DJ-1 Expression Might Serve as a Biologic Marker in Patients with Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement of Serum DJ-1
2.3. Immunohistochemistry and Scoring
2.4. Statistical Analyses
3. Results
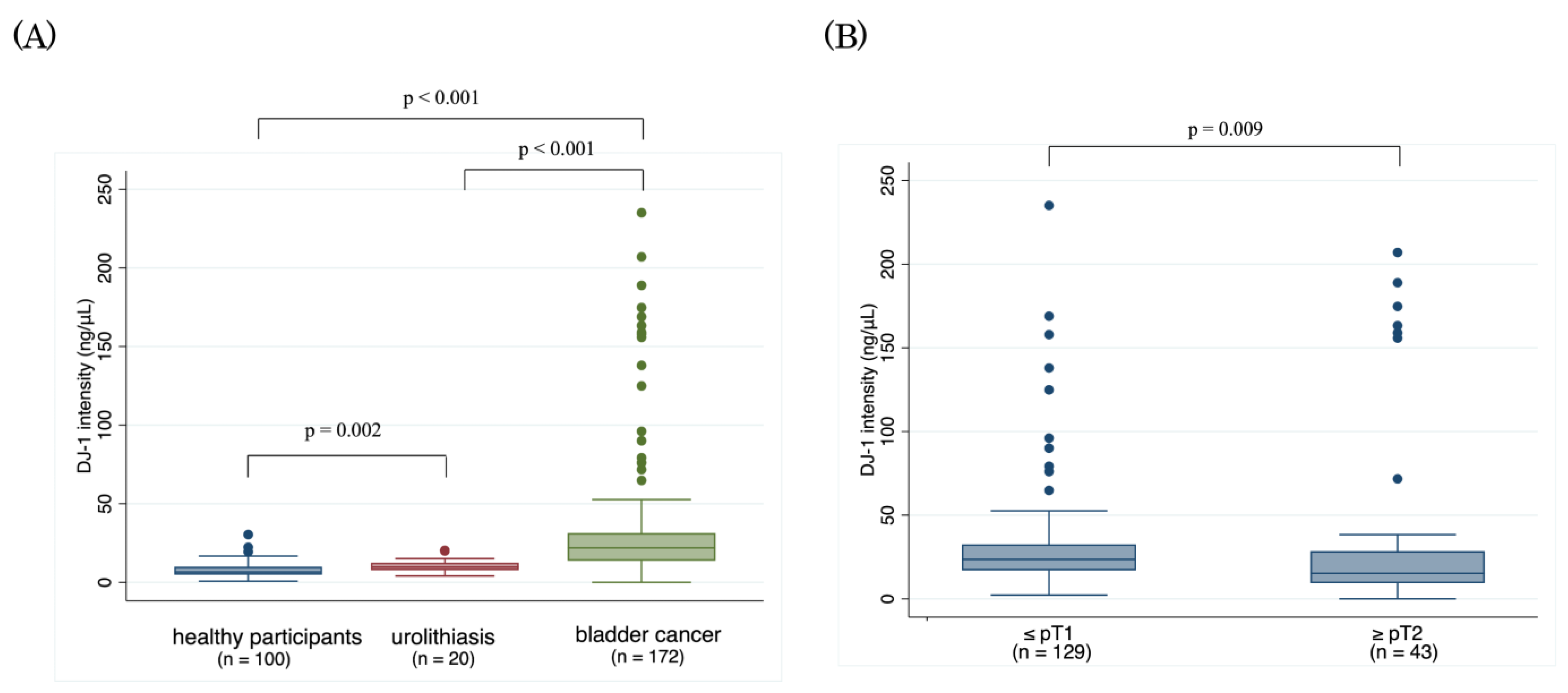
3.1. Serum DJ-1
3.2. DJ-1 Expression by Immunohistochemistry and Clinicopathologic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [Green Version]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guerin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Nakamura, K.; Kasraeian, A.; Iczkowski, K.A.; Chang, M.; Pendleton, J.; Anai, S.; Rosser, C.J. Utility of serial urinary cytology in the initial evaluation of the patient with microscopic hematuria. BMC Urol. 2009, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Van der Aa, M.N.; Steyerberg, E.W.; Sen, E.F.; Zwarthoff, E.C.; Kirkels, W.J.; van der Kwast, T.H.; Essink-Bot, M.L. Patients’ perceived burden of cystoscopic and urinary surveillance of bladder cancer: A randomized comparison. BJU Int. 2008, 101, 1106–1110. [Google Scholar] [CrossRef]
- Mowatt, G.; Zhu, S.; Kilonzo, M.; Boachie, C.; Fraser, C.; Griffiths, T.R.L.; N’Dow, J.; Nabi, G.; Cook, J.; Vale, L. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol. Assess. 2010, 14, 1–331. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef]
- Soria, F.; Droller, M.J.; Lotan, Y.; Gontero, P.; D’Andrea, D.; Gust, K.M.; Roupret, M.; Babjuk, M.; Palou, J.; Shariat, S.F. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J. Urol. 2018, 36, 1981–1995. [Google Scholar] [CrossRef] [Green Version]
- Goodison, S.; Rosser, C.J.; Urquidi, V. Bladder cancer detection and monitoring: Assessment of urine- and blood-based marker tests. Mol. Diagn. Ther. 2013, 17, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Washino, S.; Hirai, M.; Matsuzaki, A.; Kobayashi, Y. Clinical usefulness of CEA, CA19-9, and CYFRA 21-1 as tumor markers for urothelial bladder carcinoma. Urol. Int. 2011, 87, 420–428. [Google Scholar] [CrossRef]
- Gakis, G.; Todenhofer, T.; Stenzl, A. The prognostic value of hematological and systemic inflammatory disorders in invasive bladder cancer. Curr. Opin. Urol. 2011, 21, 428–433. [Google Scholar] [CrossRef]
- Okusa, H.; Kodera, Y.; Oh-Ishi, M.; Minamida, Y.; Tsuchida, M.; Kavoussi, N.; Matsumoto, K.; Sato, T.; Iwamura, M.; Maeda, T.; et al. Searching for new biomarkers of bladder cancer based on proteomic analysis. J. Electrophor. 2008, 52, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Shimura, S.; Matsumoto, K.; Shimizu, Y.; Mochizuki, K.; Shiono, Y.; Hirano, S.; Koguchi, D.; Ikeda, M.; Sato, Y.; Iwamura, M. Serum epiplakin might be a potential serodiagnostic biomarker for bladder cancer. Cancers 2021, 13, 5150. [Google Scholar] [CrossRef]
