Risk Factors of Incident Lung Cancer in Patients with Non-Cystic Fibrosis Bronchiectasis: A Korean Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Definitions of Bronchiectasis and Lung Cancer
2.3. Definitions of Covariates
2.4. Main Outcomes
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Risk Factors of Lung Cancer Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, H.; Yang, B.; Nam, H.; Kyoung, D.S.; Sim, Y.S.; Park, H.Y.; Lee, J.S.; Lee, S.W.; Oh, Y.M.; Ra, S.W.; et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 2019, 54, 1900194. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Millett, E.R.; Joshi, M.; Navaratnam, V.; Thomas, S.L.; Hurst, J.R.; Smeeth, L.; Brown, J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016, 47, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Joish, V.N.; Spilsbury-Cantalupo, M.; Operschall, E.; Luong, B.; Boklage, S. Economic burden of non-cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Appl Health Econ. Health Policy 2013, 11, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Goeminne, P.C.; Hernandez, F.; Diel, R.; Filonenko, A.; Hughes, R.; Juelich, F.; Solomon, G.M.; Upton, A.; Wichmann, K.; Xu, W.; et al. The economic burden of bronchiectasis—known and unknown: A systematic review. BMC Pulm. Med. 2019, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.S.; Lin, C.L.; Lin, C.L.; Kao, C.H. Bronchiectasis and the risk of cancer: A nationwide retrospective cohort study. Int. J. Clin. Pract. 2015, 69, 682–688. [Google Scholar] [CrossRef]
- Chung, W.S.; Lin, C.L.; Hsu, W.H.; Kao, C.H. Increased risk of lung cancer among patients with bronchiectasis: A nationwide cohort study. QJM Int. J. Med. 2016, 109, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Park, H.Y.; Han, K.; Yoo, J.; Sin, S.; Yang, B.; Kim, Y.; Park, T.S.; Park, D.W.; Moon, J.Y.; et al. Non-cystic Fibrosis Bronchiectasis Increases the Risk of Lung Cancer Independent of Smoking Status. Ann Am Thorac Soc. 2022. [Google Scholar] [CrossRef]
- Sin, S.; Yun, S.Y.; Kim, J.M.; Park, C.M.; Cho, J.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, S.M.; Yoo, C.G.; et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir. Res. 2019, 20, 271. [Google Scholar] [CrossRef]
- Choi, H.; Yang, B.; Kim, Y.J.; Sin, S.; Jo, Y.S.; Kim, Y.; Park, H.Y.; Ra, S.W.; Oh, Y.-M.; Chung, S.J.; et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci. Rep. 2021, 11, 7126. [Google Scholar] [CrossRef]
- Hovanec, J.; Siemiatycki, J.; Conway, D.I.; Olsson, A.; Stücker, I.; Guida, F.; Jöckel, K.H.; Pohlabeln, H.; Ahrens, W.; Brüske, I.; et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS ONE 2018, 13, e0192999. [Google Scholar] [CrossRef]
- Zhong, S.; Ma, T.; Chen, L.; Chen, W.; Lv, M.; Zhang, X.; Zhao, J. Physical Activity and Risk of Lung Cancer: A Meta-analysis. Clin. J. Sport Med. 2016, 26, 173–181. [Google Scholar] [CrossRef] [PubMed]
- NHIS. National Health Examination Statistical Yearbook 2014; National Health Insurence Service: Seoul, Korea, 2014.
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.Y.; Kwon, H.S.; Cha, B.S.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Lee, B.W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef] [PubMed]
- International Ethical Guidelines for Health-Related Research Involving Humans. Geneva: Council for International Organizations of Medical Sciences. 2016. Available online: https://cioms.ch/publications/product/international-ethical-guidelines-f (accessed on 30 August 2020).
