Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. VitC Is an Example of Repurposed Drugs with Anticancer Activity
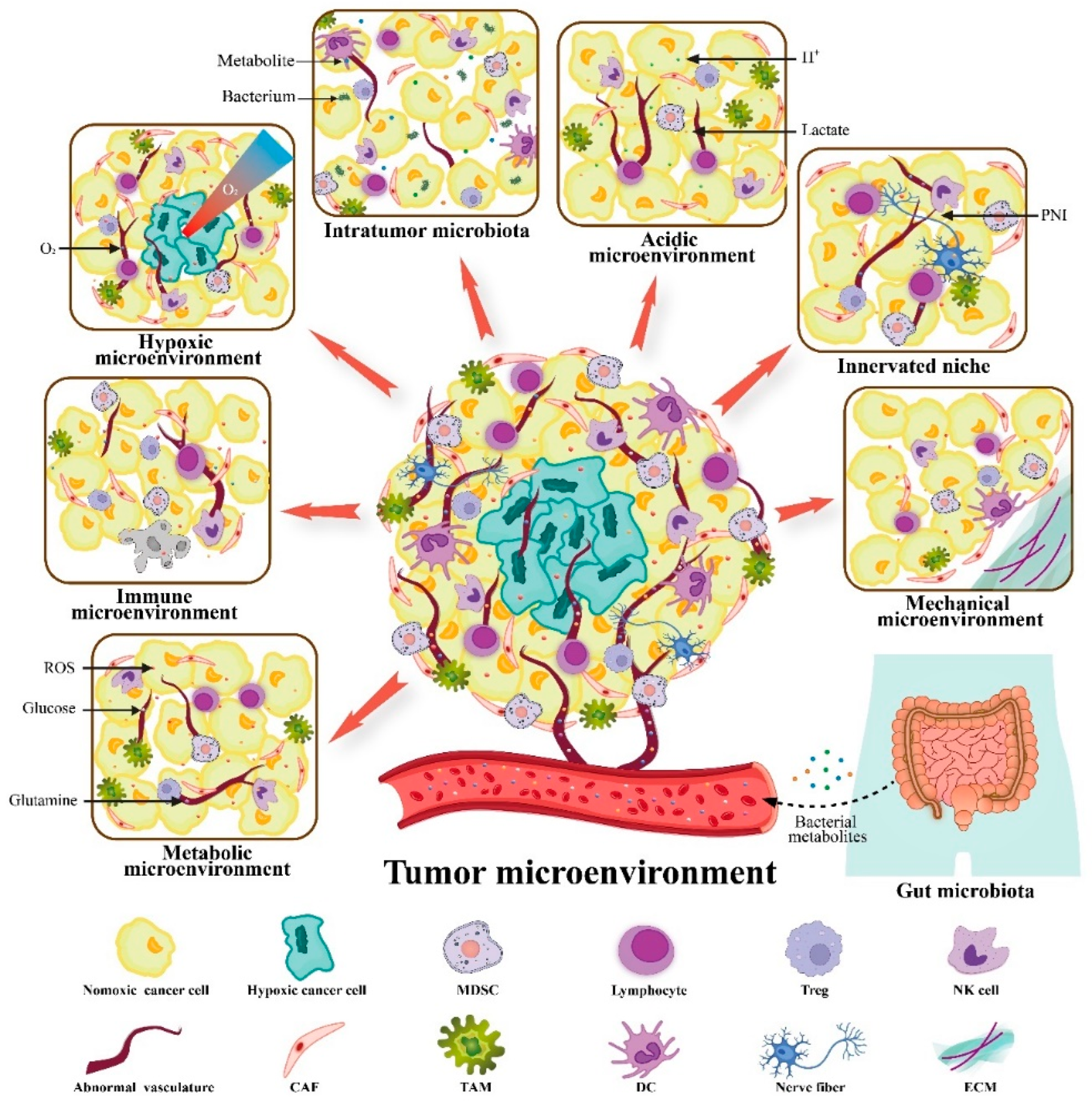
3. VitC Targets Not Only Cancers but also the TME to Exert Anticancer Activity
3.1. VitC and Immune Microenvironment
3.2. VitC and Metabolic Microenvironment
3.3. VitC and Hypoxic Microenvironment
3.4. VitC and Acidic Microenvironment
3.5. VitC and Innerved Niche
3.6. VitC and Mechanical Microenvironment
3.7. VitC and Microbial Microenvironment
3.8. The Interplay between VitC and the Complicated Metastatic TME
4. Application of VitC as a Single Agent or Adjuvant to Target the TME
4.1. Monotherapy
4.2. Combination Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of High-Dose Vitamin C (Ascorbic Acid) Therapy to Benefit Patients with Advanced Cancer: A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Y.; Cao, D.; Qiu, S.; Chen, B.; Li, J.; Bao, Y.; Wei, Q.; Han, P.; Liu, L. Vitamin C Intake and Cancers: An Umbrella Review. Front. Nutr. 2021, 8, 812394. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Peng, R.; Zou, Y.; Jiang, X.; Sun, Q.; Song, C. Vitamin C intake and multiple health outcomes: An umbrella review of systematic reviews and meta-analyses. Int. J. Food Sci. Nutr. 2022, 73, 1–12. [Google Scholar] [CrossRef]
- Cadeau, C.; Farvid, M.S.; Rosner, B.A.; Willett, W.C.; Eliassen, A.H. Dietary and Supplemental Vitamin C Intake and Risk of Breast Cancer: Results from the Nurses’ Health Studies. J. Nutr. 2022, 152, 835–843. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.-J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenet. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.; Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013, 42, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.A.; Ali, R.; Wani, Z.A.; Khanday, F.A. Pro-oxidant vitamin C mechanistically exploits p66Shc/Rac1 GTPase pathway in inducing cytotoxicity. Int. J. Biol. Macromol. 2022, 205, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; He, J.; Jin, Y.; Zhang, R.; Dong, W.; Lin, M.; Yang, Y.; Tian, T.; Zhou, Y.; et al. TET2 Suppresses VHL Deficiency-Driven Clear Cell Renal Cell Carcinoma by Inhibiting HIF Signaling. Cancer Res. 2022, 82, 1–13. [Google Scholar] [CrossRef]
- Smith-Díaz, C.C.; Magon, N.J.; McKenzie, J.L.; Hampton, M.B.; Vissers, M.C.M.; Das, A.B. Ascorbate Inhibits Proliferation and Promotes Myeloid Differentiation in TP53-Mutant Leukemia. Front. Oncol. 2021, 11, 709543. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Yue, X.; Trifari, S.; Äijö, T.; Tsangaratou, A.; Pastor, W.A.; Zepeda-Martínez, J.A.; Lio, C.W.; Li, X.; Huang, Y.; Vijayanand, P.; et al. Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 2016, 213, 377–397. [Google Scholar] [CrossRef] [Green Version]
- Kouakanou, L.; Peters, C.; Sun, Q.; Floess, S.; Bhat, J.; Huehn, J.; Kabelitz, D. Vitamin C supports conversion of human γδ T cells into FOXP3-expressing regulatory cells by epigenetic regulation. Sci. Rep. 2020, 10, 6550. [Google Scholar] [CrossRef]
- Kouakanou, L.; Xu, Y.; Peters, C.; He, J.; Wu, Y.; Yin, Z.; Kabelitz, D. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell. Mol. Immunol. 2020, 17, 462–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.H.; Nair, V.S.; Oh, K.I. Vitamin C enhances the expression of IL17 in a Jmjd2-dependent manner. BMB Rep. 2017, 50, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, T.; Sun, M.; Zhang, C.; Chen, P.; Xiao, C.; Chang, X. Ascorbic Acid Promotes Plasma Cell Differentiation through Enhancing TET2/3-Mediated DNA Demethylation. Cell Rep. 2020, 33, 108452. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Zhang, B.; Kim, H.; Anderson, S.K.; Miller, J.S.; Cichocki, F. Ascorbic Acid Promotes KIR Demethylation during Early NK Cell Differentiation. J. Immunol. 