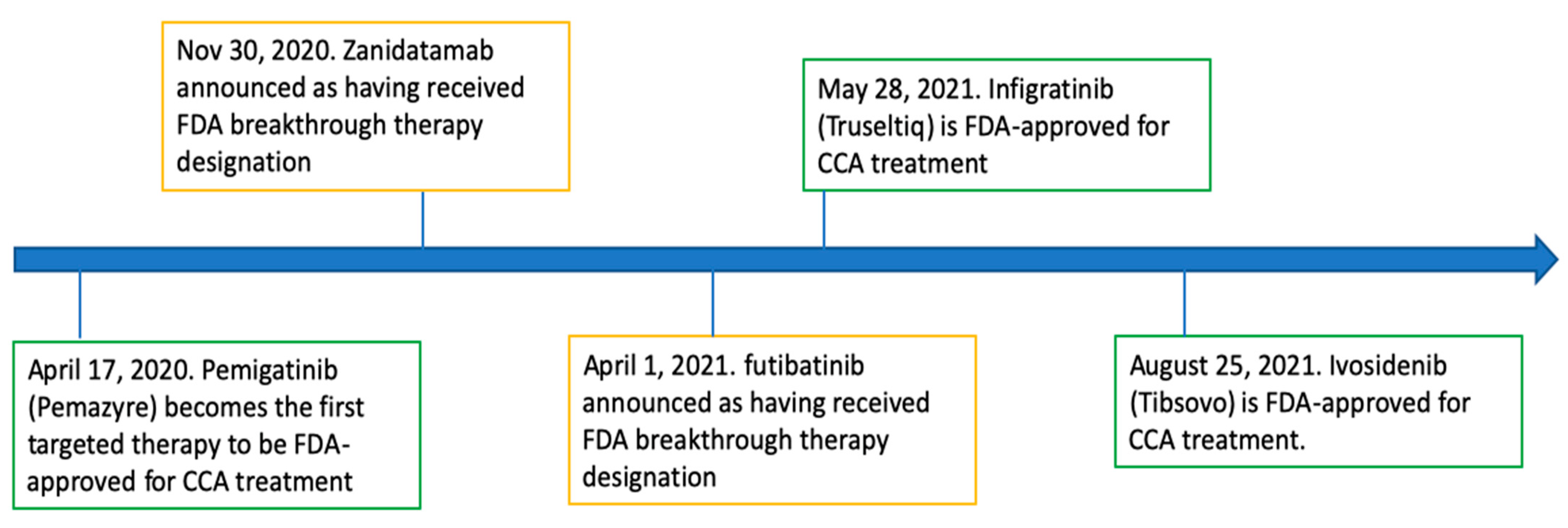
Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Targeted Therapies with FDA Approval
2.1. Pemigatinib
2.2. Infigratinib
2.3. Ivosidenib
3. Targeted Therapies with FDA Breakthrough Therapy Designation
3.1. Zanidatamab
3.2. Futibatinib
4. Mechanism of Action of Targeted Therapies for CCA
5. Future Steps
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Yao, K.J.; Jabbour, S.; Parekh, N.; Lin, Y.; Moss, R.A. Increasing mortality in the United States from cholangiocarcinoma: An analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016, 16, 117. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.-B.; Chen, Y.-D.; Liang, S.; Yang, G.-J.; Zhou, X.-N. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect. Dis. Poverty 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A. Helminths in the induction of cancer: Opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop. Geogr. Med. 1980, 32, 95–100. [Google Scholar]
- Ghouri, Y.A.; Mian, I.; Blechacz, B. Cancer review: Cholangiocarcinoma. J. Carcinog. 2015, 14, 1. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Aklan, H.M.; Esmail, A.A.; Al-Sadeq, A.A.; Eissa, G.A.; Hassan, O.A.; Al-Mikhlafy, A.A.; Al-Goshae, H.A.A. Frequency of Gallbladder Stones Among Patients Underwent Abdominal Ultrasound in a Tertiary Hospital in Sana’a City, Yemen. Malays. J. Med. Health Sci. 2020, 16, 36–39. [Google Scholar]
- Baidoun, F.; Sarmini, M.T.; Merjaneh, Z.; Moustafa, M.A. Controversial risk factors for cholangiocarcinoma. Eur. J. Gastroenterol. Hepatol. 2022, 34, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Benzing, C.; Schmelzle, M.; Atik, C.F.; Krenzien, F.; Mieg, A.; Haiden, L.M.; Wolfsberger, A.; Schöning, W.; Fehrenbach, U.; Pratschke, J. Factors associated with failure to rescue after major hepatectomy for perihilar cholangiocarcinoma: A 15-year single-center experience. Surgery 2022, 171, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.-J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Sahu, R.; Sharma, P.; Kumar, A. An Insight into Cholangiocarcinoma and Recent Advances in its Treatment. J. Gastrointest. Cancer 2022, 1–14. [Google Scholar] [CrossRef]
