Quantitative Synthetic Magnetic Resonance Imaging for Brain Metastases: A Feasibility Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phantom Selection
2.2. Patient Selection
2.3. MRI Data Acquisition
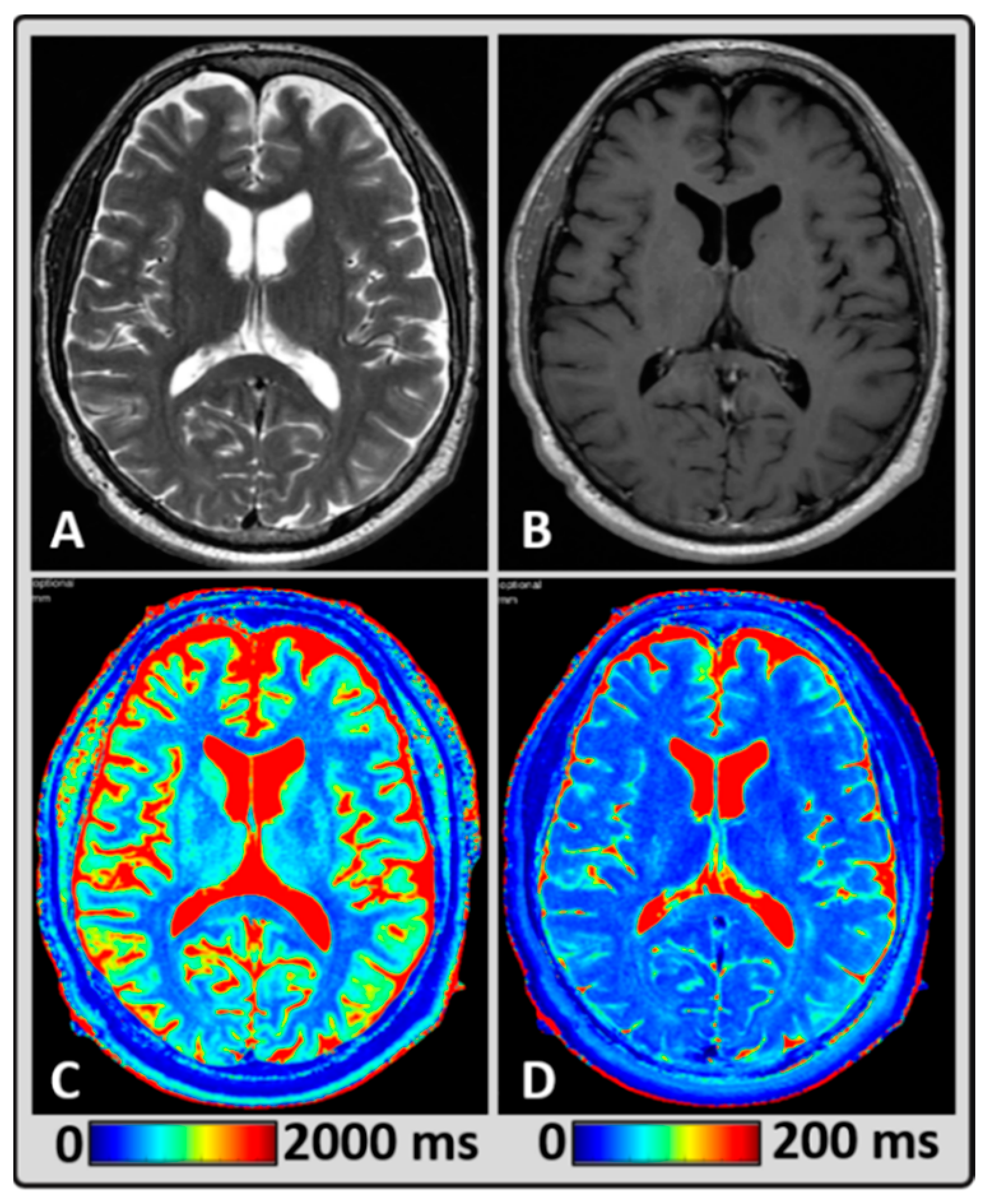
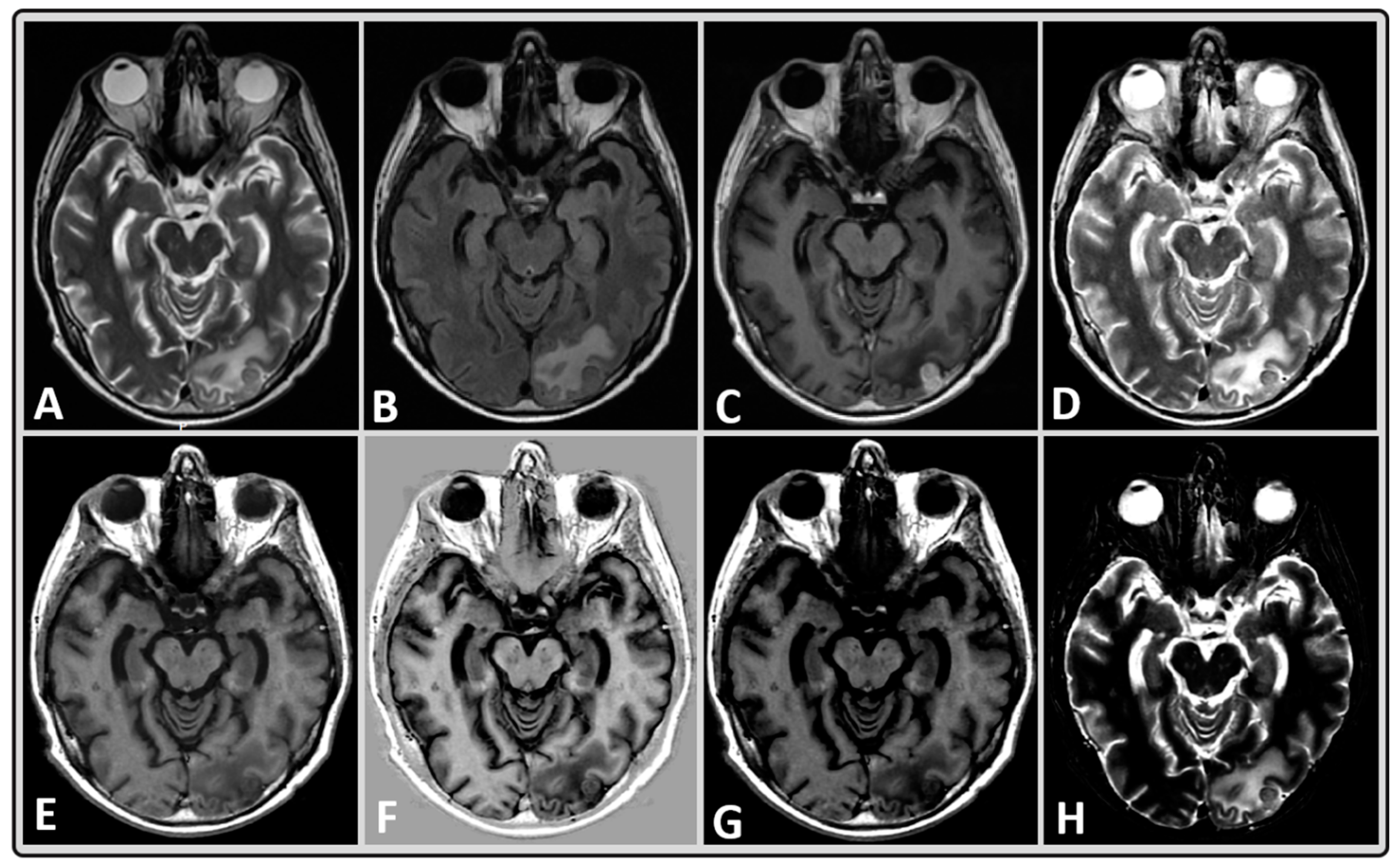
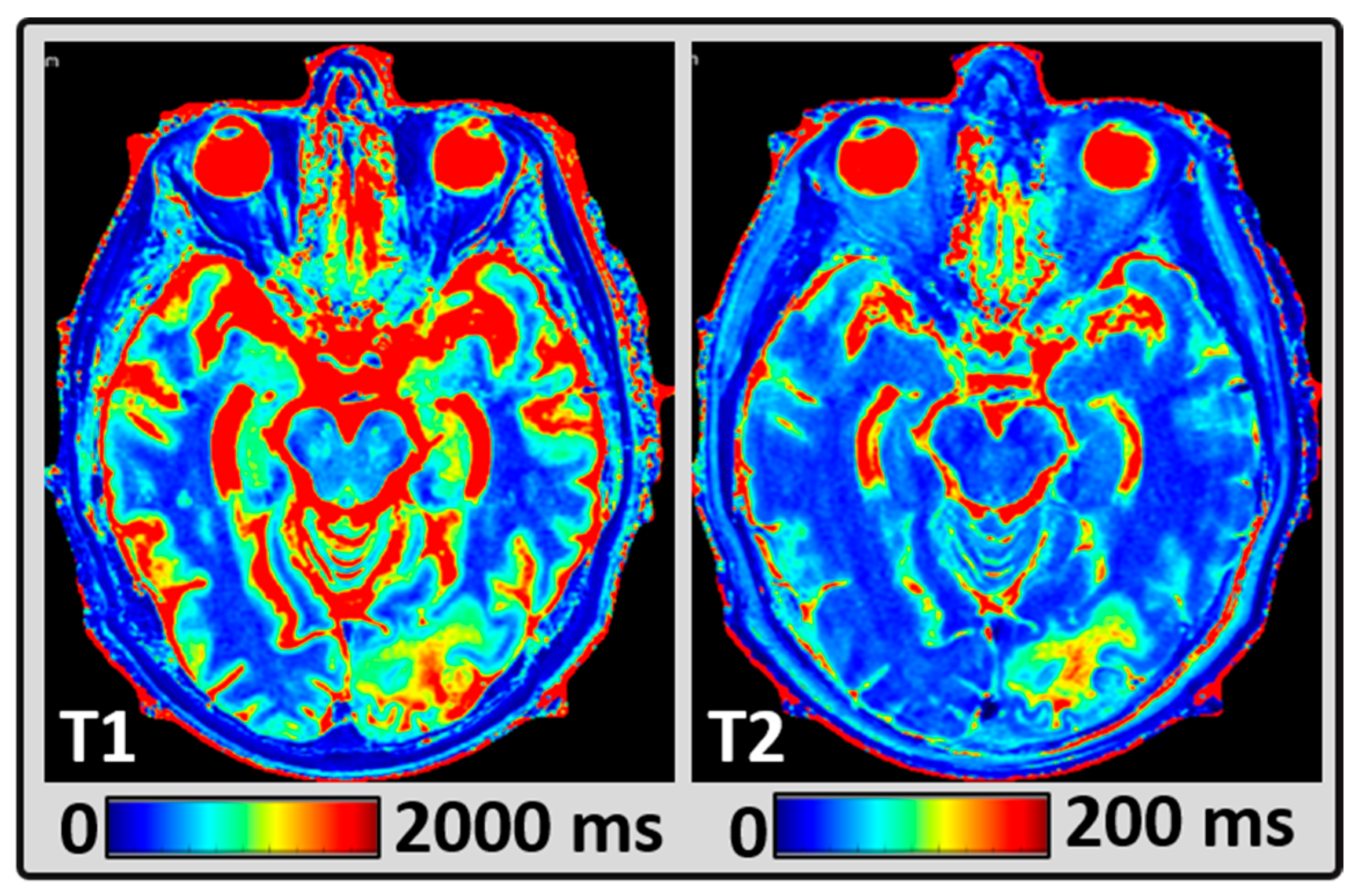
2.4. MRI Data Post-Processing
2.5. Regions of Interest Delineation
2.6. Statistical Analysis
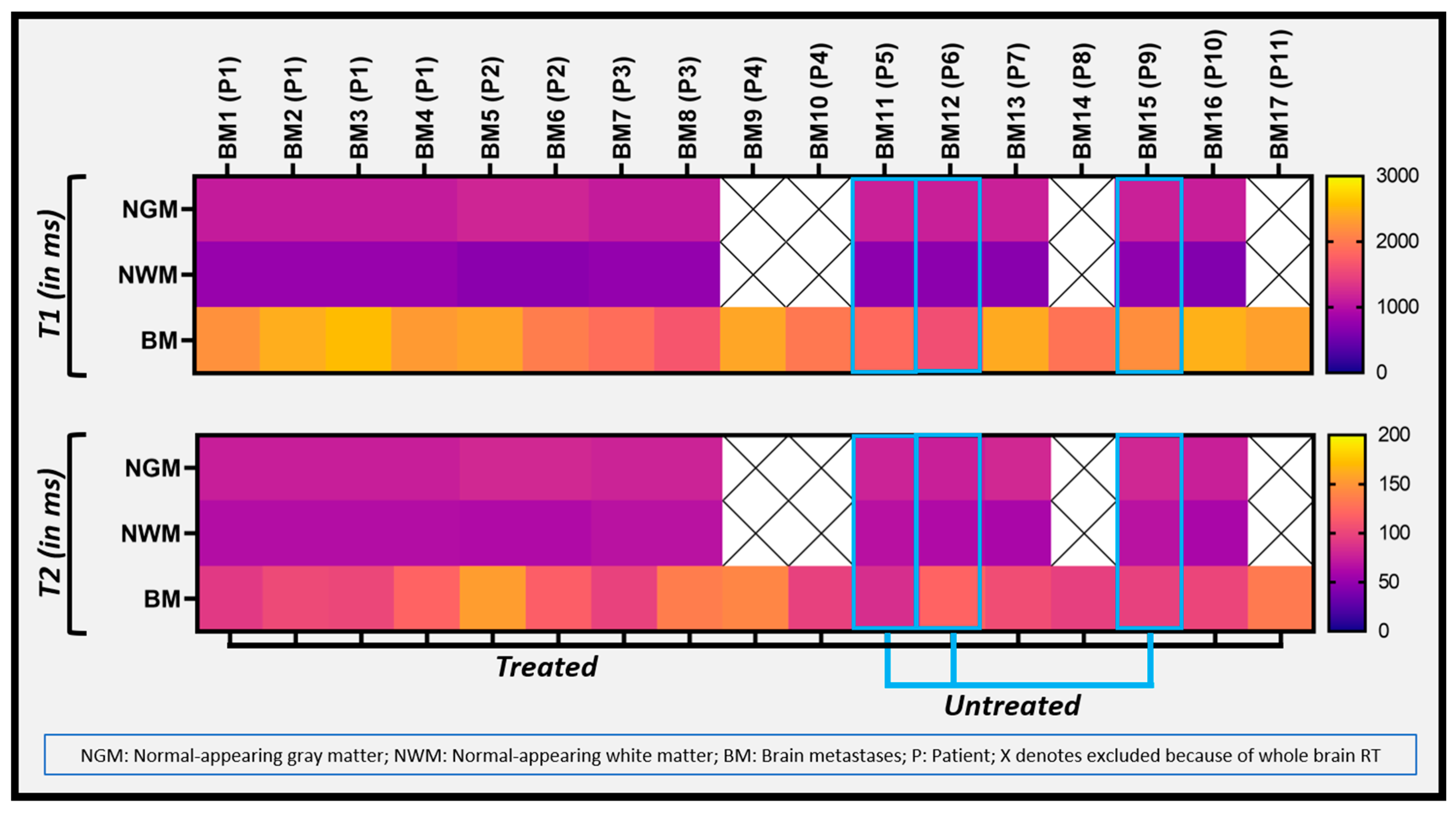
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawlani, V.; Patel, M.D.; Davies, N.; Flintham, R.; Wesolowski, R.; Ughratdar, I.; Pohl, U.; Nagaraju, S.; Petrik, V.; Kay, A.; et al. Multiparametric MRI: Practical approach and pictorial review of a useful tool in the evaluation of brain tumours and tumour-like lesions. Insights Imaging 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, H.; Detsky, J.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Advanced Magnetic Resonance Imaging Techniques in Management of Brain Metastases. Front. Oncol. 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Tong, E.; McCullagh, K.L.; Iv, M. Advanced Imaging of Brain Metastases: From Augmenting Visualization and Improving Diagnosis to Evaluating Treatment Response. Front. Neurol. 2020, 11, 270. [Google Scholar] [CrossRef]
- Deoni, S.C.; Peters, T.M.; Rutt, B.K. Determination of optimal angles for variable nutation proton magnetic spin-lattice, T1, and spin-spin, T2, relaxation times measurement. Magn. Reson. Med. 2004, 51, 194–199. [Google Scholar] [CrossRef]
- Ngo, F.Q.; Bay, J.W.; Kurland, R.J.; Weinstein, M.A.; Hahn, J.F.; Glassner, B.J.; Woolley, C.A.; Dudley, A.W., Jr.; Ferrario, C.M.; Meaney, T.F. Magnetic resonance of brain tumors: Considerations of imaging contrast on the basis of relaxation measurements. Magn. Reson. Imaging 1985, 3, 145–155. [Google Scholar] [CrossRef]