2020, 205, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.D.; Vissers, M.C.M.; Burgess, E.R.; Currie, M.J.; Dachs, G.U. Gene and Protein Expression Is Altered by Ascorbate Availability in Murine Macrophages Cultured under Tumour-Like Conditions. Antioxidants 2021, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, X.; Wang, G.; Zhou, C. Vitamin C Inhibits Metastasis of Peritoneal Tumors By Preventing Spheroid Formation in ID8 Murine Epithelial Peritoneal Cancer Model. Front. Pharmacol. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A., 3rd; Natarajan, R. Vitamin C: A Novel Regulator of Neutrophil Extracellular Trap Formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K.; Hiramoto, K.; Sato, E.F.; Ooi, K. High-Dose Vitamin C Administration Inhibits the Invasion and Proliferation of Melanoma Cells in Mice Ovary. Biol. Pharm. Bull. 2021, 44, 75–81. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Hong, S.-W.; Kim, J.-H.; Jin, D.-H.; Kang, J.S.; Lee, W.J.; Hwang, Y.-I. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell. Immunol. 2011, 266, 192–199. [Google Scholar] [CrossRef]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef]
- Panday, S.; Kar, S.; Kavdia, M. How does ascorbate improve endothelial dysfunction?—A computational analysis. Free Radic. Biol. Med. 2021, 165, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, L.; Barahona, M.J.; Salazar, K.; Vandenabeele, P.; Nualart, F. Vitamin C controls neuronal necroptosis under oxidative stress. Redox Biol. 2020, 29, 101408. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.-P.; Lv, L.; Liu, Y.; Smith, M.D.; Li, W.-C.; Tan, X.-M.; Cheng, M.; Li, Z.; Bovino, M.; Aubé, J.; et al. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J. Clin. Investig. 2019, 129, 4316–4331. [Google Scholar] [CrossRef]
- Luchtel, R.A.; Bhagat, T.; Pradhan, K.; Jacobs, W.R., Jr.; Levine, M.; Verma, A.; Shenoy, N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc. Natl. Acad. Sci. USA 2020, 117, 1666–1677. [Google Scholar] [CrossRef] [Green Version]
- Someya, K.; Nakatsukasa, H.; Ito, M.; Kondo, T.; Tateda, K.-I.; Akanuma, T.; Koya, I.; Sanosaka, T.; Kohyama, J.; Tsukada, Y.-I.; et al. Improvement of Foxp3 stability through CNS2 demethylation by TET enzyme induction and activation. Int. Immunol. 2017, 29, 365–375. [Google Scholar] [CrossRef] [Green Version]
- El Hassouni, B.; Granchi, C.; Vallés-Martí, A.; Supadmanaba, I.G.P.; Bononi, G.; Tuccinardi, T.; Funel, N.; Jimenez, C.R.; Peters, G.J.; Giovannetti, E.; et al. The dichotomous role of the glycolytic metabolism pathway in cancer metastasis: Interplay with the complex tumor microenvironment and novel therapeutic strategies. Semin. Cancer Biol. 2020, 60, 238–248. [Google Scholar] [CrossRef]
- Bonuccelli, G.; De Francesco, E.M.; De Boer, R.; Tanowitz, H.B.; Lisanti, M.P. NADH autofluorescence, a new metabolic biomarker for cancer stem cells: Identification of Vitamin C and CAPE as natural products targeting “stemness”. Oncotarget 2017, 8, 20667–20678. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Qiu, J.; Zhang, B.; He, P.; Shi, X.; He, Q.; Chen, Z.; Shen, W.; Li, Z.; Zhang, X. Pharmacological Vitamin C Treatment Impedes the Growth of Endogenous Glutamine-Dependent Cancers by Targeting Glutamine Synthetase. Front. Pharmacol. 2021, 12, 671902. [Google Scholar] [CrossRef]
- Costa, A.; Scholer-Dahirel, A.; Mechta-Grigoriou, F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol. 2014, 25, 23–32. [Google Scholar] [CrossRef]
- Shimura, T.; Ando, T.; Narao, M.; Sasatani, M.; Kamiya, K.; Ushiyama, A. Mechanism of turnover or persistence of radiation-induced myofibroblast in vitro. Cell Cycle 2020, 19, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef] [PubMed]
- García-Román, J.; Zentella-Dehesa, A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013, 335, 259–269. [Google Scholar] [CrossRef]
- Nakanishi, K.; Hiramoto, K.; Ooi, K. High-Dose Vitamin C Exerts Its Anti-cancer Effects in a Xenograft Model of Colon Cancer by Suppressing Angiogenesis. Biol. Pharm. Bull. 2021, 44, 884–887. [Google Scholar] [CrossRef]