- Park, H.Y.; Kang, D.; Shin, S.H.; Choi, H.; Jang, S.H.; Lee, C.H.; Kim, H.; Kwon, O.J.; Rhee, C.K.; Cho, J. Pulmonary Tuberculosis and the Incidence of Lung Cancer among Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2021, 19, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, W.Y.; Kang, J.H.; Kang, J.H.; Kim, B.T.; Kim, S.M.; Kim, E.M.; Suh, S.H.; Shin, H.J.; Lee, K.R.; et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. 2014, 29, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Yoo, J.E.; Han, K.; Choi, W.; Rhee, S.Y.; Lee, H.; Shin, D.W. Body Mass Index, Diabetes, and Risk of Tuberculosis: A Retrospective Cohort Study. Front. Nutr. 2021, 8, 739766. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ryu, J.; Nam, E.; Chung, S.J.; Yeo, Y.; Park, D.W.; Park, T.S.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; et al. Increased mortality in patients with corticosteroid-dependent asthma: A nationwide population-based study. Eur. Respir. J. 2019, 54, 1900804. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Han, K.; Yang, B.; Shin, D.W.; Sohn, J.W.; Lee, H. Female reproductive factors and incidence of non-tuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin. Infect. Dis. 2022, ciac134. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kim, K.H. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J. Prev. Med. Public Health 2010, 43, 42–49. [Google Scholar] [CrossRef]
- Leduc, C.; Antoni, D.; Charloux, A.; Falcoz, P.E.; Quoix, E. Comorbidities in the management of patients with lung cancer. Eur. Respir. J. 2017, 49, 1601721. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.; Mulshine, J.L. The potential contributions of chronic inflammation to lung carcinogenesis. Clin. Lung Cancer 2003, 5, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Gibiot, Q.; Monnet, I.; Levy, P.; Brun, A.L.; Antoine, M.; Chouaid, C.; Cadranel, J.; Naccache, J.M. Interstitial Lung Disease Associated with Lung Cancer: A Case-Control Study. J. Clin. Med. 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scrimini, S.; Pons, J.; Agusti, A.; Clemente, A.; Sallan, M.C.; Bauca, J.M.; Soriano, J.B.; Cosio, B.G.; Lopez, M.; Crespi, C.; et al. Expansion of myeloid-derived suppressor cells in chronic obstructive pulmonary disease and lung cancer: Potential link between inflammation and cancer. Cancer Immunol. Immun. 