- McSheehy, P.M.; Weidensteiner, C.; Cannet, C.; Ferretti, S.; Laurent, D.; Ruetz, S.; Stumm, M.; Allegrini, P.R. Quantified tumor T1 is a generic early-response imaging biomarker for chemotherapy reflecting cell viability. Clin. Cancer Res. 2010, 16, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Lee, E.; Plummer, C.; Gil, S.; Popel, A.S.; Pathak, A.P. Vasculature-specific MRI reveals differential anti-angiogenic effects of a biomimetic peptide in an orthotopic breast cancer model. Angiogenesis 2015, 18, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Look, D.C.; Locker, D.R. Time saving in measurement of NMR and EPR relaxation times. Rev. Sci. Instrume 1970, 41, 250–251. [Google Scholar] [CrossRef] [Green Version]
- Wansapura, J.P.; Holland, S.K.; Dunn, R.S.; Ball, W.S., Jr. NMR relaxation times in the human brain at 3.0 tesla. J. Magn. Reson. Imaging 1999, 9, 531–538. [Google Scholar] [CrossRef]
- Gelman, N.; Ewing, J.R.; Gorell, J.M.; Spickler, E.M.; Solomon, E.G. Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: Relation to estimated iron and water contents. Magn. Reson. Med. 2001, 45, 71–79. [Google Scholar] [CrossRef]
- Schmitt, P.; Griswold, M.A.; Jakob, P.M.; Kotas, M.; Gulani, V.; Flentje, M.; Haase, A. Inversion recovery TrueFISP: Quantification of T1, T2, and spin density. Magn. Reson. Med. 2004, 51, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Rutt, B.K.; Peters, T.M. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn. Reson. Med. 2003, 49, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Peters, T.M.; Rutt, B.K. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn. Reson. Med. 2005, 53, 237–241. [Google Scholar] [CrossRef]
