against Pancreatic Adenocarcinomas. Int. J. Nanomed. 2021, 16, 6825–6841. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gao, Y.; Yin, S.; Yao, Y.; Yu, H.; Wang, G.; Li, J. Co-delivery of paclitaxel and STAT3 siRNA by a multifunctional nanocomplex for targeted treatment of metastatic breast cancer. Acta Biomater. 2021, 134, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Hou, Y.; Quan, X.; Chen, L.; Gao, Z.; Huang, W. Smart Polymeric Nanoparticles with pH-Responsive and PEG-Detachable Properties (II): Co-Delivery of Paclitaxel and VEGF siRNA for Synergistic Breast Cancer Therapy in Mice. Int. J. Nanomed. 2021, 16, 5479–5494. [Google Scholar] [CrossRef]
- Du, J.; Shao, Y.; Hu, Y.; Chen, Y.; Cang, J.; Chen, X.; Pei, W.; Miao, F.; Shen, Y.; Muddassir, M.; et al. Multifunctional Liposomes Enable Active Targeting and Twinfilin 1 Silencing to Reverse Paclitaxel Resistance in Brain Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 23396–23409. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, Y.; Long, W.; Zhang, A.; Zhang, Y.; An, Y.; Miao, F.; Nie, F.; Li, M.; He, Y.; et al. Identification and characterization of a novel phage display-derived peptide with affinity for human brain metastatic breast cancer. Biotechnol. Lett. 2014, 36, 2291–2301. [Google Scholar] [CrossRef]
- Chen, C.; Shen, M.; Liao, H.; Guo, Q.; Fu, H.; Yu, J.; Duan, Y. A paclitaxel and microRNA-124 coloaded stepped cleavable nanosystem against triple negative breast cancer. J. Nanobiotechnol. 2021, 19, 55. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional Lipid-Based Nanoparticles for Codelivery of Anticancer Drugs and siRNA for Treatment of Non-Small Cell Lung Cancer with Different Level of Resistance and EGFR Mutations. Pharmaceutics 2021, 13, 1063. [Google Scholar] [CrossRef]
- Gu, X.G.; Schmitt, M.; Hiasa, A.; Nagata, Y.; Ikeda, H.; Sasaki, Y.; Akiyoshi, K.; Sunamoto, J.; Nakamura, H.; Kuribayashi, K.; et al. A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 1998, 58, 3385–3390. [Google Scholar]
- Xu, Y.; Ma, S.; Si, X.; Zhao, J.; Yu, H.; Ma, L.; Song, W.; Tang, Z. Polyethyleneimine-CpG Nanocomplex as an In Situ Vaccine for Boosting Anticancer Immunity in Melanoma. Macromol. Biosci. 2021, 21, e2000207. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Park, K.S.; Moon, J.J. Modularly Programmable Nanoparticle Vaccine Based on Polyethyleneimine for Personalized Cancer Immunotherapy. Adv. Sci. 2021, 8, 2002577. [Google Scholar] [CrossRef]
- Van Lysebetten, D.; Malfanti, A.; Deswarte, K.; Koynov, K.; Golba, B.; Ye, T.; Zhong, Z.; Kasmi, S.; Lamoot, A.; Chen, Y.; et al. Lipid-Polyglutamate Nanoparticle Vaccine Platform. ACS Appl. Mater. Interfaces 2021, 13, 6011–6022. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Qiu, Q.; Feng, Z.; Tong, Z.; Guo, W.; Zou, F.; Yue, N.; Huang, W.; Qian, H. Design, synthesis and immunological evaluation of self-assembled antigenic peptides from dual-antigen targets: A broad-spectrum candidate for an effective antibreast cancer therapy. J. Immunother. Cancer 2021, 9, e002523. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Zhang, F.; Li, M.; Tu, Z.; Mu, L.; Dawulieti, J.; Lao, Y.H.; Xiao, Z.; Yan, H.; et al. A Versatile and Robust Platform for the Scalable Manufacture of Biomimetic Nanovaccines. Adv. Sci. 2021, 8, 2002020. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, J.; Pan, J.; Liu, C.; Guo, X.; Zhou, S. Personalized Nanovaccine Coated with Calcinetin-Expressed Cancer Cell Membrane Antigen for Cancer Immunotherapy. Nano Lett. 2021, 21, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.; Quinones-Perez, M.; Wang, J.; Wang, X.; Basava, V.S.; Gao, J. Antigen folding improves loading efficiency and antitumor efficacy of PC7A nanoparticle vaccine. J. Control. Release 2021, 329, 353–360. [Google Scholar] [CrossRef]
- Tsai, S.J.; Amerman, A.; Jewell, C.M. Altering Antigen Charge to Control Self-Assembly and Processing of Immune Signals During Cancer Vaccination. Front. Immunol. 2020, 11, 613830. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Ramirez-Valdez, R.A.; Tobin, K.K.S.; Yamane, H.; Dutertre, C.A.; Khalilnezhad, A.; Reynoso, G.V.; Coble, V.L.; Lynn, G.M.; Mule, M.P.; et al. Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat. Immunol. 2021, 22, 41–52. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Yu, F.; Li, M.; Zhu, H.; Wang, K.; Meng, M.; Zhao, W. The Adjuvant of alpha-Galactosylceramide Presented by Gold Nanoparticles Enhances Antitumor Immune Responses of MUC1 Antigen-Based Tumor Vaccines. Int. J. Nanomed. 2021, 16, 403–420. [Google Scholar] [CrossRef]
- Trabbic, K.R.; Kleski, K.A.; Barchi, J.J., Jr. A Stable Gold Nanoparticle-Based Vaccine for the Targeted Delivery of Tumor-Associated Glycopeptide Antigens. ACS Bio. Med. Chem. Au 2021, 1, 31–43. [Google Scholar] [CrossRef]