- Koguchi, D.; Matsumoto, K.; Shimizu, Y.; Kobayashi, M.; Hirano, S.; Ikeda, M.; Sato, Y.; Iwamura, M. Prognostic impact of AHNAK2 expression in patients treated with radical cystectomy. Cancers 2021, 13, 1748. [Google Scholar] [CrossRef]
- Nagakubo, D.; Taira, T.; Kitaura, H.; Ikeda, M.; Tamai, K.; Iguchi-Ariga, S.M.; Ariga, H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997, 231, 509–513. [Google Scholar] [CrossRef]
- Taira, T.; Saito, Y.; Niki, T.; Iguchi-Ariga, S.M.; Takahashi, K.; Ariga, H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004, 5, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Wang, J.; Gao, J.; Feng, S.; Zhu, Y.; Li, X.; Xiao, T.; Qi, J.; Cui, W. DJ-1 as a potential biomarker for the early diagnosis in lung cancer patients. Tumour. Biol. 2017, 39, 1010428317714625. [Google Scholar] [CrossRef] [Green Version]
- Kawate, T.; Iwaya, K.; Koshikawa, K.; Moriya, T.; Yamasaki, T.; Hasegawa, S.; Kaise, H.; Fujita, T.; Matsuo, H.; Nakamura, T.; et al. High levels of DJ-1 protein and isoelectric point 6.3 isoform in sera of breast cancer patients. Cancer Sci. 2015, 106, 938–943. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, B.Y.; Yao, W.Y.; Zhao, X.J.; Zheng, Z.; Li, J.F.; Yu, B.Q.; Yuan, Y.Z. Serum DJ-1 as a diagnostic marker and prognostic factor for pancreatic cancer. J. Dig. Dis. 2011, 12, 131–137. [Google Scholar] [CrossRef]
- Soukup, V.; Capoun, O.; Pesl, M.; Vavrova, L.; Sobotka, R.; Levova, K.; Hanus, T.; Zima, T.; Kalousova, M. The significance of calprotectin, CD147, APOA4 and DJ-1 in non-invasive detection of urinary bladder carcinoma. Neoplasma 2019, 66, 1019–1023. [Google Scholar] [CrossRef]
- Srougi, V.; Reis, S.T.; Viana, N.; Gallucci, F.P.; Leite, K.R.; Srougi, M.; Nahas, W.C. Prospective evaluation of a urinary biomarker panel to detect and predict recurrence of non-muscle-invasive bladder cancer. World J. Urol. 2021, 39, 453–459. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tabata, K.; Hirayama, T.; Shimura, S.; Nishi, M.; Ishii, D.; Fujita, T.; Iwamura, M. Robot-assisted laparoscopic radical cystectomy is a safe and effective procedure for patients with bladder cancer compared to laparoscopic and open surgery: Perioperative outcomes of a single-center experience. Asian J. Surg. 2019, 42, 189–196. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nagashio, R.; Jiang, S.X.; Saito, K.; Tsuchiya, B.; Ryuge, S.; Katono, K.; Nakashima, H.; Fukuda, E.; Goshima, N.; et al. Calnexin is a novel sero-diagnostic marker for lung cancer. Lung Cancer 2015, 90, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, J.; Ono, M.; Honda, K.; Negishi, A.; Ueno, H.; Okusaka, T.; Furuse, J.; Furuta, K.; Sugiyama, E.; Saito, Y.; et al. Survival prediction for pancreatic cancer patients receiving gemcitabine treatment. Mol. Cell Proteom. 2010, 9, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Yanagita, K.; Nagashio, R.; Jiang, S.X.; Kuchitsu, Y.; Hachimura, K.; Ichinoe, M.; Igawa, S.; Nakashima, H.; Fukuda, E.; Goshima, N.; et al. Cytoskeleton-Associated Protein 4 Is a Novel Serodiagnostic Marker for Lung Cancer. Am. J. Pathol. 2018, 188, 1328–1333. [Google Scholar] [CrossRef]
- Goshima, N.; Kawamura, Y.; Fukumoto, A.; Miura, A.; Honma, R.; Satoh, R.; Wakamatsu, A.; Yamamoto, J.; Kimura, K.; Nishikawa, T.; et al. Human protein factory for converting the transcriptome into an in vitro-expressed proteome. Nat. Methods 2008, 5, 1011–1017. [Google Scholar] [CrossRef]
- Miyajima, Y.; Sato, Y.; Oka, H.; Utsuki, S.; Kondo, K.; Tanizaki, Y.; Nagashio, R.; Tsuchiya, B.; Okayasu, I.; Fujii, K. Prognostic significance of nuclear DJ-1 expression in astrocytoma. Anticancer Res. 2010, 30, 265–269. [Google Scholar]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.H.; Peters, M.; Jang, Y.; Shi, W.; Pintilie, M.; Fletcher, G.C.; DeLuca, C.; Liepa, J.; Zhou, L.; Snow, B.; et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 2005, 7, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Rosser, C.J.; Ross, S.; Chang, M.; Dai, Y.; Mengual, L.; Zhang, G.; Kim, J.; Urquidi, V.; Alcaraz, A.; Goodison, S. Multiplex protein signature for the detection of bladder cancer in voided urine samples. J. Urol. 2013, 190, 2257–2262. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nandi, S.; Tan, T.Z.; Ler, S.G.; Chia, K.S.; Lim, W.Y.; Butow, Z.; Vordos, D.; De la Taille, A.; Al-Haddawi, M.; et al. Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma. Oncotarget 2015, 6, 13539–13549. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S.K.; Ro, J.Y. Overexpression of DJ-1 and HSP90alpha, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers. Oncol. Lett. 2012, 3, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseur, S.; Afzal, S.; Tardivel-Lacombe, J.; Park, D.S.; Iovanna, J.L.; Mak, T.W. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc. Natl. Acad. Sci. USA 2009, 106, 1111–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, S.; Paskeh, M.D.A.; Hashemi, F.; Zabolian, A.; Hashemi, M.; Entezari, M.; Tabari, T.; Ashrafizadeh, M.; Raee, P.; Aghamiri, S.; et al. Long non-coding RNAs as new players in bladder cancer: Lessons from pre-clinical and clinical studies. Life Sci. 2022, 288, 119948. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Patients (n) | Serum DJ-1 (ng/µL) | p Value a | |
|---|---|---|---|---|
| Median | Range | |||
| Sex | ||||
| Men | 135 | 21.5 | 0–235.1 | |
| Women | 37 | 27.2 | 0.81–207.0 | 0.37 |
| Age group | ||||
| <65 Years | 137 | 20.6 | 0–235.1 | |
| ≥65 Years | 35 | 22.0 | 0–188.9 | 0.50 |
| Pathology stage | ||||
| <pT1 | 129 | 23.5 | 2.3–235.1 | |
| ≥pT2 | 43 | 15.2 | 0–207.0 | 0.009 |
| Pathology grade | ||||
| 1, 2 | 122 | 22.4 | 1.8–235.1 | |
| 3 | 50 | 20.2 | 0–188.9 | 0.49 |
| Lymph node status | ||||
| N0 | 166 | 22.0 | 0–235.1 | |
| N1–2 | 6 | 16.1 | 7.6–29.9 | 0.34 |
| Characteristic | Patients (n) | DJ-1 Expression (n (%)) | p Value b | |
|---|---|---|---|---|
| Group 1 a | Groups 2–4 a | |||
| Total | 92 | 28 (30.4) | 64 (69.6) | |
| Sex | ||||
| Male | 72 (78.3) | 22 (30.6) | 50 (69.4) | |
| Female | 20 (21.7) | 6 (30.0) | 14 (70.0) | 0.96 |
| Age group | ||||
| <65 | 44 (47.8) | 19 (43.2) | 25 (56.8) | |
| ≥65 | 48 (52.2) | 9 (18.8) | 39 (81.2) | 0.011 |
| Pathology stage | ||||
| pTa, pTis, pT1 | 20 (21.7) | 2 (10.0) | 18 (90.0) | |
| pT2–4 | 72 (78.3) | 26 (36.1) | 46 (63.9) | 0.025 |
| Pathology grade | ||||
| 1, 2 | 36 (39.1) | 10 (27.8) | 26 (72.2) | |
| 3 | 56 (60.9) | 18 (32.1) | 38 (67.9) | 0.66 |
| Carcinoma in situ | ||||
| Negative | 80 (87.0) | 24 (30.0) | 56 (70.0) | |
| Positive | 12 (13.0) | 4 (33.3) | 8 (66.6) | 0.89 |
| Lymph node status c | ||||
| N0 | 65 (70.7) | 17 (26.1) | 48 (73.9) | |
| N1, N2 | 22 (23.9) | 11 (50.0) | 11 (50.0) | 0.039 |
| Lymphovascular invasion d | ||||
| Negative | 32 (37.6) | 8 (25.0) | 24 (75.0) | |
| Positive | 53 (62.4) | 19 (35.8) | 34 (64.2) | 0.30 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| (A) Overall survival | ||||||
| DJ-1 nucleus−, cytoplasm+ | 2.60 | 1.48 to 4.59 | 0.001 | 4.2 | 2.14 to 8.49 | <0.001 |
| Age (<65, ≥65) | 1.11 | 0.64 to 1.92 | 0.72 | 1.83 | 0.94 to 3.55 | 0.075 |
| Pathology stage | 3.62 | 1.56 to 8.40 | 0.003 | 2.79 | 0.96 to 8.12 | 0.06 |
| Pathology grade | 1.84 | 1.01 to 3.35 | 0.046 | 1.29 | 0.66 to 2.55 | 0.46 |