- Riederer, S.J.; Lee, J.N.; Farzaneh, F.; Wang, H.Z.; Wright, R.C. Magnetic resonance image synthesis. Clinical implementation. Acta Radiol. Suppl. 1986, 369, 466–468. [Google Scholar] [PubMed]
- Riederer, S.J.; Suddarth, S.A.; Bobman, S.A.; Lee, J.N.; Wang, H.Z.; MacFall, J.R. Automated MR image synthesis: Feasibility studies. Radiology 1984, 153, 203–206. [Google Scholar] [CrossRef]
- Glad, I.K.; Sebastiani, G. A Bayesian approach to synthetic magnetic resonance imaging. Biometrika 1995, 82, 237–250. [Google Scholar] [CrossRef]
- Maitra, R.; Riddles, J.J. Synthetic magnetic resonance imaging revisited. IEEE Trans. Med. Imaging 2010, 29, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Blystad, I.; Warntjes, J.B.; Smedby, O.; Landtblom, A.M.; Lundberg, P.; Larsson, E.M. Synthetic MRI of the brain in a clinical setting. Acta Radiol. 2012, 53, 1158–1163. [Google Scholar] [CrossRef]
- Andica, C.; Hagiwara, A.; Hori, M.; Kamagata, K.; Koshino, S.; Maekawa, T.; Suzuki, M.; Fujiwara, H.; Ikeno, M.; Shimizu, T.; et al. Review of synthetic MRI in pediatric brains: Basic principle of MR quantification, its features, clinical applications, and limitations. J. Neuroradiol. 2019, 46, 268–275. [Google Scholar] [CrossRef]
- Hagiwara, A.; Warntjes, M.; Hori, M.; Andica, C.; Nakazawa, M.; Kumamaru, K.K.; Abe, O.; Aoki, S. SyMRI of the Brain: Rapid Quantification of Relaxation Rates and Proton Density, With Synthetic MRI, Automatic Brain Segmentation, and Myelin Measurement. Investig. Radiol. 2017, 52, 647–657. [Google Scholar] [CrossRef]