- Liu, L.; Kshirsagar, P.; Christiansen, J.; Gautam, S.K.; Aithal, A.; Gulati, M.; Kumar, S.; Solheim, J.C.; Batra, S.K.; Jain, M.; et al. Polyanhydride nanoparticles stabilize pancreatic cancer antigen MUC4beta. J. Biomed. Mater. Res. A 2021, 109, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Dolen, Y.; Gileadi, U.; Chen, J.L.; Valente, M.; Creemers, J.H.A.; Van Dinther, E.A.W.; van Riessen, N.K.; Jager, E.; Hruby, M.; Cerundolo, V.; et al. PLGA Nanoparticles Co-encapsulating NY-ESO-1 Peptides and IMM60 Induce Robust CD8 and CD4 T Cell and B Cell Responses. Front. Immunol. 2021, 12, 641703. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pan, N.; Wei, Y.; Zhao, J.; Aldarouish, M.; Wang, X.; Sun, X.; Wen, Z.; Chen, Y.; Wang, L. Effects of Combinatorial Ubiquitinated Protein-Based Nanovaccine and STING Agonist in Mice With Drug-Resistant and Metastatic Breast Cancer. Front. Immunol. 2021, 12, 707298. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Teoh, S.M.; Kuo, P.; Tolley, L.; Bashaw, A.A.; Tuong, Z.K.; Liu, Y.; Chen, Z.; Wells, J.W.; Yu, C.; et al. Manganese-Doped Silica-Based Nanoparticles Promote the Efficacy of Antigen-Specific Immunotherapy. J. Immunol. 2021, 206, 987–998. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Roh, V.; Hiou-Feige, A.; Galliverti, G.; Simon, C.; Tolstonog, G.V.; Nardelli-Haefliger, D. Vaccination with a nanoparticle E7 vaccine can prevent tumor recurrence following surgery in a human papillomavirus head and neck cancer model. Oncoimmunology 2021, 10, 1912473. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Ma, C.; Zhang, Y.; Tan, H.; Qing, G.; Zhang, J.; Wang, Y.; Wang, J.; Chen, S.; et al. An amphiphilic dendrimer as a light-activable immunological adjuvant for in situ cancer vaccination. Nat. Commun. 2021, 12, 4964. [Google Scholar] [CrossRef]
- Xia, M.; Cheng, Y.; Meng, Z.; Jiang, X.; Chen, Z.; Theato, P.; Zhu, M. A novel nanocomposite hydrogel with precisely tunable UCST and LCST. Macromol. Rapid Commun. 2015, 36, 477–482. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Y.; She, J.; Zhou, X.; Xu, J.; Han, X.; Wang, C.; Zhu, M.; Liu, Z. Ultrasound-Mediated Remotely Controlled Nanovaccine Delivery for Tumor Vaccination and Individualized Cancer Immunotherapy. Nano Lett. 2021, 21, 1228–1237. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.; Liu, X.; Xu, Y.; Zhao, J.; Si, X.; Li, H.; Huang, Z.; Wang, Z.; Tang, Z.; et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv. Mater. 2021, 33, e2007293. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Xiao, M.; Huang, X.; Wu, X. A versatile photothermal vaccine based on acid-responsive glyco-nanoplatform for synergistic therapy of cancer. Biomaterials 2021, 273, 120792. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Xu, L.; Zhuang, Z.; Liu, J.; Liu, S.; Wu, Y.; Gong, A.; Zhang, M.; Du, F. NIR responsive tumor vaccine in situ for photothermal ablation and chemotherapy to trigger robust antitumor immune responses. J. Nanobiotechnol. 2021, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Choi, C.; Cho, H.; Ahn, W.G.; Kim, S.Y.; Shin, S.W.; Kim, Y.; Jang, T.; Lee, N.; Park, H.C. Antigen-Capturing Mesoporous Silica Nanoparticles Enhance the Radiation-Induced Abscopal Effect in Murine Hepatocellular Carcinoma Hepa1-6 Models. Pharmaceutics 2021, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R.; et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2021, 266, 120431. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Yin, Y.; Hu, Y.; Lu, Y.; Zou, H.; Lu, G.; Wang, Q.; Lian, J.; Gao, J.; Shen, X. Tumor RNA-loaded nanoliposomes increases the anti-tumor immune response in colorectal cancer. Drug Deliv. 2021, 28, 1548–1561. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Wu, Y.; Cao, P.; Movahedi, F.; Liu, J.; Wang, J.; Xu, Z.P.; Gu, W. Mannose-Functionalized Biodegradable Nanoparticles Efficiently Deliver DNA Vaccine and Promote Anti-tumor Immunity. ACS Appl. Mater. Interfaces 2021, 13, 14015–14027. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Gu, W.; Cao, P.; Movahedi, F.; Wu, Y.; Xu, Z.P.; Gu, W. ATP stabilised and sensitised calcium phosphate nanoparticles as effective adjuvants for a DNA vaccine against cancer. J. Mater. Chem. B 2021, 9, 7435–7446. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Ni, B.; Jing, T.; Tang, J. Tumor targeting nanoparticle E749-57-HSP110-RGD elicits potent anti-tumor immune response in a CD8-dependent manner in cervical cancer-bearing mouse model. Hum. Vaccin. Immunother. 2021, 17, 3529–3538. [Google Scholar] [CrossRef]
- Chai, D.; Zhang, Z.; Jiang, N.; Ding, J.; Qiu, D.; Shi, S.Y.; Wang, G.; Fang, L.; Li, H.; Tian, H.; et al. Co-immunization with L-Myc enhances CD8(+) or CD103(+) DCs mediated tumor-specific multi-functional CD8(+) T cell responses. Cancer Sci. 2021, 112, 3469–3483. [Google Scholar] [CrossRef]