2015, 64, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.E.; Kim, H.R.; Chung, Y.K.; Kang, S.K.; Kim, E.A. Mortality rates by occupation in Korea: A nationwide, 13-year follow-up study. Occup. Env. Med. 2016, 73, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Go, U.; Park, M.; Kim, U.N.; Lee, S.; Han, S.; Lee, J.; Yang, J.; Kim, J.; Park, S.; Kim, Y.; et al. Tuberculosis prevention and care in Korea: Evolution of policy and practice. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 11, 28–36. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Li, C.; Ma, H.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. Biomed. Res. Int. 2019, 2019, 2025636. [Google Scholar] [CrossRef]
- Park, B.; Kim, Y.; Lee, J.; Lee, N.; Jang, S.H. Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population. Cancers 2020, 13, 113. [Google Scholar] [CrossRef]
- Mavridis, K.; Michaelidou, K. The obesity paradox in lung cancer: Is there a missing biological link? J. Thorac. Dis. 2019, 11, S363–S366. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Hashimoto, N.; Nakayama, A.; Suzuki, S.; Miyabayashi, Y.; Yamato, A.; Higuchi, S.; Fujimoto, M.; Sakuma, I.; Beppu, M.; et al. p53-inducible DPYSL4 associates with mitochondrial supercomplexes and regulates energy metabolism in adipocytes and cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8370–8375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, B.; Yu, M.; Sang, C.; Bi, B.; Chen, J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: A case-control study. Lipids Health Dis. 2018, 17, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.X.; Zheng, W.; Johansson, M.; Lan, Q.; Park, Y.; White, E.; Matthews, C.E.; Sawada, N.; Gao, Y.T.; Robien, K.; et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. JNCI J. Natl. Cancer I. 2018, 110, 831–842. [Google Scholar] [CrossRef]
- Westeel, V.; Pitard, A.; Martin, M.; Thaon, I.; Depierre, A.; Dalphin, J.C.; Arveux, P. Negative impact of rurality on lung cancer survival in a population-based study. J. Thorac. Oncol. 2007, 2, 613–618. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Tang, W.; Sun, Q.; Chen, Y.; Yang, C.; Yan, B.; Wang, Y.; Wang, J.; Wang, S.; et al. Urban-Rural Disparity in Cancer Incidence, Mortality, and Survivals in Shanghai, China, During 2002 and 2015. Front. Oncol. 2018, 8, 579. [Google Scholar] [CrossRef]