- Tanenbaum, L.N.; Tsiouris, A.J.; Johnson, A.N.; Naidich, T.P.; DeLano, M.C.; Melhem, E.R.; Quarterman, P.; Parameswaran, S.X.; Shankaranarayanan, A.; Goyen, M.; et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. Am. J. Neuroradiol. 2017, 38, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giuliano, F.; Minosse, S.; Picchi, E.; Marfia, G.A.; Da Ros, V.; Muto, M.; Muto, M.; Pistolese, C.A.; Laghi, A.; Garaci, F. Comparison between synthetic and conventional magnetic resonance imaging in patients with multiple sclerosis and controls. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hori, M.; Suzuki, M.; Andica, C.; Nakazawa, M.; Tsuruta, K.; Takano, N.; Sato, S.; Hamasaki, N.; Yoshida, M.; et al. Contrast-enhanced synthetic MRI for the detection of brain metastases. Acta Radiol. Open 2016, 5, 2058460115626757. [Google Scholar] [CrossRef] [PubMed]
- Blystad, I.; Warntjes, J.B.M.; Smedby, Ö.; Lundberg, P.; Larsson, E.M.; Tisell, A. Quantitative MRI using relaxometry in malignant gliomas detects contrast enhancement in peritumoral oedema. Sci. Rep. 2020, 10, 17986. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Jurcoane, A.; Kebir, S.; Ditter, P.; Schrader, F.; Herrlinger, U.; Tzaridis, T.; Mädler, B.; Schild, H.H.; Glas, M.; et al. Quantitative T1-mapping detects cloudy-enhancing tumor compartments predicting outcome of patients with glioblastoma. Cancer Med. 2017, 6, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Sacks, P.; Rahman, M. Epidemiology of Brain Metastases. Neurosurg. Clin. N. Am. 2020, 31, 481–488. [Google Scholar] [CrossRef]
- Kelly, P.J. Gliomas: Survival, origin and early detection. Surg. Neurol. Int. 2010, 1, 96. [Google Scholar] [CrossRef] [Green Version]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef]
- Nowosielski, M.; Radbruch, A. The emerging role of advanced neuroimaging techniques for brain metastases. Chin. Clin. Oncol. 2015, 4, 23. [Google Scholar]