| Lymphovascular invasion | 2.27 | 1.19 to 4.32 | 0.012 | 1.65 | 0.79 to 3.45 | 0.18 |
| Lymph node metastasis | 2.95 | 1.59 to 5.48 | 0.001 | 2.55 | 1.28 to 5.08 | 0.008 |
| (B) Recurrence-free survival | ||||||
| DJ-1 nucleus−, cytoplasm+ | 1.94 | 1.06 to 3.58 | 0.03 | 2.43 | 1.20 to 4.95 | 0.01 |
| Age (<65, ≥65) | 0.85 | 0.48 to 1.51 | 0.58 | 1.05 | 0.53 to 2.08 | 0.89 |
| Pathology stage | 2.64 | 1.12 to 6.25 | 0.027 | 2.03 | 0.67 to 6.14 | 0.21 |
| Pathology grade | 1.28 | 0.70 to 2.35 | 0.42 | 0.94 | 0.47 to 1.88 | 0.86 |
| Lymphovascular invasion | 2.66 | 1.31 to 5.43 | 0.007 | 1.87 | 0.83 to 4.22 | 0.13 |
| Lymph node metastasis | 3.21 | 1.73 to 5.98 | < 0.001 | 2.60 | 1.31 to 5.16 | 0.006 |
| (C) Cancer-specific survival | ||||||
| DJ-1 nucleus−, cytoplasm+ | 2.28 | 1.21 to 4.30 | 0.01 | 3.53 | 1.61 to 7.57 | 0.001 |
| Age (<65, ≥65) | 0.90 | 0.49 to 1.67 | 0.75 | 1.25 | 0.60 to 2.63 | 0.55 |
| Pathology stage | 2.37 | 0.99 to 5.66 | 0.052 | 1.55 | 0.50 to 4.84 | 0.45 |
| Pathology grade | 1.39 | 0.73 to 2.65 | 0.32 | 1.02 | 0.48 to 2.14 | 0.96 |
| Lymphovascular invasion | 2.50 | 1.18 to 5.32 | 0.017 | 1.68 | 0.70 to 4.07 | 0.25 |
| Lymph node metastasis | 3.70 | 1.92 to 7.13 | < 0.001 | 3.45 | 1.63 to 7.29 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, S.; Matsumoto, K.; Tanaka, K.; Amano, N.; Koguchi, D.; Ikeda, M.; Shimizu, Y.; Tsuchiya, B.; Nagashio, R.; Sato, Y.; et al. DJ-1 Expression Might Serve as a Biologic Marker in Patients with Bladder Cancer. Cancers 2022, 14, 2535. https://doi.org/10.3390/cancers14102535
Hirano S, Matsumoto K, Tanaka K, Amano N, Koguchi D, Ikeda M, Shimizu Y, Tsuchiya B, Nagashio R, Sato Y, et al. DJ-1 Expression Might Serve as a Biologic Marker in Patients with Bladder Cancer. Cancers. 2022; 14(10):2535. https://doi.org/10.3390/cancers14102535
Chicago/Turabian StyleHirano, Shuhei, Kazumasa Matsumoto, Kei Tanaka, Noriyuki Amano, Dai Koguchi, Masaomi Ikeda, Yuriko Shimizu, Benio Tsuchiya, Ryo Nagashio, Yuichi Sato, and et al. 2022. "DJ-1 Expression Might Serve as a Biologic Marker in Patients with Bladder Cancer" Cancers 14, no. 10: 2535. https://doi.org/10.3390/cancers14102535
APA StyleHirano, S., Matsumoto, K., Tanaka, K., Amano, N., Koguchi, D., Ikeda, M., Shimizu, Y., Tsuchiya, B., Nagashio, R., Sato, Y., & Iwamura, M. (2022). DJ-1 Expression Might Serve as a Biologic Marker in Patients with Bladder Cancer. Cancers, 14(10), 2535. https://doi.org/10.3390/cancers14102535