- Na Kim, H.; Kim, H.; Kong, J.M.; Bae, S.; Kim, Y.S.; Lee, N.; Cho, B.J.; Lee, S.K.; Kim, H.-R.; Hwang, Y.-I.; et al. Vitamin C down-regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J. Cell. Biochem. 2011, 112, 894–901. [Google Scholar] [CrossRef]
- Ashino, H.; Shimamura, M.; Nakajima, H.; Dombou, M.; Kawanaka, S.; Oikawa, T.; Iwaguchi, T.; Kawashima, S. Novel Function of Ascorbic Acid as an Angiostatic Factor. Angiogenesis 2003, 6, 259–269. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Xu, Y.; Guo, X.; Wang, B.; Sun, L.; Liu, L.; Cui, F.; Zhuang, Q.; Bao, X.; et al. The Hypoxia-inducible Factor Renders Cancer Cells More Sensitive to Vitamin C-induced Toxicity. J. Biol. Chem. 2014, 289, 3339–3351. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Kang, J.-I.; Han, S.; Kim, Y.R.; Jo, J.; Kang, Y.W.; Choo, D.R.; Hyun, J.W.; Koh, Y.S.; Yoo, E.-S.; et al. L-Ascorbic Acid Inhibits Breast Cancer Growth by Inducing IRE/JNK/CHOP-Related Endoplasmic Reticulum Stress-Mediated p62/SQSTM1 Accumulation in the Nucleus. Nutrients 2020, 12, 1351. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Jóźwiak, P.; Ciesielski, P.; Zaczek, A.; Lipińska, A.; Pomorski, L.; Wieczorek, M.; Bryś, M.; Forma, E.; Krześlak, A. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J. Biomed. Sci. 2017, 24, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuyyuri, S.B.; Rinkinen, J.; Worden, E.; Shim, H.; Lee, S.; Davis, K.R. Ascorbic Acid and a Cytostatic Inhibitor of Glycolysis Synergistically Induce Apoptosis in Non-Small Cell Lung Cancer Cells. PLoS ONE 2013, 8, e67081. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.J.; Dachs, G.U.; Morrin, H.R.; Davey, V.C.; Robinson, B.A.; Vissers, M.C.M. Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels. BMC Cancer 2019, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, P.; Long, Y.; Yuan, G.; Shen, W.; Chen, Z.; Zhang, B.; Wang, Y.; Yue, D.; Seidl, C.; et al. Drug Repurposing of Pantoprazole and Vitamin C Targeting Tumor Microenvironment Conditions Improves Anticancer Effect in Metastatic Castration-Resistant Prostate Cancer. Front. Oncol. 2021, 11, 660320. [Google Scholar] [CrossRef]
- Gąbka, M.; Dałek, P.; Przybyło, M.; Gackowski, D.; Oliński, R.; Langner, M. The Membrane Electrical Potential and Intracellular pH as Factors Influencing Intracellular Ascorbate Concentration and Their Role in Cancer Treatment. Cells 2021, 10, 2964. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Englard, A.S.; Seifter, S. The Biochemical Functions of Ascorbic Acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Rebec, G.V.; Pierce, R.C. A vitamin as neuromodulator: Ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog. Neurobiol. 1994, 43, 537–565. [Google Scholar] [CrossRef]
- Glembotski, C.C. The Role of Ascorbic Acid in the Biosynthesis of the Neuroendocrine Peptides α-MSH and TRH. Ann. N. Y. Acad. Sci. 1987, 498, 54–62. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Hata, F.; Yoshida, H.; Yamatodani, A.; Wada, H. Effect of ascorbic acid on release of acetylcholine from synaptic vesicles prepared from different species of animals and release of noradrenaline from synaptic vesicles of rat brain. Life Sci. 1979, 24, 911–915. [Google Scholar] [CrossRef]
- Zhang, W.; He, R.; Yang, W.; Zhang, Y.; Yuan, Q.; Wang, J.; Liu, Y.; Chen, S.; Zhang, S.; Zhang, W.; et al. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J. Exp. Clin. Cancer Res. 2022, 41, 48. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.C.; Sant, D.W.; Camarena, V.; Van Booven, D.; Andrade, N.S.; Mustafi, S.; Monje, P.V.; Wang, G. Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes. J. Neurochem. 2021, 157, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, F.; Magdalena, R.; Saldivia, N.; Jara, N.; Martínez, F.; Ferrada, L.; Salazar, K.; Ávila, F.; Nualart, F. Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Nualart, F.; Mack, L.; Garcia, A.; Cisternas, P.; Bongarzone, E.R.; Heitzer, M.; Jara, N.; Martinez, F.; Ferrada, L.; Espinoza, F.; et al. Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J. Stem. Cell Res. Ther. 2014, 4, 209. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Holmes, C. Reciprocal effects of ascorbate on cancer cell growth and the expression of matrix metalloproteinases and transforming growth factor-β. Cancer Lett. 2007, 256, 49–55. [Google Scholar] [CrossRef]