- Shah, A.D.; Shridhar Konar, A.; Paudyal, R.; Oh, J.H.; LoCastro, E.; Nuñez, D.A.; Swinburne, N.; Vachha, B.; Ulaner, G.A.; Young, R.J.; et al. Diffusion and Perfusion MRI Predicts Response Preceding and Shortly After Radiosurgery to Brain Metastases: A Pilot Study. J. Neuroimaging 2021, 31, 317–323. [Google Scholar] [CrossRef]
- Taunk, N.K.; Oh, J.H.; Shukla-Dave, A.; Beal, K.; Vachha, B.; Holodny, A.; Hatzoglou, V. Early posttreatment assessment of MRI perfusion biomarkers can predict long-term response of lung cancer brain metastases to stereotactic radiosurgery. Neuro-Oncology 2018, 20, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.M.; Choi, S.H.; Hwang, M.; Yoo, R.E.; Yun, T.J.; Kim, J.H.; Sohn, C.H. Application of Synthetic MRI for Direct Measurement of Magnetic Resonance Relaxation Time and Tumor Volume at Multiple Time Points after Contrast Administration: Preliminary Results in Patients with Brain Metastasis. Korean J. Radiol. 2018, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Russek, S.; Boss, M.; Jackson, E.; Jennings, D.; Evelhoch, J.; Gunter, J.; Sorensen, A. Characterization of NIST/ISMRM MRI system phantom. In Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia, 5–11 May 2012; p. 2456. [Google Scholar]
- Keenan, K.E.; Stupic, K.F.; Boss, M.A.; Russek, S.E.; Chenevert, T.L.; Prasad, P.V.; Reddick, W.E.; Zheng, J.; Hu, P.; Jackson, E.F. Comparison of T1 measurement using ISMRM/NIST system phantom. In Proceedings of the 24th Annual Meeting of ISMRM, Singapore, 7–13 May 2016; p. 3290. [Google Scholar]