- Songserm, N.; Butprom, S.; Thongchai, C.; Ruksilp, M.; Charoenbut, P.; Woradet, S.; Souvanaa, T.; Buonhoseng, V.; Ali, A. Effectiveness of Village Health Volunteer Parallel Program for Proactive Action to Reduce Risk Factors for Cholangiocarcinoma in Two High-Risk Countries in the Greater Mekong Subregion. Nutr. Cancer 2021, 74, 1724–1733. [Google Scholar] [CrossRef]
- Songserm, N.; Woradet, S.; Kankarn, W.; Pintakham, K.; Vanhnivongkham, P.; Uyen, N.T.T.; Cuu, N.C.; Cua, L.N.; Sripa, B.; Ali, A. Cholangiocarcinoma protective factors in Greater Mekong Subregion: Critical issues for joint planning to sustainably solve regional public health problems. PLoS ONE 2022, 17, e0262589. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mori, T.; Momose, H.; Matsuki, R.; Kogure, M.; Abe, N.; Isayama, H.; Tazuma, S.; Tanaka, A.; Takikawa, H.; et al. Predictive factors for subsequent intrahepatic cholangiocarcinoma associated with hepatolithiasis: Japanese National Cohort Study for 18 years. J. Gastroenterol. 2022, 57, 387–395. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; Bhati, C.; Olliff, S.; Hübscher, S.; Gunson, B.; Mayer, D.; Mirza, D.; Buckels, J.; Bramhall, S. Cholangiocarcinoma Complicating Primary Sclerosing Cholangitis: A 24-Year Experience. Dig. Surg. 2008, 25, 126–132. [Google Scholar] [CrossRef]
- Nooijen, L.E.; Swijnenburg, R.-J.; Klümpen, H.-J.; Verheij, J.; Kazemier, G.; van Gulik, T.M.; Erdmann, J.I. Surgical Therapy for Perihilar Cholangiocarcinoma: State of the Art. Visc. Med. 2021, 37, 18–25. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Fong, J.V.N.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; Esmail, A.; Chang, J.C.; Ghobrial, R.M.; Abdelrahim, M. Utility of Cell-Free DNA Detection in Transplant Oncology. Cancers 2022, 14, 743. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Al-Rawi, H.; Esmail, A.; Xu, J.; Umoru, G.; Ibnshamsah, F.; Abudayyeh, A.; Victor, D.; Saharia, A.; McMillan, R.; et al. Gemcitabine and Cisplatin as Neo-Adjuvant for Cholangiocarcinoma Patients Prior to Liver Transplantation: Case-Series. Curr. Oncol. 2022, 29, 3585–3594. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abudayyeh, A.; Victor, D.; McMillan, R.; Kodali, S.; et al. Gemcitabine Plus Cisplatin Versus Non-Gemcitabine and Cisplatin Regimens as Neo-adjuvant Treatment for Cholangiocarcinoma Patients Prior to Liver Transplantation: An Institution Experience. Front. Oncol. 2022, in press.