- Lee, K.-Y. Relationship between Public Service Satisfaction and Intention of Continuous Residence of Younger Generations in Rural Areas: The Case of Jeonbuk, Korea. Land 2021, 10, 1203. [Google Scholar] [CrossRef]
- Bagnardi, V.; Randi, G.; Lubin, J.; Consonni, D.; Lam, T.K.; Subar, A.F.; Goldstein, A.M.; Wacholder, S.; Bergen, A.W.; Tucker, M.A.; et al. Alcohol consumption and lung cancer risk in the Environment and Genetics in Lung Cancer Etiology (EAGLE) study. Am. J. Epidemiol. 2010, 171, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Troche, J.R.; Mayne, S.T.; Freedman, N.D.; Shebl, F.M.; Abnet, C.C. The Association between Alcohol Consumption and Lung Carcinoma by Histological Subtype. Am. J. Epidemiol. 2016, 183, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Rohrmann, S.; Linseisen, J.; Boshuizen, H.C.; Whittaker, J.; Agudo, A.; Vineis, P.; Boffetta, P.; Jensen, M.K.; Olsen, A.; Overvad, K.; et al. Ethanol intake and risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Epidemiol. 2006, 164, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Emberson, J.R.; Bennett, D.A. Effect of alcohol on risk of coronary heart disease and stroke: Causality, bias, or a bit of both? Vasc. Health Risk Manag. 2006, 2, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kang, D.; Shin, S.H.; Yoo, K.-H.; Rhee, C.K.; Suh, G.Y.; Kim, H.; Shim, Y.M.; Guallar, E.; Cho, J.; et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: A cohort study. Thorax 2020, 75, 506. [Google Scholar] [CrossRef] [Green Version]
- Dhar, R.; Singh, S.; Talwar, D.; Mohan, M.; Tripathi, S.K.; Swarnakar, R.; Trivedi, S.; Rajagopala, S.; D’Souza, G.; Padmanabhan, A.; et al. Bronchiectasis in India: Results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob. Health 2019, 7, e1269–e1279. [Google Scholar] [CrossRef]
- Visser, S.K.; Bye, P.T.P.; Fox, G.J.; Burr, L.D.; Chang, A.B.; Holmes-Liew, C.L.; King, P.; Middleton, P.G.; Maguire, G.P.; Smith, D.; et al. Australian adults with bronchiectasis: The first report from the Australian Bronchiectasis Registry. Respir. Med. 2019, 155, 97–103. [Google Scholar] [CrossRef]