- Kuo, S.-M.; Burl, L.R.; Hu, Z. Cellular Phenotype-Dependent and -Independent Effects of Vitamin C on the Renewal and Gene Expression of Mouse Embryonic Fibroblasts. PLoS ONE 2012, 7, e32957. [Google Scholar] [CrossRef] [Green Version]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [Green Version]
- Zeller, K.S.; Riaz, A.; Sarve, H.; Li, J.; Tengholm, A.; Johansson, S. The Role of Mechanical Force and ROS in Integrin-Dependent Signals. PLoS ONE 2013, 8, e64897. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ye, L.; Li, Q.; Wu, X.; Wang, B.; Ouyang, Y.; Yuan, Z.; Li, J.; Lin, C. Synaptopodin-2 suppresses metastasis of triple-negative breast cancer via inhibition of YAP/TAZ activity. J. Pathol. 2018, 244, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Camarena, V.; Mustafi, S.; Wang, G. Vitamin C Inhibits Triple-Negative Breast Cancer Metastasis by Affecting the Expression of YAP1 and Synaptopodin 2. Nutrients 2019, 11, 2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes 2021, 13, 1875774. [Google Scholar] [CrossRef] [PubMed]
- Otten, A.T.; Bourgonje, A.R.; Peters, V.; Alizadeh, B.Z.; Dijkstra, G.; Harmsen, H.J.M. Vitamin C Supplementation in Healthy Individuals Leads to Shifts of Bacterial Populations in the Gut—A Pilot Study. Antioxidants 2021, 10, 1278. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenet. 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhang, C.; Shi, G.; Yue, D.; Shu, Y.; Hu, S.; Qi, Z.; Chen, Y.; Zhang, B.; Zhang, Y.; et al. High-dose VitC plus oncolytic adenoviruses enhance immunogenic tumor cell death and reprogram tumor immune microenvironment. Mol. Ther. 2022, 30, 644–661. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.-J.; Yoo, H.-S.; Song, D.H.; Chung, K.-H.; Lee, K.-J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-M.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Kwon, S.-Y.; Lee, H.-S.; Park, J.-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, R.C.; Cheng, S.; Mohan, S. Vitamin C effects on 5-hydroxymethylcytosine and gene expression in osteoblasts and chondrocytes: Potential involvement of PHD2. PLoS ONE 2019, 14, e0220653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolhe, R.; Mondal, A.K.; Pundkar, C.; Periyasamy-Thandavan, S.; Mendhe, B.; Hunter, M.; Isales, C.M.; Hill, W.D.; Hamrick, M.W.; Fulzele, S. Modulation of miRNAs by Vitamin C in Human Bone Marrow Stromal Cells. Nutrients 2018, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hie, M.; Tsukamoto, I. Vitamin C-deficiency stimulates osteoclastogenesis with an increase in RANK expression. J. Nutr. Biochem. 2011, 22, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zunich, S.M.; Valdovinos, M.; Douglas, T.; Walterhouse, D.; Iannaccone, P.; Lamm, M.L.G. Osteoblast-secreted collagen upregulates paracrine Sonic hedgehog signaling by prostate cancer cells and enhances osteoblast differentiation. Mol. Cancer 2012, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Peng, D.; He, A.; He, S.; Ge, G.; Wang, S.; Ci, W.; Li, X.; Xia, D.; Zhou, L. Ascorbic acid induced TET2 enzyme activation enhances cancer immunotherapy efficacy in renal cell carcinoma. Int. J. Biol. Sci. 2022, 18, 995–1007. [Google Scholar] [CrossRef]
- Fan, P.; Zhao, J.; Meng, Z.; Wu, H.; Wang, B.; Wu, H.; Jin, X. Overexpressed histone acetyltransferase 1 regulates cancer immunity by increasing programmed death-ligand 1 expression in pancreatic cancer. J. Exp. Clin. Cancer Res. 2019, 38, 47. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.-J.; Kim, J.-H.; Hong, J.-M.; Kang, J.S.; Kim, H.-R.; Lee, W.J.; Hwang, Y.-I. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8+ memory T cell production capacity of these cells in vivo. Immunobiology 2014, 219, 554–564. [Google Scholar] [CrossRef]