- Rossmann, E.; Osterborg, A.; Lofvenberg, E.; Choudhury, A.; Forssmann, U.; von Heydebreck, A.; Schroder, A.; Mellstedt, H. Mucin 1-specific active cancer immunotherapy with tecemotide (L-BLP25) in patients with multiple myeloma: An exploratory study. Hum. Vaccin. Immunother. 2014, 10, 3394–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.E.; Bosquee, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Thatcher, N.; Socinski, M.A.; Wasilewska-Tesluk, E.; Horwood, K.; Szczesna, A.; Martin, C.; Ragulin, Y.; Zukin, M.; Helwig, C.; et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: Updated overall survival and biomarker analyses. Ann. Oncol. 2015, 26, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Hida, T.; Nokihara, H.; Imamura, F.; Sakai, H.; Atagi, S.; Nishio, M.; Kashii, T.; Satouchi, M.; Helwig, C.; et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer 2017, 105, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Lee, J.W.; Carbone, D.P.; Wagner, H.; Shanker, A.; de Aquino, M.T.P.; Horn, L.; Johnson, M.L.; Gerber, D.E.; Liu, J.J.; et al. Phase II Study of Immunotherapy With Tecemotide and Bevacizumab After Chemoradiation in Patients With Unresectable Stage III Non-Squamous Non-Small-Cell Lung Cancer (NS-NSCLC): A Trial of the ECOG-ACRIN Cancer Research Group (E6508). Clin. Lung Cancer 2020, 21, 520–526. [Google Scholar] [CrossRef]
- Schimanski, C.C.; Kasper, S.; Hegewisch-Becker, S.; Schroder, J.; Overkamp, F.; Kullmann, F.; Bechstein, W.O.; Vohringer, M.; Ollinger, R.; Lordick, F.; et al. Adjuvant MUC vaccination with tecemotide after resection of colorectal liver metastases: A randomized, double-blind, placebo-controlled, multicenter AIO phase II trial (LICC). Oncoimmunology 2020, 9, 1806680. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F.; Pfeiler, G.; Hubalek, M.; Bartsch, R.; Stoger, H.; Pichler, A.; Petru, E.; Bjelic-Radisic, V.; Greil, R.; Rudas, M.; et al. Efficacy and safety of the therapeutic cancer vaccine tecemotide (L-BLP25) in early breast cancer: Results from a prospective, randomised, neoadjuvant phase II study (ABCSG 34). Eur. J. Cancer 2020, 132, 43–52. [Google Scholar] [CrossRef]
- Najafabadi, A.H.; Abadi, Z.I.N.; Aikins, M.E.; Foulds, K.E.; Donaldson, M.M.; Yuan, W.; Okeke, E.B.; Nam, J.; Xu, Y.; Weerappuli, P.; et al. Vaccine nanodiscs plus polyICLC elicit robust CD8+ T cell responses in mice and non-human primates. J. Control. Release 2021, 337, 168–178. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kageyama, S.; Miyahara, Y.; Okayama, T.; Kokura, S.; Wang, L.; Sato, E.; Yagita, H.; Itoh, Y.; Shiku, H. Safety and antibody immune response of CHP-NY-ESO-1 vaccine combined with poly-ICLC in advanced or recurrent esophageal cancer patients. Cancer Immunol. Immunother. 2021, 70, 3081–3091. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J. Angiogenic zip code. Nat. Biotechnol. 1999, 17, 749. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkila, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; et al. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774. [Google Scholar] [CrossRef] [PubMed]
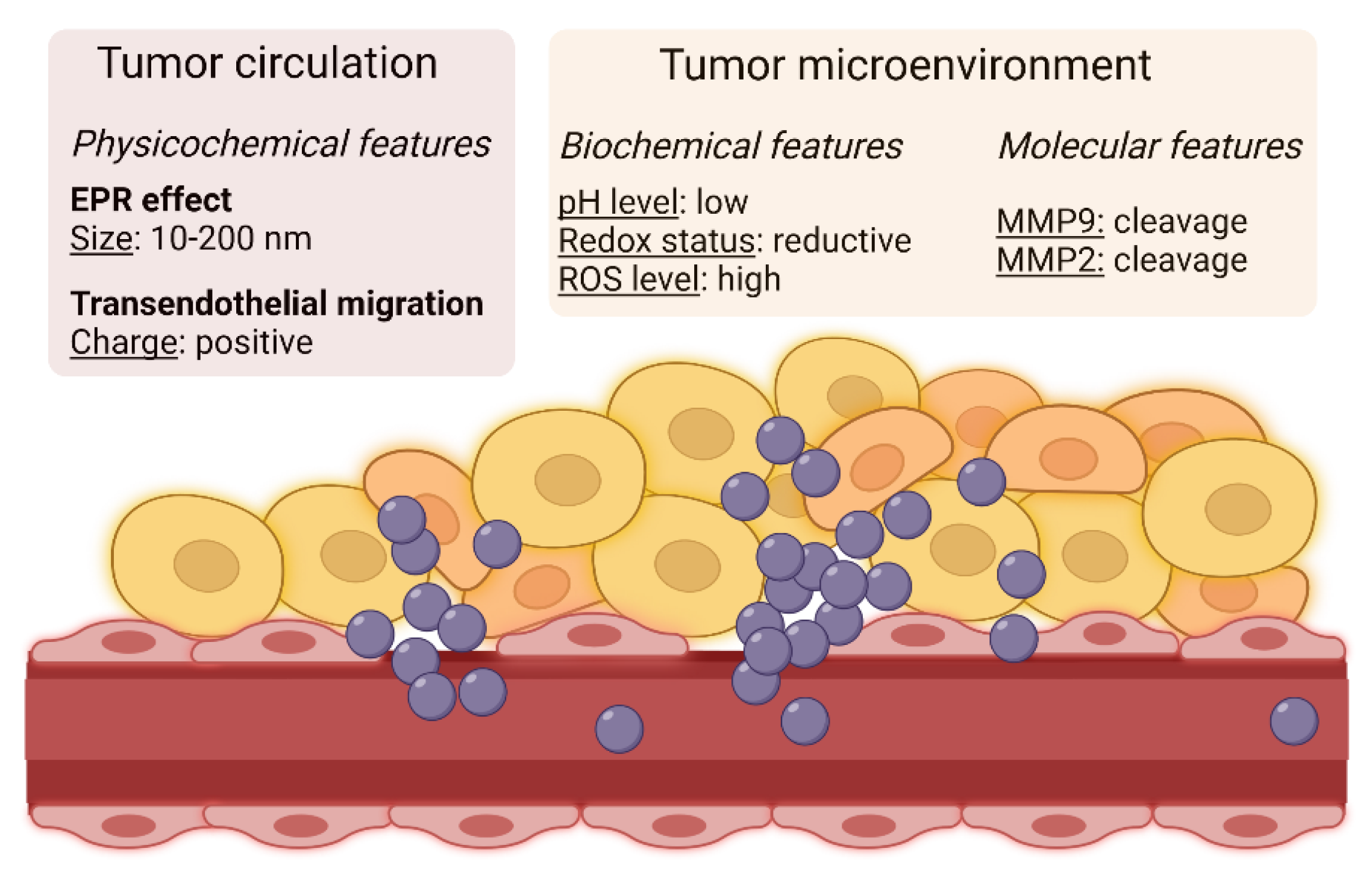


| Carrier | Drug | Strategy | Achievement | Optimized Step | Ref. |
|---|---|---|---|---|---|
| Liposome | Doxorubicin + simvastatin | Hydrophilic and hydrophobic portions | Coencapsulation of drugs with opposite solubility | Loading | [11] |
| Double-emulsion nanoparticle | Doxorubicin + erlotinib | Hydrophilic and Hydrophobic portions | Coencapsulation of drugs with opposite solubility | Loading | [12] |
| Liposome | Doxorubicin | Different lipid composition and internal pH | Slow, medium, or fast drug release | Release | [13] |
| Lipid nanoparticle | Doxorubicin | Shell with different thickness | Slow, tunable (thickness) release | Release | [14] |
| Polymeric nanomicelle | Doxorubicin | Hydrophobic and cationic portions | Slow, sustained release | Release | [15] |
| Calcium carbonate microcapsule | Doxorubicin | Alternate layers of polyanions and polycations | Slow, tunable release | Release | [16] |
| Polymeric nanomicelle | Camptothecin | Different backbone length | Fast/high or slow/low uptake | Uptake | [17] |
| Amphiphilic nanoparticle | Doxorubicin | Redox/pH-responsive | Activable intracellular unloading | Delivery | [18] |
| Vitamicelle | Doxorubicin | Redox/pH-responsive | Activable intracellular unloading | Delivery | [19] |
| Lipid nanomicelle | Doxorubicin | Artificial cytosol | Favored intracellular unloading | Delivery | [20] |
| Carrier | Drug | Modality | Strategy | Responsive to | Ref. |
|---|---|---|---|---|---|
| Starch | Doxorubicin | Unbound drug | Ionizable portion | Low pH | [33] |