- Araújo, D.; Shteinberg, M.; Aliberti, S.; Goeminne, P.C.; Hill, A.T.; Fardon, T.C.; Obradovic, D.; Stone, G.; Trautmann, M.; Davis, A.; et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur. Respir. J. 2018, 51, 1701953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksamit, T.R.; O’Donnell, A.E.; Barker, A.; Olivier, K.N.; Winthrop, K.L.; Daniels, M.L.A.; Johnson, M.; Eden, E.; Griffith, D.; Knowles, M.; et al. Adult Patients with Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017, 151, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Olveira, C.; Padilla, A.; Martínez-García, M.; de la Rosa, D.; Girón, R.M.; Vendrell, M.; Máiz, L.; Borderías, L.; Polverino, E.; Martínez-Moragón, E.; et al. Etiology of Bronchiectasis in a Cohort of 2047 Patients. An Analysis of the Spanish Historical Bronchiectasis Registry. Arch. Bronconeumol. 2017, 53, 366–374. [Google Scholar] [CrossRef]

| Participants with Bronchiectasis (n = 7425) | Incident Lung Cancer | p-Value | ||
|---|---|---|---|---|
| No (n = 7286) | Yes (n = 138) | |||
| Age, years | <0.001 | |||
| <60 | 3825 (51.5) | 3803 (52.2) | 22 (15.9) | |
| ≥60 | 3600 (48.5) | 3484 (47.8) | 116 (84.1) | |
| Male sex | 3720 (50.1) | 3611 (49.6) | 109 (79.0) | <0.001 |
| Body mass index | 0.215 | |||
| <18.5 | 356 (4.8) | 348 (4.8) | 8 (5.8) | |
| ≥18.5–<23.0 | 2882 (38.8) | 2834 (38.9) | 48 (34. 8) | |
| ≥23.0–<25.0 | 1791 (24.1) | 1748 (24.0) | 43 (31.2) | |
| ≥25.0 | 2396 (32.3) | 2357 (32.3) | 39 (28.2) | |
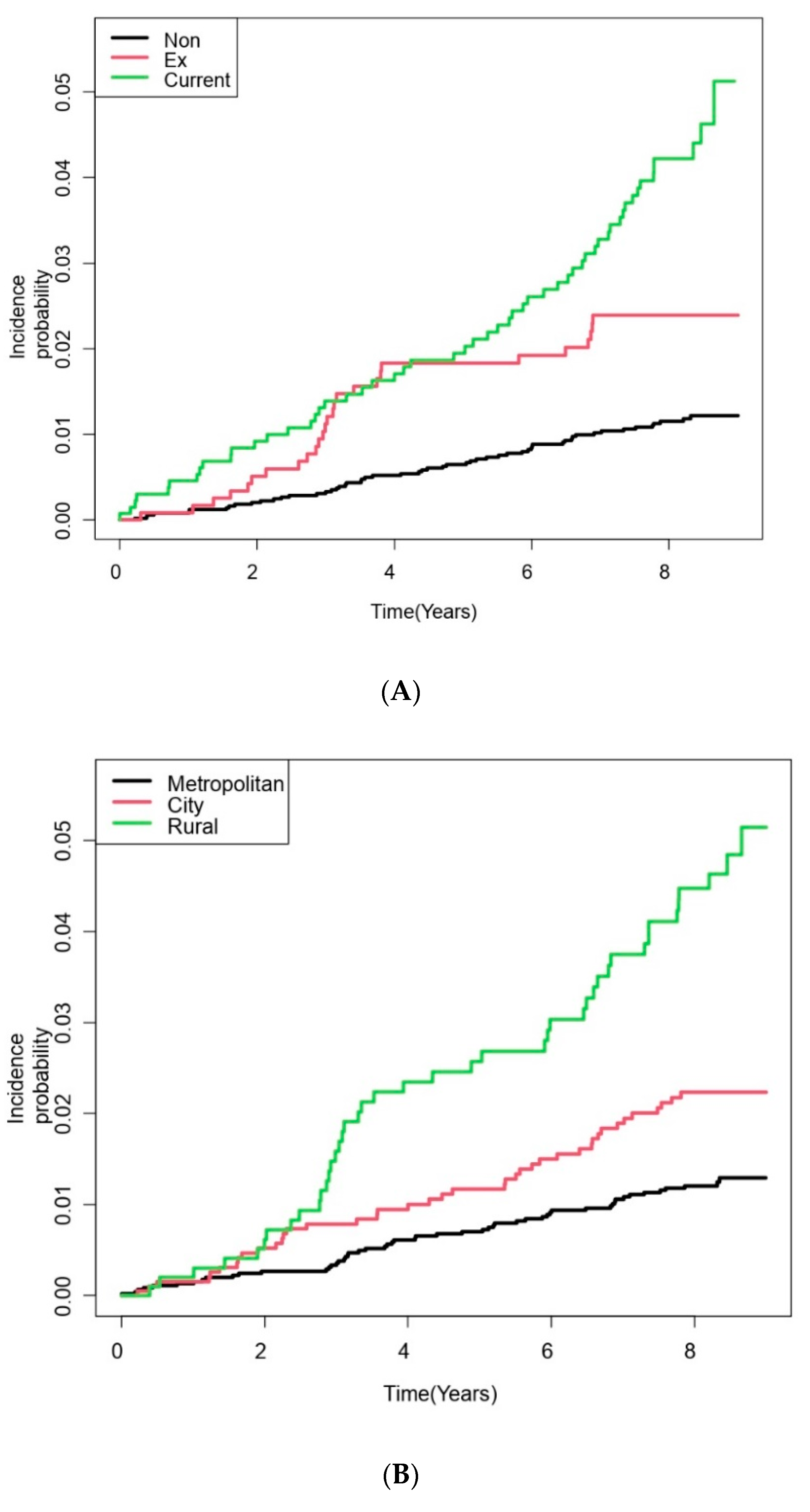
| Smoking status | <0.001 | |||
| Never-smoker | 4913 (66.2) | 4857 (66.6) | 56 (40.6) | |
| Ever-smoker | 1197 (16.1) | 1170 (16.1) | 27 (19.6) | |
| Current smoker | 1315 (17.7) | 1260 (17.3) | 55 (39.8) | |
| Alcohol consumption | 0.007 | |||
| None | 4927 (66.4) | 4828 (66.3) | 99 (71.7) | |
| Mild | 2066 (27.8) | 2041 (28.0) | 25 (18.1) | |
| Heavy | 432 (5.8) | 418 (5.7) | 14 (10.2) | |
| Residential area | <0.001 | |||
| Metropolitan | 4493 (60.5) | 4439 (60.9) | 54 (39.1) | |
| City | 1940 (26.1) | 1899 (26.1) | 41 (29.7) | |
| Rural | 992 (13.4) | 949 (13.0) | 43 (31.2) | |
| Income | 0.200 | |||
| High | 6264 (84.4) | 6153 (84.4) | 111 (80.4) | |
| Low | 1161 (15.6) | 1134 (15.6) | 27 (19.6) | |
| Physical activity | 0.701 | |||
| on-regular | 6040 (81.3) | 5926 (81.3) | 114 (82.6) | |
| Regular | 1385 (18.7) | 1361 (18.7) | 24 (17.4) | |
| Charlson Comorbidity Index | 0.001 | |||
| 0 | 571 (7.7) | 565 (7.8) | 6 (4.4) | |
| 1 | 2339 (31.5) | 2312 (31.7) | 27 (19.6) | |
| ≥2 | 4515 (60.8) | 4410 (60.5) | 105 (76.0) | |
| Asthma | 2451 (33.0) | 2400 (32.9) | 51 (37.0) | 0.320 |