- Warntjes, J.B.; Leinhard, O.D.; West, J.; Lundberg, P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn. Reson. Med. 2008, 60, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Stikov, N.; Ghugre, N.R.; Wright, G.A. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012, 36, 805–824. [Google Scholar] [CrossRef]
- Callaghan, M.F.; Mohammadi, S.; Weiskopf, N. Synthetic quantitative MRI through relaxometry modelling. NMR Biomed. 2016, 29, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Bobman, S.; Riederer, S.; Lee, J.; Suddarth, S.; Wang, H.; MacFall, J. Synthesized MR images: Comparison with acquired images. Radiology 1985, 155, 731–738. [Google Scholar] [CrossRef]
- Granberg, T.; Uppman, M.; Hashim, F.; Cananau, C.; Nordin, L.; Shams, S.; Berglund, J.; Forslin, Y.; Aspelin, P.; Fredrikson, S. Clinical feasibility of synthetic MRI in multiple sclerosis: A diagnostic and volumetric validation study. Am. J. Neuroradiol. 2016, 37, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Betts, A.M.; Leach, J.L.; Jones, B.V.; Zhang, B.; Serai, S. Brain imaging with synthetic MR in children: Clinical quality assessment. Neuroradiology 2016, 58, 1017–1026. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, Y.H.; Cheon, J.-E.; Kim, I.-O.; Cho, S.H.; Kim, W.H.; Kim, H.J.; Cho, H.-H.; You, S.-K.; Park, S.-H. Image quality at synthetic brain magnetic resonance imaging in children. Pediatr. Radiol. 2017, 47, 1638–1647. [Google Scholar] [CrossRef]
- Cui, Y.; Han, S.; Liu, M.; Wu, P.Y.; Zhang, W.; Zhang, J.; Li, C.; Chen, M. Diagnosis and Grading of Prostate Cancer by Relaxation Maps From Synthetic MRI. J. Magn. Reson. Imaging 2020, 52, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Gho, S.M.; Back, S.N.; Ha, T.; Kang, D.K.; Kim, T.H. The feasibility of synthetic MRI in breast cancer patients: Comparison of T(2) relaxation time with multiecho spin echo T(2) mapping method. Br. J. Radiol. 