| Albumin | Doxorubicin | Unbound drug | Ionizable portion | Low pH | [34] |
| PEG-CaCO3 nanoparticle | Doxorubicin | Unbound drug | Degradation | Low pH | [35] |
| Nanocapsule with ferrocene | Paclitaxel | Unbound drug | Ferrocene oxidation, surface charges | Presence of ROS | [36] |
| Carbon nanotube | Doxorubicin + paclitaxel | Unbound drug | Ionizable portion, degradation | Low pH | [37] |
| PEG-drug | Doxorubicin | Prodrug | Ionizable portion | Low pH | [38] |
| PEG-drug, lecithin | Cisplatin + paclitaxel | Prodrug | Ionizable portion, disulfide bonds | Low pH, reductive conditions | [39] |
| Drug dimer-F127, DSPE-PEG, albumin, or Fe-tannic acid | Paclitaxel | Prodrug | Diselenide bond | Reductive conditions | [40] |
| Drug-dextran | Paclitaxel | Prodrug | Disulfide bond | Reductive conditions | [41] |
| Drug-tetramethyl pyrazine | Paclitaxel | Prodrug | Disulfide bond | Reductive conditions | [42] |
| Carrier | Drug | Additional Functions | Mechanism | Responsive to | Ref. |
|---|---|---|---|---|---|
| Upconversion nanoparticle-albumin | Doxorubicin Chlorin 6, Fe2+ | Photodynamic Chemodynamic | Production of ROS by NIR and H2O2 | NIR irradiation Low pH | [43] |
| Copolymer nanoparticle | Doxorubicin prodrug Chlorin 6 | Photodynamic Chemodynamic | Production of ROS by NIR | NIR irradiation Reductive conditions | [44] |
| Nano-donuts | Doxorubicin Prussian Blue Fe3+ ion | Chemodynamic Photothermal MRI | Production of ROS by H2O2 Induction of hyperthermia | NIR irradiation, Presence of ROS | [45] |
| PEG-PEI-based nanoparticle | Docetaxel IR825 | Photothermal | Induction of hyperthermia | NIR irradiation, Low pH | [46] |
| Semiconducting polymer | Doxorubicin prodrug | Photothermal | Induction of hyperthermia | NIR irradiation, Low pH | [47] |
| Dendrimer/ Lentinan nanoparticle | Paclitaxel | Photodynamic | Production of ROS by NIR | NIR irradiation, Low pH, Reductive conditions, Presence of ROS | [48] |
| Carrier | Drug | Antibody | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| PEG-nanomicelle | Taxol | α-nucleosome | Nucleosome | Lewis lung carcinoma (in vitro and in vivo) | [51] |
| Liposome | Paclitaxel + salinomycin | Clone 2C5 | Nucleolin | Breast cancer (in vitro) | [52] |
| Nanorod | Paclitaxel | Trastuzumab | HER2 | Breast cancer (in vitro) | [55] |
| Gold nanorod/ PLGA-PEG nanoparticle | Paclitaxel | Trastuzumab | HER2 | Breast cancer (in vitro) | [56] |
| Chitosan/PLGA nanoparticle | Doxorubicin | Trastuzumab | HER2 | Breast cancer (in vitro) | [57] |
| Fucoidan/chitosan nanoparticle | Gemcitabine | Clone 7H3L20 | HER2 | Breast cancer (in vitro and in vivo) | [58] |
| Gold nanoparticle | Cetuximab | EGFR | Colorectal cancer (in vitro) | [59] | |
| PLGA-PEG nanoparticle | 5-FU | α- EGFR | EGFR | Colorectal cancer (in vitro) | [60] |
| Chitosan/PEG nanocapsule | Docetaxel | Chi-Tn | Tn-antigen | Lung cancer (in vitro) | [61] |
| Lipid nanocapsule | Paclitaxel | α-CD44 | CD44 | Pancreatic cancer (in vitro and in vivo) | [62] |
| Solid lipid nanoparticle | γ-secretase inhibitor | α-DR5 | DR5 | Breast cancer (in vitro and in vivo) | [64] |
| Halloysite nanotube | Docetaxel+ gold | Clone 2D8-E3 | Sortilin | Ovarian carcinoma (in vitro) | [66] |
| PEG-polymer/ prodrug | Camptothecin | α-CD147 | CD147 | Liver cancer (in vitro) | [67] |
| Carrier | Drug | Antibody Fragment | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| Prodrug nanoparticle | DM1 | Trastuzumab scFv | HER2 | Breast cancer (in vitro and in vivo) | [71] |
| Mesoporous silica nanoparticle | Doxorubicin | α-HER2 scFv | HER2 | Breast cancer (in vitro and in vivo) | [72] |
| Polystyrene nanoparticle | α-CD44v6 half antibody | CD44 | Gastric cancer (in vitro) | [73] | |
| F127 nanomicelle | Niclosamide | α-CD44v6 Fab | CD44 | Colorectal cancer (in vitro and in vivo) | [74] |
| LipoDox® | Doxorubicin | α-HER2 scFv + α-mPEG Fab | HER2 | Ovarian cancer (in vitro and in vivo) | [75] |
| LipoDox® | Doxorubicin | Ofatumumab + α-mPEG Fab | CD20 | B-cell malignancy (in vitro and in vivo) | [76] |
| mPEG/lecithin nanomicelle | Docetaxel | α-EGFR scFv + α-FAP scFv + α-mPEG Fab | EGFR, FAP | Pancreatic and colorectal cancer (in vitro and in vivo) | [77] |
| Carrier | Drug | Peptide | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| Albumin/red cell membranes | Gefitinib | cRGD | αvβ3/αvβ5 integrins | Lung cancer (in vitro and in vivo) | [86] |
| Lipid nanoparticle | GNA002 | cRGD-Arg6 | αvβ3/αvβ5 integrins + cell penetration | Squamous cell Carcinoma-tongue (in vitro and in vivo) | [87] |
| Silk fibroin nanoparticle | Curcumin | cRGD | αvβ3/αvβ5 integrins | Different cell models (in vitro) | [88] |
| Liposome | Doxorubicin | cRGD | αvβ3/αvβ5 integrins | Colon cancer (in vitro and in vivo) | [89] |