- Thongprasert, S. The role of chemotherapy in cholangiocarcinoma. Ann. Oncol. 2005, 16 (Suppl. S2), ii93–ii96. [Google Scholar] [CrossRef]
- Holster, J.J.; El Hassnaoui, M.; Franssen, S.; Ijzermans, J.N.M.; de Jonge, J.; Mostert, B.; Polak, W.G.; de Wilde, R.F.; Homs, M.Y.V.; Koerkamp, B.G. Hepatic Arterial Infusion Pump Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 11, 44. [Google Scholar] [CrossRef]
- Bisello, S.; Camilletti, A.C.; Bertini, F.; Buwenge, M.; Arcelli, A.; Macchia, G.; Deodato, F.; Cilla, S.; Mattiucci, G.; Autorino, R.; et al. Stereotactic radiotherapy in intrahepatic cholangiocarcinoma: A systematic review. Mol. Clin. Oncol. 2021, 15, 152. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bednar, F.; Cho, C.S.; Nathan, H. Survival benefit with adjuvant radiotherapy after resection of distal cholangiocarcinoma: A propensity-matched National Cancer Database analysis. Cancer 2020, 127, 1266–1274. [Google Scholar] [CrossRef]
- Ren, B.; Guo, Q.; Yang, Y.; Liu, L.; Wei, S.; Chen, W.; Tian, Y. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat. Oncol. 2020, 15, 15. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; Abudayyeh, A.; Abdel-Wahab, N.; Diab, A.; Murakami, N.; Kaseb, A.O.; Chang, J.C.; Gaber, A.O.; et al. Utilization of Immunotherapy for the Treatment of Hepatocellular Carcinoma in the Peri-Transplant Setting: Transplant Oncology View. Cancers 2022, 14, 1760. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Doherty, B.; Nambudiri, V.E.; Palmer, W.C. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shen, W. Latest evidence on immunotherapy for cholangiocarcinoma (Review). Oncol. Lett. 2020, 20, 381. [Google Scholar] [CrossRef]
- Charalampakis, N.; Papageorgiou, G.; Tsakatikas, S.; Fioretzaki, R.; Kole, C.; Kykalos, S.; Tolia, M.; Schizas, D. Immunotherapy for cholangiocarcinoma: A 2021 update. Immunotherapy 2021, 13, 1113–1134. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Phys. 2008, 77, 311–319. [Google Scholar]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. TRK inhibition in cholangiocarcinoma: Trying to teach an old dog new tricks. Cancer Treat. Res. Commun. 2021, 27, 100351. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.E.; Law, L.; Nguyen, V.D.; Qiao, W.; Javle, M.M.; Kaseb, A.; Shroff, R.T. Second-line systemic treatment for advanced cholangiocarcinoma. J. Gastrointest. Oncol. 2014, 5, 408–413. [Google Scholar] [CrossRef]
- Brieau, B.; Dahan, L.; De Rycke, Y.; Boussaha, T.; Vasseur, P.; Tougeron, D.; Lecomte, T.; Coriat, R.; Bachet, J.-B.; Claudez, P.; et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015, 121, 3290–3297. [Google Scholar] [CrossRef]
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Association. FDA Grants Accelerated Approval to Pemigatinib for Cholangiocarcinoma with an FGFR2 Rearrangement or Fusion; US Food & Drug Association: Silver Spring, MD, USA, 2020.
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Jain, A.; Borad, M.J.; Kelley, R.K.; Wang, Y.; Abdel-Wahab, R.; Meric-Bernstam, F.; Baggerly, K.A.; Kaseb, A.O.; Al-Shamsi, H.O.; Ahn, D.H.; et al. Cholangiocarcinoma with FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2018, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. FDA Grants Accelerated Approval to Infigratinib for Metastatic Cholangiocarcinoma; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2021.
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Ivosidenib for Advanced or Metastatic Cholangiocarcinoma; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2021.
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Zymeworks Receives FDA Breakthrough Therapy Designation for HER2-Targeted Bispecific Antibody Zanidatamab. In Patients with Biliary Tract Cancer; Zymeworks Inc.: Vancouver, BC, Canada, 2020.
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Oh, D.-Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J. Clin. Oncol. 2021, 39, 299. [Google Scholar] [CrossRef]
- Tahio Oncology Inc. FDA Grants Breakthrough Therapy Designation for Taiho Oncology’s Futibatinib for Treatment of Advanced Cholangiocarcinoma. News Release; Tahio Oncology Inc.: Princeton, NJ, USA, 2021. [Google Scholar]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; He, Y.; Soni, N.; et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J. Clin. Oncol. 2020, 38 (Suppl. S15), 108. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (FGFRs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Liu, P.C.C.; Koblish, H.; Wu, L.; Bowman, K.; Diamond, S.; DiMatteo, D.; Zhang, Y.; Hansbury, M.; Rupar, M.; Wen, X.; et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS ONE 2020, 15, e0231877. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 2021, 95, 102170. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Biological Role and Therapeutic Potential of IDH Mutations in Cancer. Cancer Cell 2018, 34, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.; Yen, K.; Attar, E.C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. 