- Mustafi, S.; Camarena, V.; Qureshi, R.; Sant, D.W.; Wilkes, Z.; Bilbao, D.; Slingerland, J.; Kesmodel, S.B.; Wang, G. Vitamin C sensitizes triple negative breast cancer to PI3K inhibition therapy. Theranostics 2021, 11, 3552–3564. [Google Scholar] [CrossRef]
- Rouleau, L.; Antony, A.N.; Bisetto, S.; Newberg, A.; Doria, C.; Levine, M.; Monti, D.A.; Hoek, J.B. Synergistic effects of ascorbate and sorafenib in hepatocellular carcinoma: New insights into ascorbate cytotoxicity. Free Radic. Biol. Med. 2016, 95, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef] [PubMed]
- Lorenzato, A.; Magrì, A.; Matafora, V.; Audrito, V.; Arcella, P.; Lazzari, L.; Montone, M.; Lamba, S.; Deaglio, S.; Siena, S.; et al. Vitamin C Restricts the Emergence of Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer. Cancers 2020, 12, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017, 8, 67269–67286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorillo, M.; Tóth, F.; Sotgia, F.; Lisanti, M.P. Doxycycline, Azithromycin and Vitamin C (DAV): A potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs). Aging 2019, 11, 2202–2216. [Google Scholar] [CrossRef]
- Liu, M.; Ohtani, H.; Zhou, W.; Ørskov, A.D.; Charlet, J.; Zhang, Y.W.; Shen, H.; Baylin, S.B.; Liang, G.; Grønbæk, K.; et al. Vitamin C increases viral mimicry induced by 5-aza-2′-deoxycytidine. Proc. Natl. Acad. Sci. USA 2016, 113, 10238–10244. [Google Scholar] [CrossRef] [Green Version]
- Mustafi, S.; Camarena, V.; Qureshi, R.; Yoon, H.; Volmar, C.-H.; Huff, T.C.; Sant, D.W.; Zheng, L.; Brothers, S.P.; Wahlestedt, C.; et al. Vitamin C supplementation expands the therapeutic window of BETi for triple negative breast cancer. eBioMedicine 2019, 43, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Mustafi, S.; Camarena, V.; Volmar, C.-H.; Huff, T.C.; Sant, D.W.; Brothers, S.P.; Liu, Z.-J.; Wahlestedt, C.; Wang, G. Vitamin C Sensitizes Melanoma to BET Inhibitors. Cancer Res. 2018, 78, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, S.; Paul, A.; Joby, J.; Saijan, S.; Vilapurathu, J.K. Effect of high-dose intravenous ascorbic acid on cancer patients following ketogenic diet. J. Cancer Res. Ther. 2021, 17, 1583–1586. [Google Scholar] [CrossRef]
- Di Tano, M.; Raucci, F.; Vernieri, C.; Caffa, I.; Buono, R.; Fanti, M.; Brandhorst, S.; Curigliano, G.; Nencioni, A.; De Braud, F.; et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020, 11, 2332. [Google Scholar] [CrossRef]
- Bánvölgyi, A.; Lőrincz, K.; Kiss, N.; Avci, P.; Fésűs, L.; Szipőcs, R.; Krenács, T.; Gyöngyösi, N.; Wikonkál, N.; Kárpáti, S.; et al. Efficiency of long-term high-dose intravenous ascorbic acid therapy in locally advanced basal cell carcinoma—A pilot study. Postȩpy Dermatol. Alergol. 2020, 37, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Jørgensen, N.R.; Zerahn, B.; Kristensen, B.; Henriksen, T.; Lykkesfeldt, J.; Mikines, K.J.; Poulsen, H.E. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: A single-arm phase II trial. Transl. Androl. Urol. 2017, 6, 517–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zasowska-Nowak, A.; Nowak, P.J.; Ciałkowska-Rysz, A. High-Dose Vitamin C in Advanced-Stage Cancer Patients. Nutrients 2021, 13, 735. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Wan, J.; Zhou, J.; Fu, L.; Li, Y.; Zeng, H.; Xu, X.; Lv, C.; Jin, H. Ascorbic Acid Inhibits Liver Cancer Growth and Metastasis in vitro and in vivo, Independent of Stemness Gene Regulation. Front. Pharmacol. 2021, 12, 726015. [Google Scholar] [CrossRef]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Bedhiafi, T.; Inchakalody, V.P.; Fernandes, Q.; Mestiri, S.; Billa, N.; Uddin, S.; Merhi, M.; Dermime, S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022, 146, 112553. [Google Scholar] [CrossRef]


| Category | Study Type(s) | VitC Dose and Administration | Main Mechanism | Findings | Reference | |
|---|---|---|---|---|---|---|