| DSPE/PEG nanoparticle | TQs-PEG4 | cRGD | αvβ3/αvβ5 integrins | Breast cancer (in vitro and in vivo) | [90] |
| Silver sulfide nanoparticle | Doxorubicin | cRGD | αvβ3/αvβ5 integrins | Breast cancer (in vitro and in vivo) | [91] |
| Gold-iron oxide nanoparticle | Multimodal | cRGD | αvβ3/αvβ5 integrins | Breast cancer (in vitro and in vivo) | [92] |
| Gallic acid/Fe3+ nanoparticle | Doxorubicin, multimodal | cRGD-Platinum prodrug | αvβ3/αvβ5 integrins | Glioblastoma (in vitro and in vivo) | [93] |
| DSPE nanoparticle | Siponimod | cRGD-PEG | αvβ3/αvβ5 integrins | Breast cancer (in vitro and in vivo) | [94] |
| PEG-lipid nanoparticle | Wee1 siRNA | cRGD-Arg8 | αvβ3/αvβ5 integrins + cell penetration | Melanoma (in vitro and in vivo) | [95] |
| Chitosan nanoparticle | VEGF siRNA | cRGD-albumin | αvβ3/αvβ5 integrins + protective corona | Liver cancer (in vitro and in vivo) | [96] |
| PLGA nanoparticle | Paclitaxel | iRGD co-administration | αvβ3/αvβ5 integrins + internalization | Colorectal cancer (in vitro and in vivo) | [100] |
| Fe-metal organic framework | Sorafenib | iRGD co-administration | αvβ3/αvβ5 integrins + internalization | Liver cancer (in vitro and in vivo) | [101] |
| Polylysine nanoparticle | FGL-1 siRNA PD-L1 siRNA | iRGD co-administration | αvβ3/αvβ5 integrins + internalization | Lewis lung cancer (in vitro and in vivo) | [102] |
| Mesoporous silica nanoparticle | Doxorubicin | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Breast cancer (in vitro and in vivo) | [103] |
| Tetrahedral framework | Doxorubicin | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Breast cancer (in vitro and in vivo) | [104] |
| Polymersome | Doxorubicin | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Breast cancer (in vitro and in vivo) | [105] |
| DSPE-PEG nanoparticle | Ursolic acid prodrug | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Gastric cancer (in vitro and in vivo) | [106] |
| Lipid nanoparticle | CRM1 inhibitor | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Melanoma (in vitro and in vivo) | [107] |
| Lipid nanoparticle | Camptothecin Indocyanine green | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Liver carcinoma (in vitro and in vivo) | [108] |
| DSPE-PEG nanoparticle | Doxorubicin prodrug | iRGD conjugate | αvβ3/αvβ5 integrins + internalization | Prostate and breast cancer (in vitro) | [109] |
| Mesoporous silica nanoparticle | Paclitaxel | Angiopep-2 | LRP1 | Glioma (in vitro and in vivo) | [110] |
| Hyaluronic acid nanoparticle | Irinotecan | Angiopep-2 | LRP1 | Glioma (in vitro) | [111] |
| Caelyx® | Doxorubicin | Leptin peptide Lp31 | Leptin receptor Ob-R | Colon cancer (in vitro and in vivo) | [112] |
| Multicomponent nanostructure | Cisplatin Alpelisib Indocyanine green | dYNH | EphA2 | Lung cancer (in vitro and in vivo) | [113] |
| PEG-liposome | Docetaxel | AE147 | uPAR | Breast cancer (in vitro and in vivo) | [114] |
| PEG-dendritic nanomicelle | Paclitaxel | (D-Lys)-LHRH | LHRH receptor | Breast cancer (in vitro and in vivo) | [115] |
| Prodrug-peptide nanoparticle | Doxorubicin | FRRG | Cathepsin B substrate | Breast cancer (in vitro and in vivo) | [116] |
| Branched polymer nanoparticle | AZD2281 Pyropheophorbide a | GFLG | Cathepsin B substrate | Breast cancer (in vitro and in vivo) | [117] |
| Caelyx® | Doxorubicin | CGKRK | Breast cancer (in vitro and in vivo) | [118] | |
| Liposome | Curcumin Indocyanine green | GE11 (phage display) | Lung cancer (in vitro) | [119] | |
| Polydopamine nanoparticle | Doxorubicin Phthalo- cyanine | QRH (phage display) | Breast cancer (in vitro and in vivo) | [120] | |
| Polymersome | Doxorubicin | SP94 (phage display) | Cancer cell surface | Liver cancer (in vitro and in vivo) | [121] |
| DSPE-prodrug nanoparticle | Cisplatin Paclitaxel | Cyclic TMTP1 (bacterial display) | Cancer cell surface | Squamous cell carcinoma-uterus (in vitro and in vivo) | [122] |
| Graphene oxide-PEG nanoparticle | Doxorubicin | HN-1 (phage display) | Cancer cell surface | Squamous cell carcinoma-oral (in vitro and in vivo) | [123] |
| Liposome | Doxorubicin Sorafenib | LinTT-1 (phage display) | gC1qR M2 macrophages | Breast cancer (in vitro and in vivo) | [124] |
| Hyaluronic acid PEG-nanoparticle | miR125b | M2 peptide (phage display) | M2 macrophages | Pancreatic cancer (in vitro and in vivo) | [125] |
| PEG-prodrug nanoparticle | Irinotecan (SN38) | IELLQAR | E-selectin Endothelial cells | Colon cancer and melanoma (in vitro and in vivo) | [126] |
| Lipoprotein nanoparticle | Paclitaxel GANT61 | tLyP-1 | Neuropilin-1 Endothelial cells | Breast cancer (in vitro and in vivo) | [127] |
| Carrier | Drug | Protein | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| Liposome | Vincristine | Transferrin-mimetic | TfR1 | Glioma (in vitro and in vivo) | [129] |