2016, 27, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Biaglow, J.E.; Miller, R.A. The thioredoxin reductase/thioredoxin system: Novel redox targets for cancer therapy. Cancer Biol. Ther. 2005, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010, 462, 739–744, Erratum in Nature 2010, 465, 966. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; VasanthaKumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Kernytsky, A.; Wang, F.; Hansen, E.; Schalm, S.; Straley, K.; Gliser, C.; Yang, H.; Travins, J.; Murray, S.; Dorsch, M.; et al. IDH2 mutation-induced histone and DNA hypermethylation is progressively reversed by small-molecule inhibition. Blood 2015, 125, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.; et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef]
- Wang, F.; Travins, J.; DeLaBarre, B.; Penard-Lacronique, V.; Schalm, S.; Hansen, E.; Straley, K.; Kernytsky, A.; Liu, W.; Gliser, C.; et al. Targeted Inhibition of Mutant IDH2 in Leukemia Cells Induces Cellular Differentiation. Science 2013, 340, 622–626. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Wang, Y.; Zhang, Z.; Cao, G.; Song, T.; Zhang, T.; Li, Q. Systemic treatment of advanced or recurrent biliary tract cancer. Biosci. Trends 2020, 14, 328–341. [Google Scholar] [CrossRef]
- Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann Oncol. 2001, 12 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef]
- Kahraman, S.; Yalcin, S. Recent Advances in Systemic Treatments for HER-2 Positive Advanced Gastric Cancer. OncoTargets Ther. 2021, 14, 4149–4162. [Google Scholar] [CrossRef]
- Lee, K.W.; Im, Y.-H.; Cho, J.Y.; Oh, D.-Y.; Chung, H.C.C.; Chao, Y.; Bai, L.-Y.; Yen, C.J.; Kim, I.-H.; Oh, S.C.; et al. Zanidatamab, an anti-HER2 bispecific antibody, plus chemotherapy with/without tislelizumab as first-line treatment for patients with advanced HER2-positive breast cancer or gastric/gastroesophageal junction adenocarcinoma: A phase 1B/2 trial-in-progress. J. Clin. Oncol. 2021, 39, TPS2656. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef] [Green Version]
- Palomba, G.; Doneddu, V.; Cossu, A.; Paliogiannis, P.; Manca, A.; Casula, M.; Colombino, M.; Lanzillo, A.; DeFraia, E.; Pazzola, A.; et al. Prognostic impact of KRAS, NRAS, BRAF, and PIK3CA mutations in primary colorectal carcinomas: A population-based study. J. Transl. Med. 2016, 14, 292. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, G.; Ombra, M.; Colombino, M.; Casula, M.; Sini, M.; Manca, A.; Paliogiannis, P.; Eascierto, P.A.; Cossu, A. Multiple Molecular Pathways in Melanomagenesis: Characterization of Therapeutic Targets. Front. Oncol. 2015, 5, 183. [Google Scholar] [CrossRef]
- Cho, S.M.; Esmail, A.; Abdelrahim, M. Triple-Regimen of Vemurafenib, Irinotecan, and Cetuximab for the Treatment of BRAF. Front. Pharmacol. 2021, 12, 795381. [Google Scholar] [CrossRef]
- Targeted Therapy for Advanced Cholangiocarcinoma & BRAF Mutations. Oncol. Times 2020, 42, 33.
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Liu, B.; Lv, D.; Zhai, J.; Guan, X.; Yi, Z.; Ma, F. Antibody-drug conjugates in HER2-positive breast cancer. Chin. Med. J. 2021, 135, 261–267. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.A.; Wasan, H.S.; et al. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J. Clin. Oncol. 2020, 38, TPS592. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Zhu, C.; Zhuang, W.; Chen, L.; Yang, W.; Ou, W.B. Frontiers of ctDNA, targeted therapies, and immunotherapy in non-small-cell lung cancer. Transl. Lung Cancer Res. 2020, 9, 111–138. [Google Scholar] [CrossRef]

| Drug | Drug Class | Study | Treatment Group | ORR | DCR | PFS | OS |
|---|---|---|---|---|---|---|---|
| Pemigatinib | FGFR inhibitor | NCT03656536 | 107 participants | 35.50% | 82% | 6.9 months | 21.1 months |
| Infigratinib | FGFR inhibitor | NCT02150967 | 61 participants | 14.80% | 75.40% | 5.8 months | Not reported |
| Ivosidenib | IDH1 inhibitor | NCT02989857 | 124 participants | 2% | 53% | 2.7 months | 10.3 months |
| Zanidatamab | HER2-targeted antibody | NCT04466891 | 20 participants | 47% | 65% | Not reported | Not reported |
| Futibatinib | FGFR inhibitor | NCT02052778 | 67 participants | 34.30% | 76.10% | Not reported | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers 2022, 14, 2641. https://doi.org/10.3390/cancers14112641
Cho SM, Esmail A, Raza A, Dacha S, Abdelrahim M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers. 2022; 14(11):2641. https://doi.org/10.3390/cancers14112641
Chicago/Turabian StyleCho, Su Min, Abdullah Esmail, Ali Raza, Sunil Dacha, and Maen Abdelrahim. 2022. "Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma" Cancers 14, no. 11: 2641. https://doi.org/10.3390/cancers14112641
APA StyleCho, S. M., Esmail, A., Raza, A., Dacha, S., & Abdelrahim, M. (2022). Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers, 14(11), 2641. https://doi.org/10.3390/cancers14112641