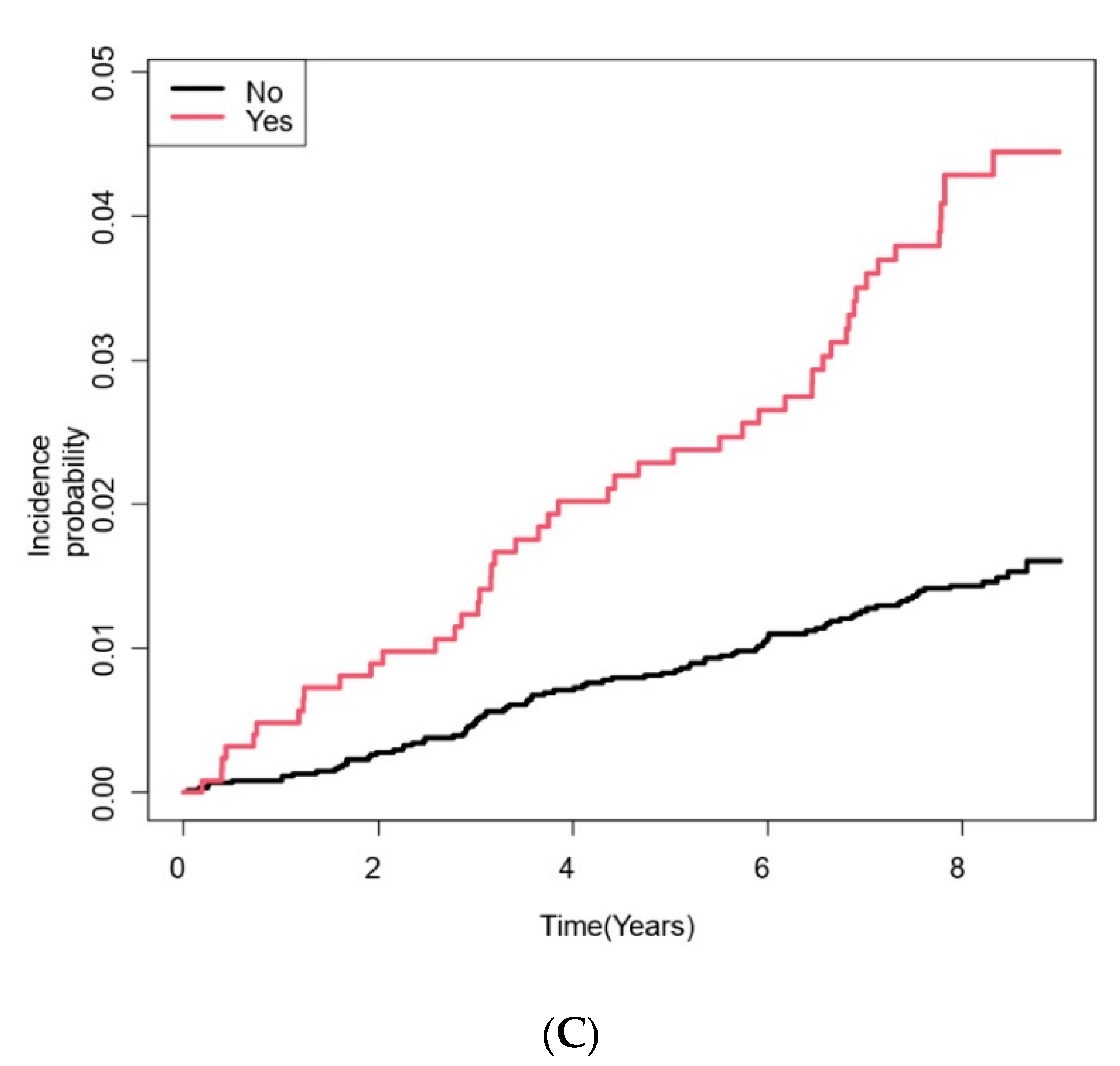
| COPD | 1261 (17.0) | 1212 (16.6) | 49 (35.5) | <0.001 |
| Tuberculosis | 1336 (18.0) | 1310 (18.0) | 26 (18.8) | 0.794 |
| Number of Participants | Follow-Up Duration (PY) | Incidence Rate (/1000 PY) | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Crude Model | Model 1 | Model 2 | Model 3 | ||||
| Age, years | |||||||
| <60 | 3825 | 31,470 | 0.699 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| ≥60 | 3600 | 27,375 | 4.237 | 6.11 (3.87–9.64) | 1.61 (0.81–3.17) | 1.59 (0.81–3.13) | 1.61 (0.81–3.18) |
| Sex | |||||||
| Female | 3705 | 30,065 | 0.965 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Male | 3720 | 28,780 | 3.787 | 3.94 (2.62–5.94) | 3.60 (2.21–5.86) | 3.61 (2.22–5.88) | 3.54 (2.17–5.79) |
| Body mass index | |||||||
| <18.5 | 356 | 2561 | 3.124 | 1.48 (0.70–3.14) | 1.19 (0.56–2.54) | 1.23 (0.58–2.62) | 1.21(0.57–2.57) |
| ≥18.5–<23.0 | 2882 | 22,674 | 2.117 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| ≥23.0–<25.0 | 1791 | 14,329 | 3.001 | 1.42 (0.94–2.14) | 1.58 (1.05–2.39) | 1.58 (1.04–2.39) | 1.55 (1.03–2.35) |
| ≥25.0 | 2396 | 19,281 | 2.023 | 0.95 (0.63–1.46) | 1.21 (0.79–1.86) | 1.17 (0.76–1.80) | 1.21 (0.79–1.86) |
| Smoking status | |||||||
| Never smoker | 4913 | 39,361 | 1.423 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Ex-smoker | 1197 | 9272 | 2.912 | 2.05 (1.30–3.25) | 1.25 (0.75–2.10) | 1.25 (0.75–2.10) | 1.21 (0.72–2.03) |
| Current smoker | 1315 | 10,213 | 5.385 | 3.80 (2.62–5.51) | 3.21 (2.08–4.95) | 3.22 (2.09–4.97) | 3.10 (2.00–4.79) |
| Alcohol drinking | |||||||
| None | 4927 | 38,830 | 2.550 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Mild | 2066 | 16,639 | 1.503 | 0.59 (0.38–0.91) | 0.46 (0.29–0.73) | 0.46 (0.29–0.73) | 0.47 (0.29–0.74) |
| Heavy | 432 | 3376 | 4.147 | 1.63 (0.93–2.85) | 0.95 (0.53–1.70) | 0.96 (0.54–1.73) | 0.94 (0.52–1.69) |
| Residential area | |||||||
| Metropolitan | 4493 | 36,001 | 1.500 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| City | 1940 | 15,336 | 2.673 | 1.79 (1.19–2.68) | 1.52 (1.01–2.29) | 1.50 (0.99–2.25) | 1.49 (0.98–2.24) |
| Rural | 992 | 7508 | 5.727 | 3.85 (2.58–5.74) | 2.65 (1.76–4.01) | 2.64 (1.74–3.98) | 2.54 (1.68–3.85) |
| Income | |||||||
| High | 6264 | 49,704 | 2.233 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Low | 1161 | 9142 | 2.954 | 1.32 (0.87–2.02) | 1.45 (0.95–2.21) | 1.44 (0.95–2.20) | 1.44 (0.95–2.21) |