| PLGA-PEG nanoparticle | Seliciclib | Transferrin-mimetic | TfR1 | Different cell models (in vitro) | [130] |
| Chitosan nanoparticle | Docetaxel Curcumin | Transferrin-mimetic + phenylboronic acid | TfR1 | Lung cancer (in vitro and in vivo) | [131] |
| Carbon-nitride dot | Doxorubicin | Transferrin | TfR1 | B-cell malignancy (in vitro and in vivo) | [132] |
| PEG-albumin nanoparticle | Doxorubicin | Transferrin | TfR1 | Breast cancer (in vitro and in vivo) | [133] |
| Mesoporous silica nanoparticle | Doxorubicin | Transferrin (+ RGD) | TfR1 | Different cell models (in vitro) | [134] |
| Glycyrrhizic acid/PLGA nanoparticle | Piperine | Transferrin | TfR1 | Different cell models Breast cancer (in vitro and in vivo) | [135] |
| PC/DSPE-PEG nanoparticle | Plumbagin | Transferrin | TfR1 | Melanoma (in vitro and in vivo) | [136] |
| Liposome | Erianin | Transferrin | TfR1 | Liver cancer (in vitro and in vivo) | [137] |
| Different formulations | Thymoquinone Gefitinib | Transferrin | TfR1 | Lung cancer (in vitro and in vivo) | [138] |
| Ferritin nanocage | Paclitaxel ERK inhibitor | Ferritin | TfR1 | Breast cancer (in vitro) | [140] |
| Ferritin nanocage | Doxorubicin | Ferritin (+α2β1 integrin-binding) | TfR1 | Glioma (in vitro and in vivo) | [141] |
| Ferritin nanocage | Paclitaxel | Ferritin (+ tLyP-1 peptide) | TfR1 | Breast cancer (in vitro and in vivo) | [142] |
| Lactoferrin nanoparticle | Docetaxel Celastrol | Lactoferrin | TfR1 and others | Breast cancer (in vitro and in vivo) | [143] |
| Lactoferrin nanoparticle | Carboplatin Etoposide | Lactoferrin | TfR1 and others | Retinoblastoma (in vitro) | [144] |
| Ultra-small silica nanoparticle | Doxorubicin | Lactoferrin | TfR1 and others | Glioma (in vitro) | [145] |
| PEG-liquid crystalline nanoparticle | Imatinib | Lactoferrin | TfR1 and others | Liver cancer (in vitro and in vivo) | [146] |
| Nanogel | Doxorubicin | Lactoferrin + phenylboronic acid | TfR1 and others | Brain tumors (in vitro and in vivo) | [147] |
| Iron oxide nanoparticle | Paclitaxel | Folate | Folate receptor | Tumor cell line (in vitro) | [148] |
| Albumin nanoparticle | Paclitaxel 2-methoxy- estradiol | Folate | Folate receptor | Squamous cell carcinoma- esophagus (in vitro) | [149] |
| PEG-prodrug nanoparticle | Paclitaxel | Folate | Folate receptor | Breast cancer (in vitro and in vivo) | [150] |
| Liposome | Paclitaxel Vinorelbine | Folate | Folate receptor | Lewis lung cancer (in vitro and in vivo) | [151] |
| DSPE-PEG nanoparticle | Doxorubicin Berberine | Folate + hyaluronic acid | Folate receptor | Breast cancer (in vitro and in vivo) | [152] |
| Polymeric nanoparticle | Paclitaxel Baicalein | Folate | Folate receptor | Breast cancer (in vitro and in vivo) | [153] |
| PEI-cyclodextrin nanoparticle | Doxorubicin hTERT siRNA | Folate | Folate receptor | Breast cancer (in vitro) | [154] |
| Carrier | Drug | Aptamer | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| Gold nanoparticle | Doxorubicin | By SELEX | PrPC | Colon cancer (in vitro) | [159] |
| PLA-PEG/dextran nanoparticle | A10 | PSMA | Prostate cancer (in vitro) | [160] | |
| DSPE-PEG nanoparticle | Paclitaxel + siRNAs | α-PSMA | PSMA | Prostate cancer (in vitro and in vivo) | [161] |
| Mesoporous polydopamine nanoparticle | Docetaxel | AS1411 | Nucleolin | Prostate cancer (in vitro and in vivo) | [163] |
| Mesoporous silica nanoparticle | Doxorubicin + Tie2 siRNA | AS1411 | Nucleolin | Breast cancer (in vitro and in vivo) | [164] |
| Mesoporous silica/ chitosan nanoparticle | Doxorubicin + α-miR21 | AS1411 | Nucleolin | Colon cancer (in vitro and in vivo) | [165] |
| Gold/chitosan nanoparticle | Doxorubicin + paclitaxel | AS1411 | Nucleolin | Glioblastoma (in vitro) | [166] |
| Gold/DNA nanocage | Doxorubicin + VEGF siRNA | AS1411 | Nucleolin | Lung cancer (in vitro and in vivo) | [167] |
| PEG/PAMAM dendrimer nanoparticle | Doxorubicin + HSP70/90 siRNA | AS1411 | Nucleolin | Breast cancer (in vitro and in vivo) | [168] |
| Polymersome | Camptothecin | AS1411 | Nucleolin | Colon cancer (in vitro and in vivo) | [169] |
| PLA-PEI nanomicelle | Camptothecin + survivin shRNA | AS1411 | Nucleolin | Colon cancer (in vitro and in vivo) | [170] |
| Starch nanoparticle | Coumaric acid | AS1411 | Nucleolin | Breast cancer (in vitro) | [171] |
| Nanocapsule + gel | 5-FU | AS1411 | Nucleolin | Basal carcinoma cells + healthy animals (in vitro and in vivo) | [172] |
| Gold nanoparticle + gel | C8 + imiquimod | AS1411 | Nucleolin | Various tests (in vitro) | [173] |
| Mesoporous silica nanoparticle | Doxorubicin | α-mucin 1 α-ATP | Mucin 1 ATP | Breast/colon cancer (in vitro and in vivo) | [174] |
| Iron oxide-pyoverdine nanoparticle | Doxorubicin | α-mucin 1 | Mucin 1 | Colon cancer (in vitro and in vivo) | [175] |