| cancer cells | Multiple cancers | cell line | 0–20 mM | pro-oxidant | Pharmacologic VitC selectively kills multiple cancer cells by initiating the production of extracellular H2O2 | [9] |
| KRAS or BRAF mutant CRC | cell line and animal | 0–3 mM (in vitro); 4 g/kg, i.p. (in vivo) | pro-oxidant | DHA, the oxidized form of VitC, exhibits selective toxicity by elevating ROS to disrupt cancer cell metabolism | [10] | |
| Breast cancer | cell line | 0–10 mM | pro-oxidant | VitC dose-dependently regulates the p66Shc/Rac1 pathway, which in turn induces apoptosis through ROS overexpression in cancer cells | [15] | |
| Melanoma | cell line | 0–2 mM | DNA demethylation | Physiological concentrations of VitC inhibit melanoma migration and malignant transformation by increasing 5hmC levels without damaging normal melanocytes | [12] | |
| Melanoma, Breast cancer | animal | 500 ppm pVitC, 150 mg/L VitC, oral | hydroxylase cofactor | Oral VitC promotes tumor collagen encapsulation and reduces MMP-9, IL-6, and VEGF levels, thereby inhibiting tumor growth and metastasis | [13] | |
| VHL-deficient ccRCC | animal | 2 g/kg, i.p. | expression of HIF target genes is suppressed by enhanced TET2 activity | VitC inhibits HIF1/2α-mediated tumor metabolic reprogramming in a TET2-dependent manner, and increases the efficiency of glycolysis inhibitor (2-DG) to suppress ccRCC | [16] | |
| TET2 and TP53 mutant Leukemia | cell line | 0–500 μM | DNA demethylation | VitC inhibits the proliferation of SKM-1 cells and promotes their differentiation to monocytes by restoring 5hmC levels | [17] | |
| Leukemia | cell line and animal | 250 μM (in vitro); 4 g/kg, i.p. (in vivo) | DNA demethylation | VitC reverses aberrant AML self-renewal and promotes myeloid differentiation through the restoration of TET2 and TET3 | [11] | |
| tumor stromal cells | T Lymphocytes | animal | 4 g/kg, i.p. | - | IVC promotes T cell differentiation, maturation, and immune memory formation, thereby increasing intra-tumor infiltration and immune responsiveness | [18] |
| CD4+ Tregs cells | cell line and animal | 100 μg/mL (in vitro) | DNA demethylation | VitC was shown to enhance the expression and stability of Foxp3+ markers in a TET2/3-dependent manner during iTregs cell differentiation | [19] | |
| γδ T cells | cell line | 0–200 μg/mL pVitC | DNA demethylation | The derivative pVitC regulates TGF-β-induced γδ T cell expansion and promotes conversion to Foxp3+ Tregs cells | [20] | |
| γδ T cells | cell line | 12.5 μg/mL VitC, 50 μg/mL pVitC | - | High concentrations of VitC and pVitC promote restimulated Vγ9Vδ2 T cell expansion through an accelerated cell cycle and affect Th1/Th2 cytokine secretion | [21] | |
| Th17 cells | cell line | 10 μg/mL | histone demethylation | In vitro, VitC reduces H3K9me3 levels and upregulates IL17 expression in a JMJD2-dependent manner | [22] | |
| B cells | cell line and animal | 0–20 μM (in vitro); 4 g/kg, i.p. (in vivo) | DNA demethylation | VitC promotes B-cell differentiation and humoral immunity via enhancing the enzymatic activity of TET2/3 in vitro and in vivo | [23] | |
| NK cells | cell line | 50 ng/mL | DNA demethylation | Low-dose VitC promotes KIR promoter demethylation and KIR expression, representing the maturation of NK cells | [24] | |
| Monocytes | cell line | 0–500 μM | - | VitC induces alterations in monocyte surface markers, gene expression and protein secretion in a mimicked hypoxic microenvironment (1% O2) in vitro | [25] | |
| Macrophages | cell line and animal | 0–4 mM (in vitro); 2 g/kg, 4 g/kg, i.p. (in vivo) | - | High-dose VitC induces apoptosis of M2 macrophages in TME and dose-dependently inhibits EMT and metastasis in ovarian cancer | [26] | |
| Neutrophils | animal | 0.33 g/L, oral; 200 mg/kg, i.p. | multi-pathways | Oral VitC attenuates NETs formation and autophagic gene expression as well as inhibits NF-κB activation | [27] | |
| Neutrophils | animal | 4 g/kg, oral | - | Oral high-dose VitC prevents melanoma invasion and increases neutrophil infiltration within the tumor | [28] | |