2018, 92, 20180479. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, M.; Wu, P.-y.; Yang, Y.; Zhang, H.; Zhao, X. A preliminary study of synthetic magnetic resonance imaging in rectal cancer: Imaging quality and preoperative assessment. Insights Imaging 2021, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; He, N.; He, H.; Liu, K.; Ke, L.; Liu, H.; Zhong, L.; Huang, C.; Yang, A.; Zhou, C.; et al. The diagnostic performance of quantitative mapping in breast cancer patients: A preliminary study using synthetic MRI. Cancer Imaging 2020, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Delshad, S.; Macey, P.M.; Woo, M.A.; Harper, R.M. Development of T2-relaxation values in regional brain sites during adolescence. Magn. Reson. Imaging 2011, 29, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.; Hao, X.; Liu, F.; Xu, D.; Liu, J.; Peterson, B.S. The effects of changing water content, relaxation times, and tissue contrast on tissue segmentation and measures of cortical anatomy in MR images. Magn. Reson. Imaging 2013, 31, 1709–1730. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Value |
|---|---|
| Total patients | 11 |
| Total number of BM lesions | 17 |
| Demographics | |
| Median age (y) | 52 |
| Age range (y) | 25–61 |
| Male/Female | 5/6 |
| Location of primary tumor | |
| Lung | 5 |
| Colon | 1 |
| Melanoma | 2 |
| Other | 3 |
| Untreated/Treated | 3/8 |
| Vial # | T1 (in ms) | Percent of Difference (in %) | ||||
| VP | GS | MAGiC | VP and GS | VP and MAGiC | GS and MAGiC | |
| 1 | 1838 | 1779.7 | 1719 | 3.2 | 6.5 | 3.4 |
| 2 | 1398 | 1350.9 | 1179 | 3.4 | 15.7 | 12.7 |
| 3 | 998.3 | 957.7 | 852 | 4.1 | 14.7 | 11 |
| 4 | 725.8 | 678.2 | 622 | 6.6 | 14.3 | 8.3 |