| Hydrogel/quantum dot | Paclitaxel + sodium oxamate | α-mucin 1 | Mucin 1 | Breast cancer (in vitro) | [176] |
| PEG/PAMAM dendrimer nanoparticle | Gefitinib | α-mucin 1 | Mucin 1 | Breast cancer (in vitro and in vivo) | [177] |
| PEG-liposome | Doxorubicin | α-EpCAM | EpCAM | Colon cancer (in vitro and in vivo) | [178] |
| Iron oxide nanoparticle | Paclitaxel | α-EpCAM | EpCAM | Different cell models (in vitro) | [179] |
| Polymeric nanomicelle | Temozolomide + idasanutlin | α-CD133 | CD133 | Glioblastoma (in vitro) | [180] |
| PAMAM dendrimer nanoparticle | Temozolomide + paclitaxel | B19 | CD133 | Glioblastoma (in vitro) | [181] |
| PAMAM dendrimer nanoparticle | Gefitinib + hematoporphyrin | α-EGFR | EGFR | Lung cancer (in vitro) | [182] |
| PLGA-PEG nanoparticle | Cisplatin +dye | CL4 | EGFR | Breast cancer (in vitro and in vivo) | [183] |
| Mesoporous silica nanoparticle | Doxorubicin | α-HER2 | HER2 | Breast cancer (in vitro) | [184] |
| Carrier | Drug | Sugar | Target | Tumor Model | Ref. |
|---|---|---|---|---|---|
| PLGA nanoparticle | Hyaluronic acid | CD44, LYVE-1 RHAMM | Different cell models (in vitro) | [188] | |
| Liposome | Oxaliplatin | Hyaluronic acid | CD44, LYVE-1 RHAMM | Colon cancer (in vitro and in vivo) | [189] |
| Chitosan nanoparticle | Doxorubicin | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer (in vitro) | [190] |
| Chitosan nanoparticle | Doxorubicin + miR3a | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer (in vitro and in vivo) | [191] |
| Albumin nanoparticle | Doxorubicin | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer (in vitro) | [192] |
| Albumin-TPGS nanoparticle | Paclitaxel | Chondroitin sulfate | CD44 | Breast cancer (in vitro and in vivo) | [193] |
| PEI-PLGA nanoparticle | Olaparib | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer (in vitro and in vivo) | [194] |
| TPGS-prodrug nanoparticle | Dasatinib | Hyaluronic acid | CD44, LYVE-1 RHAMM | Epithelial tumor cells + healthy animals (in vitro and in vivo) | [195] |
| mPEG copolymer nanoparticle | Cantharidin | Hyaluronic acid | CD44, LYVE-1 RHAMM | Liver cancer (in vitro and in vivo) | [196] |
| Prodrug-PLGA nanoparticle | Cabazitaxel + orlistat | Hyaluronic acid | CD44, LYVE-1 RHAMM | Prostate cancer (in vitro and in vivo) | [197] |
| Silk fibroin nanoparticle | Curcumin + 5-FU | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer (in vitro and in vivo) | [198] |
| Poloxamer 407 nanoparticle | Resveratrol + tamoxifen | Hyaluronic acid | CD44, LYVE-1 RHAMM | Breast cancer cells + healthy animals (in vitro and in vivo) | [199] |
| Polymersome | Doxorubicin + captothecin + α-FOXM1 | Hyaluronic acid | CD44, LYVE-1 RHAMM | Lung cancer (in vitro and in vivo) | [200] |
| Fucoidan nanoparticle | S63845 + venetoclax | Fucoidan | P-selectin | B-cell malignancy (in vitro and in vivo) | [201] |
| Metal organic framework | Talazoparib + temozolomide | Fucoidan | P-selectin | Colon cancer (in vitro and in vivo) | [202,203] |
| Ginger lipid nanoparticle | Doxorubicin | Fucoidan | P-selectin | Colon cancer (in vitro and in vivo) | [204] |
| Carrier | Nucleic Acid | Drug | Natural Compound | Small-Molecule Inhibitor | Targeting Moiety | Ref. |
|---|---|---|---|---|---|---|
| Mesoporous silica nanoparticle | P-gp siRNA | [207] | ||||
| Prodrug nanoparticle | P-gp siRNA | Paclitaxel | [208] | |||
| Chitosan-PEI nanoparticle | P-gp shRNA | Paclitaxel | Folate | [209] | ||
| Albumin nanoparticle | P-gp siRNA | Doxorubicin | Cetuximab | [210] | ||
| RNA nanoparticle | miR-122 | Paclitaxel | Asialoglycoprotein | [211] | ||
| Silica/gold nanoparticle | miR-let-7a | Paclitaxel | Hyaluronic acid | [212] | ||
| Mesoporous silica nanoparticle | GNC5 siRNA | Doxorubicin | Hyaluronic acid | [213] | ||
| Prodrug-PLGA nanoparticle | CCAT2 siRNA | Docetaxel | Transferrin | [214] | ||
| Carbon/silica nanoparticle | Doxorubicin | HM30181A | Fucoidan | [216] | ||
| Nanomicelle | Paclitaxel | Ritonavir | Hyaluronic acid | [217] | ||
| Liposome | Paclitaxel + doxorubicin | [218] | ||||
| DSPE-PEG nanobubble | Paclitaxel + gemcitabine | [219] | ||||
| Liposome +hydrogel | Paclitaxel + gemcitabine | PR_b peptide | [221] | |||
| Nanocrystal + hydrogel | Paclitaxel + niclosamide | [222] | ||||
| Lipid nanocapsule | Paclitaxel + salinomycin | [223] | ||||
| Mesoporous silica nanoparticle | Cisplatin + gemcitabine | α-mucin 1 | [224] | |||
| PEG-PLGA nanoparticle | Paclitaxel | Curcumin | cRGD | [225] | ||
| Polymeric nanoparticle | Paclitaxel | Resveratrol | [226] | |||
| PEG-PLGA nanoparticle | Paclitaxel | Etoposide | [227] | |||
| Solid lipid nanoparticle | Paclitaxel | Naringenin | cRGD | [228] | ||
| PEG-PLGA nanoparticle | Paclitaxel + verteporfin | Combretastatin | [229] | |||
| Stealth nanoparticle | Paclitaxel | 17-AAG | [230] | |||