| DCs | cell line and animal | 0–2 mM (in vitro); 0.08 mM vcDC (in vivo) | signal molecules modulation | VitC increases IL-12 and IFN-γ secretion from DC cells, which in turn drives Th1 immunity | [29] | |
| Fibroblasts | cell line | 0–20 μM | - | VitC regulates the expression of genes related to ECM remodeling and cell adhesion, thereby affecting the phenotype of immortalized MEF | [30] | |
| Endothelial cells | cell line | 0–200 μM | multi-pathways | VitC improves endothelial cell dysfunction through multiple molecules, involving NO, ROS, RNS, biopterins, and GSH | [31] | |
| Neurons | cell line | 200 μM | signal molecules modulation | VitC oxidation induces necrotic apoptosis of neurons in a ROS-independent manner | [32] | |
| Combination Therapy | Study Type(s) | VitC Dose | Cancers (Cell Lines) | Target | Outcome(s) | Reference/NCT Number | |
|---|---|---|---|---|---|---|---|
| Immunotherapy | ICT (anti-PD-1, anti-CTLA-4) + VitC i.p. | animal | 4 g/kg | breast cancer (TS/A, 4T1), colorectal cancer (CT26, MC38), pancreatic cancer (PDAC), melanoma (B16-F10) | CD4+ and CD8+T lymphocytes, cancer cells | VitC increases the recruitment of lymphocytes in TME and improves the responsiveness of MMR-deficient tumors to ICT | [18] |
| ICT (anti-PD-1) + VitC i.p. | cell line and animal | 1 mM (in vitro); 4 g/kg (in vivo) | B-cell lymphoma (A20, SU-DHL-6, OCI-Ly1, OCI-Ly7, OCI-Ly3) | CD8+T cells, macrophages, cancer cells | VitC synergistically increases ICT efficacy by enhancing retrovirus expression, CTLs infiltration, and IL12 production in lymphoma | [35] | |
| ICT + IVC | cell line and animal | 250 μM (in vitro); 4 g/kg (in vivo) | melanoma (B16-OVA), leukemia (THP-1), colorectal cancer (MC38) | CD3+T cells, CTLs, CD56+NK cells, cancer cells | VitC upregulates TET-mediated cytokine expression to activate the IFN-γ/JAK2/STAT1 pathway, enhancing TILs infiltration, as well as ICT efficacy | [34] | |
| ICT + VitC i.p. | cell line and animal | 0.5 g/kg | renal cell carcinoma (Renca, 786-O, A498) | CD4+ and CD8+T lymphocytes, cancer cells | VitC improves ICT efficacy via upregulation of cytokine and chemokine levels in a TET2-dependent manner, and indirectly induces PD-L1 expression | [86] | |
| ICT + VitC | cell line | 0–50 μM | pancreatic cancer (PANC-1, BxPC-3 and MIA PaCa-2) | cancer cells | VitC inhibits histone acetyltransferase 1, which in turn downregulates PD-1 mRNA expression | [87] | |
| ICD (oAds) + VitC i.p. | cell line and animal | 2 mM (in vitro); 4 g/kg (in vivo) | colon cancer (CT26), breast cancer (4T1), hepatocellular carcinoma (Hepa1-6) | DC cells, CD8+T cells, CD4+ T cells, CD3+T cells | High-dose VitC and oAds exhibit a synergistic antitumor effect, with increased CD8+ T cells and DCs and decreased M2-type TAM cells in TME | [77] | |
| DC vaccines + VitC i.p. | cell line and animal | 0–2 mM (in vitro); 0.08 mM (in vivo) | melanoma (B16F10) | DC cells, CD8+T cells, CD4+ T cells | VitC promotes the secretion of co-cultured CD4+, CD8+ T cells in vitro and induces protective antitumor immunity in mice | [88] | |
| Small-molecule kinase inhibitors | PI3K inhibitor (buparlisib) + oral VitC | cell line and animal | 0, 50, 100, 300 μM (in vitro); 3.3 g/L (animal) | TNBC (BT20, MDA-MB-453) | cancer cells | Synergistically, VitC enhanced KDM5-mediated histone H3K4 demethylation and boosted the efficacy of buparlisib | [89] |
| sorafenib + IVC | cell line and clinical | 0–20 mM (in vitro); 75 g/infusion (clinical) | hepatocellular carcinoma (Hep G2, SNU-449, HuH-7), breast cancer (T47D), pancreatic cancer (MIA PaCa2) | cancer cells, angiogenesis | IVC and low-dose sorafenib exhibit synergistic cytotoxicity to suppress cancer viability and metastasis | [90] | |
| erlotinib + gemcitabine + IVC | clinical, phase I | 50, 75, 100 g/infusion | pancreatic cancer | cancer cells, angiogenesis | IVC is well tolerated with erlotinib and gemcitabine in patients with advanced cancer | [91] | |