| Physical activity | |||||||
| Non-regular | 6040 | 47,718 | 2.389 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Regular | 1385 | 11,127 | 2.157 | 0.90 (0.58–1.40) | 0.94 (0.60–1.47) | 0.93 (0.60–1.46) | 0.93 (0.60–1.45) |
| CCI | |||||||
| 0 | 571 | 4749 | 1.263 | 1 (Reference) | 1 (Reference) | ||
| 1 | 2339 | 18,980 | 1.423 | 1.13 (0.47–2.73) | 1.06 (0.44–2.56) | ||
| ≥2 | 4515 | 35,116 | 2.990 | 2.38 (1.04–5.41) | 1.59 (0.70–3.64) | ||
| Asthma | |||||||
| No | 4974 | 39,905 | 2.180 | 1 (Reference) | 1 (Reference) | ||
| Yes | 2451 | 18,941 | 2.693 | 1.24 (0.88–1.75) | 0.91 (0.63–1.31) | ||
| COPD | |||||||
| No | 6164 | 49,514 | 1.797 | 1 (Reference) | 1 (Reference) | ||
| Yes | 1261 | 9331 | 5.251 | 2.94 (2.07–4.16) | 1.46 (1.01–2.13) | ||
| Tuberculosis | |||||||
| No | 6089 | 48,515 | 2.309 | 1 (Reference) | 1 (Reference) | ||
| Yes | 1336 | 10,331 | 2.517 | 1.09 (0.71–1.67) | 0.81 (0.52–1.25) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Han, K.; Yoo, J.; Kang, H.K.; Park, T.S.; Park, D.W.; Hong, J.Y.; Moon, J.-Y.; Kim, S.-H.; Kim, T.H.; et al. Risk Factors of Incident Lung Cancer in Patients with Non-Cystic Fibrosis Bronchiectasis: A Korean Population-Based Study. Cancers 2022, 14, 2604. https://doi.org/10.3390/cancers14112604
Kim Y, Han K, Yoo J, Kang HK, Park TS, Park DW, Hong JY, Moon J-Y, Kim S-H, Kim TH, et al. Risk Factors of Incident Lung Cancer in Patients with Non-Cystic Fibrosis Bronchiectasis: A Korean Population-Based Study. Cancers. 2022; 14(11):2604. https://doi.org/10.3390/cancers14112604
Chicago/Turabian StyleKim, Youlim, Kyungdo Han, Juhwan Yoo, Hyung Koo Kang, Tai Sun Park, Dong Won Park, Ji Young Hong, Ji-Yong Moon, Sang-Heon Kim, Tae Hyung Kim, and et al. 2022. "Risk Factors of Incident Lung Cancer in Patients with Non-Cystic Fibrosis Bronchiectasis: A Korean Population-Based Study" Cancers 14, no. 11: 2604. https://doi.org/10.3390/cancers14112604
APA StyleKim, Y., Han, K., Yoo, J., Kang, H. K., Park, T. S., Park, D. W., Hong, J. Y., Moon, J.-Y., Kim, S.-H., Kim, T. H., Yoo, K. H., Sohn, J. W., Yoon, H. J., Choi, H., & Lee, H. (2022). Risk Factors of Incident Lung Cancer in Patients with Non-Cystic Fibrosis Bronchiectasis: A Korean Population-Based Study. Cancers, 14(11), 2604. https://doi.org/10.3390/cancers14112604