| Calcium carbonate nanoparticle | Paclitaxel | Gefitinib | [231] | |||
| Albumin nanoparticle | Paclitaxel | Carfilzomib | [232] | |||
| Copolymer micelle | STAT3 siRNA | Paclitaxel | Hyaluronic acid | [233] | ||
| PEI-PLGA nanoparticle | VEGF siRNA | Paclitaxel | [234] | |||
| Liposome | Twinfilin 1 siRNA | Paclitaxel | [235] | |||
| Polymeric nanoparticle | miR-124 | Paclitaxel | Hyaluronic acid | [237] | ||
| Lipid nanoparticle | EGFR siRNA | Paclitaxel | Gefitinib | LHRH | [238] |
| Carrier | Antigen(s) | Adjuvant | Route of Administration | Optimized Step | Model | Ref. |
|---|---|---|---|---|---|---|
| PEI-based nanocomplex | CpG | Intratumor injection | Adjuvant formulation | Melanoma | [240] | |
| PEI-PEG nanoparticle | Adpgk M27/M30 | CpG | Intratumor injection | Adjuvant formulation | Colon cancer, melanoma | [241] |
| Lipid-polyGlu nanoparticle | Ovalbumin | Imidazoquinoline | Subcutaneous/ intravenous injection | Adjuvant/antigen coencapsulation | Healthy animals | [242] |
| PLGA nanoparticle | Ovalbumin | Riboxxim | Intramuscular injection | Adjuvant/antigen coencapsulation | Thymoma, melanoma | [243] |
| Erythrocyte membrane DSPE-PEG nanoparticle | MAGE-1A + NY-ESO-1 (linker cleaved by cathepsin B) | Not included | Intratumor injection | Inclusion of multiple peptides | Breast carcinoma | [244] |
| PLGA, PEI-DNA, silica nanoparticle | Cancer cell membranes | CpG | Subcutaneous injection | Inclusion of multiple peptides | Melanoma | [245] |
| PLGA nanoparticle | Cancer cell membranes | Imiquimod | Intratumor injection | Inclusion of multiple peptides | Breast carcinoma | [246] |
| Carrier | Antigen(s) | Adjuvant | Model | Ref. |
|---|---|---|---|---|
| PC7A nanoparticle | Melanoma: Trp1, Tnpo3 | Melanoma | [247] | |
| Polyplexes | Melanoma: Trp1 | CpG | Melanoma | [248] |
| Nanomicelle | Melanoma: Reps1 | Imidazoquinoline | Melanoma | [249] |
| Gold nanoparticle | Mucin glycopeptide | α-galactosyl ceramide | MUC1-tumor | [250] |
| Gold nanoparticle | mucin glycopeptide + ovalbumin | Sigma Adjuvant System or TiterMax Gold | Healthy animals | [251] |
| Copolymer nanoparticle | Mucin 4β | Healthy animals | [252] | |
| PLGA nanoparticle | NY-ESO-1 peptides | α-galactosyl ceramide | Healthy animals | [253] |
| Vx3-alumina nanoparticle | Ubiquitinated proteins | STING agonist | Breast cancer | [254] |
| Silica nanoparticle | E7 GF001 peptide (HPV16) | HPV tumor | [255] | |
| Ultra-small nanoparticle | E7 long peptide (HPV16) | CpG | Head and neck cancer | [256] |
| Carrier | Antigen | Adjuvant | Additional Component | Additional Function | Model | Ref. |
|---|---|---|---|---|---|---|
| Dendrimer nanomicelle | Cancer cell antigens | 2-amino imidazole | Chlorin e6 | Activation by NIR irradiation | Breast cancer | [257] |
| PLGA nanoparticle + hydrogel | Ovalbumin | Imiquimod | Activation by ultrasounds | Breast cancer | [259] | |
| Modular nanoparticle | Cancer cell antigens | CpG | Cisplatin | Activation by ROS | Colorectal cancer | [260] |
| Dextran nanoparticle | Albumin | Imiquimod | Indocyanine green | Photothermal therapy | Thymoma | [261] |
| Polydopamine nanoparticle + hydrogel | Cancer cell antigens | Imiquimod | Hyaluronic acid Doxorubicin | Photothermal therapy | Breast cancer | [262] |
| Mesoporous silica nanoparticle | Ovalbumin/cancer cell antigens | Radiotherapy | Liver cancer | [263] |
| Carrier | Antigen | Adjuvant/Stabilizer | Nucleic Acid | Model | Ref. |
|---|---|---|---|---|---|
| DSPE-PEG-PAMAM nanoparticle | Ovalbumin | RNA | Colon cancer, melanoma | [264] | |
| Cationic polymer nanoparticle | Ovalbumin | R848 | RNA | Lymphoma, prostate cancer | [265] |
| PEG-liposome | Total RNA from cancer cells | RNA | Colon cancer | [266] | |
| Calcium phosphate nanoparticle | Ovalbumin | Biphosphonate | DNA | Thymoma | [267] |
| Calcium phosphate nanoparticle | Ovalbumin | ATP | DNA | Thymic lymphoma | [268] |
| DNA-RGD nanoparticle | E7 from HPV16 | HSP110 | DNA | HPV tumor | [269] |
| Chitosan nanoparticle | Carbonic anhydrase | L-Myc | DNA | Renal cancer | [270] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglizzo, V.; Marchiò, S. Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy. Cancers 2022, 14, 2473. https://doi.org/10.3390/cancers14102473
Foglizzo V, Marchiò S. Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy. Cancers. 2022; 14(10):2473. https://doi.org/10.3390/cancers14102473
Chicago/Turabian StyleFoglizzo, Valentina, and Serena Marchiò. 2022. "Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy" Cancers 14, no. 10: 2473. https://doi.org/10.3390/cancers14102473
APA StyleFoglizzo, V., & Marchiò, S. (2022). Nanoparticles as Physically- and Biochemically-Tuned Drug Formulations for Cancers Therapy. Cancers, 14(10), 2473. https://doi.org/10.3390/cancers14102473