| tyrosine kinase inhibitors (osimertinib or tarceva or iressa) + IVC | clinical, phase I/II | 30 g/infusion | EGFR mutant NSCLC | cancer cells | - | NCT03799094 | |
| Monoclonal antibodies | bevacizumab+ Temozolomide + oral VitC | clinical, phase I | 250 mg/d | recurrent high-grade glioma | cancer cells, angiogenesis | - | NCT01891747 |
| FOLFOXIRI +/- bevacizumab + IVC | clinical, phase III | 1.5 g/kg | peritoneal metastatic colorectal cancer | cancer cells, angiogenesis | - | NCT04516681 | |
| mFOLFOX6 +/- bevacizumab + IVC | clinical, phase III | 1.5 g/kg | colorectal neoplasms | cancer cells, angiogenesis | - | NCT02969681 | |
| cetuximab + VitC i.p. | cell line and animal | 1 mM, 2 mM (in vitro); 4 g/kg (animal) | colon cancer (RAS/BRAF wt, DiFi, CCK81, C75, IRCC-10A) | cancer cells, angiogenesis | Combination therapy delays the emergence of acquired drug resistance in EGFR mutant tumors in vitro and in vivo | [92] | |
| Metabolic inhibitors | antibiotics (doxycycline, azithromycin) + VitC | cell line | 0–500 μM | breast cancer stem cells (MCF7) | cancer cell mitochondria | VitC and glycolysis inhibitor form a synthetic lethal strategy that targets both OXPHOS and glycolysis | [93,94] |
| metformin + IVC | clinical, phrase II | 1.5 g/kg | hepatocellular carcinoma, pancreatic cancer, gastric cancer, colorectal cancer | cancer cell mitochondria and other targets | - | NCT04033107 | |
| glycolysis inhibitors (3-PO) + VitC | cell line | 0–20 mM | NSCLC (H1299, H661, A549) | cancer cells | VitC synergizes with glycolysis inhibitors to induce apoptosis in NSCLC, mainly through the upregulation of ROS | [52] | |
| Epigenetic therapies | DNMTis (5-aza-CdR) + VitC | cell line | 57 μM | colorectal cancer (HCT116), APL (HL60), breast cancer (MCF7), liver cancer (HepG2, SNU398) | cancer cells | In cooperation with DNMTis, low-dose VitC acts as a TET enzyme stimulator, which enhances viral mimicry response via endogenous retroviral gene transcription | [95] |
| DNMTis (5-azacytidine) + oral VitC | clinical | 500 mg/d | AML, MDS, CMML | cancer cells | The treatment increased 5hmC/5mC levels in patients and upregulated retroviral gene expression in DNMTi naïve patients compared to the placebo group | NCT02877277; [76] | |
| BETi + oral VitC | cell line and animal | 50–300 μM (in vitro); 3.3 g/L (in vivo) | TNBC (MDA-MB-231, BT-549, HCC1937), melanoma (A2058, SK-MEL28, SK-MEL2, C8161, 1205Lu) | cancer cells | Oral VitC and BETi collectively inhibit histone acetylation and improve tumor response to BETi treatment in vitro and in vivo. The underlying molecular mechanisms involve disruption of BRD4 and H4 interactions and upregulation of HDAC1 expression | [96,97] | |
| Diet therapy | ketogenic diet + IVC | clinical | 15–40 g/d | multiple cancers | cancer cells | VitC controls the inflammatory status of patients with advanced cancer, as well as increases ketone body content after a ketogenic diet | [98] |
| fasting-mimicking + IVC | cell line and animal | 350 μM (in vitro); 4 g/kg (in vivo) | KRAS mutant cancers: colorectal cancer (HCT116, DLD-1, CT26), lung cancer (H23, H727), pancreatic cancer (PANC1) | cancer cells | VitC and fasting-mimicking synergistically disrupt ROS and iron metabolism to enhance toxicity to KRAS-mutated tumor cells, sensitizing oxaliplatin therapy | [99] | |
| very low carbohydrate diet + IVC | clinical, phase I/II | 25, 50, 75, 100 g/infusion | KRAS and BRAF mutant colon cancer stage IV | cancer cells | - | NCT04035096 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-N.; Zhang, S.-J.; Feng, J.-Q.; Jin, W.-L. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers 2022, 14, 2608. https://doi.org/10.3390/cancers14112608
Li W-N, Zhang S-J, Feng J-Q, Jin W-L. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers. 2022; 14(11):2608. https://doi.org/10.3390/cancers14112608
Chicago/Turabian StyleLi, Wen-Ning, Shi-Jiao Zhang, Jia-Qing Feng, and Wei-Lin Jin. 2022. "Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment" Cancers 14, no. 11: 2608. https://doi.org/10.3390/cancers14112608