| 5 | 509 | 483 | 453 | 5.1 | 11 | 6.2 |
| 6 | 367 | 345.9 | 327 | 5.7 | 10.9 | 5.5 |
| 7 | 258.7 | 242.1 | 300 | 6.4 | 16 | 23.9 |
| Vial # | T2 (in ms) | Percent of Difference (in %) | ||||
| VP | GS | MAGiC | VP and GS | VP and MAGiC | GS and MAGiC | |
| 1 | 645.8 | 537.4 | 591 | 16.8 | 8.5 | 10 |
| 2 | 423.6 | 357.4 | 414 | 15.6 | 2.3 | 15.8 |
| 3 | 286 | 245.9 | 287 | 14 | 0.3 | 16.7 |
| 4 | 184.8 | 162.6 | 186 | 12 | 0.6 | 14.4 |
| 5 | 134.1 | 118.3 | 141 | 11.8 | 5.1 | 19.2 |
| 6 | 94.4 | 81.6 | 103 | 13.6 | 9.1 | 26.2 |
| 7 | 62.5 | 56.7 | 74 | 9.3 | 18.4 | 30.5 |
| Relaxometry Values | Untreated BM | Treated BM | |
|---|---|---|---|
| T1 (ms) | Median (min, max) | 1845 (1583,2177) | 2311 (1654, 2558) |
| Mean ± SD | 1868 ± 298 | 2211 ± 269 | |
| T2 (ms) | Median (min, max) | 97 (85, 119) | 104 (92, 154) |
| Mean ± SD | 100 ± 17 | 114 ± 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konar, A.S.; Shah, A.D.; Paudyal, R.; Fung, M.; Banerjee, S.; Dave, A.; Hatzoglou, V.; Shukla-Dave, A. Quantitative Synthetic Magnetic Resonance Imaging for Brain Metastases: A Feasibility Study. Cancers 2022, 14, 2651. https://doi.org/10.3390/cancers14112651
Konar AS, Shah AD, Paudyal R, Fung M, Banerjee S, Dave A, Hatzoglou V, Shukla-Dave A. Quantitative Synthetic Magnetic Resonance Imaging for Brain Metastases: A Feasibility Study. Cancers. 2022; 14(11):2651. https://doi.org/10.3390/cancers14112651
Chicago/Turabian StyleKonar, Amaresha Shridhar, Akash Deelip Shah, Ramesh Paudyal, Maggie Fung, Suchandrima Banerjee, Abhay Dave, Vaios Hatzoglou, and Amita Shukla-Dave. 2022. "Quantitative Synthetic Magnetic Resonance Imaging for Brain Metastases: A Feasibility Study" Cancers 14, no. 11: 2651. https://doi.org/10.3390/cancers14112651





