Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Imbalance in GM Is Associated with DNA Damage and Immunosuppression
2.1. Imbalance of GM Causes DNA Damage
2.2. Interaction between GM and Immune Regulation
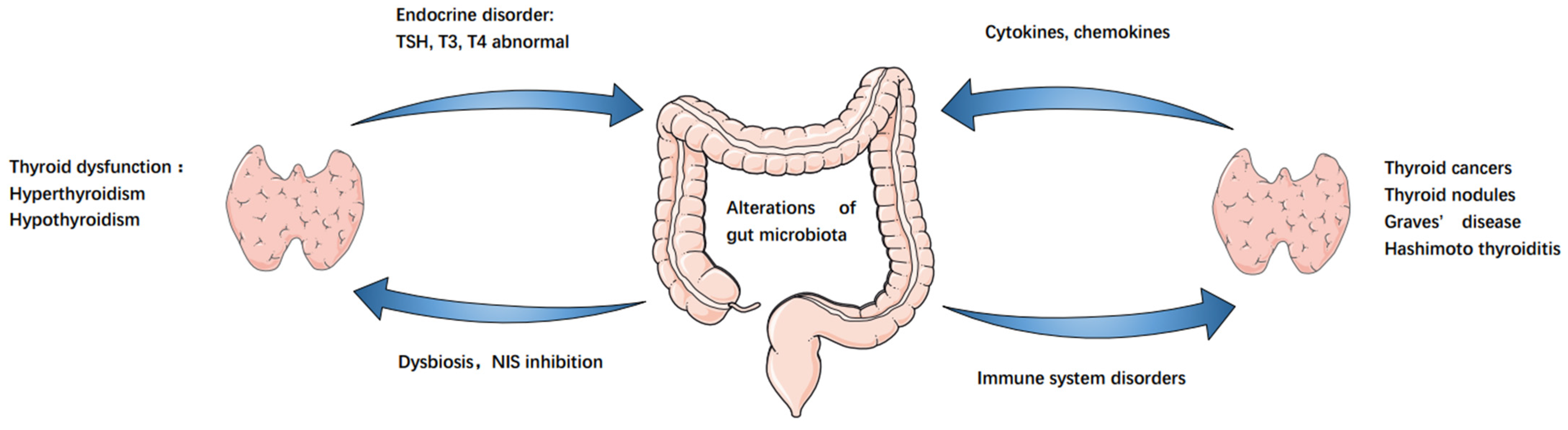
3. GM–Endocrine Homeostasis–Thyroid Axis
3.1. GM Affects Endocrine Homeostasis
3.2. GM and Autoimmune Thyroid Disease
3.3. GM and Thyroid Function Regulation
4. Correlation between GM and Cancer
4.1. Interrelationship between GM and Gastrointestinal Cancers
4.2. Interrelationship between GM and Non-Gastrointestinal Cancers
5. Can Dysbiosis of GM Promote the Development of TC?
6. TC induces Alterations in the Intestinal Microbiota
6.1. Thyroid Cancer Triggers Changes in Intestinal Microbiota
6.2. Microbiota Changes in Thyroid Cancer
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines, American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Kotwal, A.; Erickson, D.; Geske, J.R.; Hay, I.D.; Castro, M.R. Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma over Two Decades. Thyroid 2021, 31, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Canducci, F.; Nebuloni, M.; Clementi, M.; Montorsi, F.; Salonia, A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 2016, 13, 77–90. [Google Scholar] [CrossRef]
- Dai, D.; Yang, Y.; Yang, Y.; Dang, T.; Xiao, J.; Wang, W.; Teng, L.; Xu, J.; Ye, J.; Jiang, H. Alterations of thyroid microbiota across different thyroid microhabitats in patients with thyroid carcinoma. J. Transl. Med. 2021, 19, 488. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Khoruts, A.; Dicksved, J.; Jansson, J.K.; Sadowsky, M.J. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 2010, 44, 354–360. [Google Scholar] [CrossRef]
- Ma, C.; Wu, X.; Nawaz, M.; Li, J.; Yu, P.; Moore, J.E.; Xu, J. Molecular characterization of fecal microbiota in patients with viral diarrhea. Curr. Microbiol. 2011, 63, 259–266. [Google Scholar] [CrossRef]
- Liang, D.; Leung, R.K.; Guan, W.; Au, W.W. Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
- Ishaq, H.M.; Mohammad, I.S.; Guo, H.; Shahzad, M.; Hou, Y.J.; Ma, C.; Naseem, Z.; Wu, X.; Shi, P.; Xu, J. Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed Pharm. 2017, 95, 865–874. [Google Scholar] [CrossRef]
- Lauritano, E.C.; Bilotta, A.L.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Laginestra, A.; Novi, M.; Sottili, S.; Serricchio, M.; Cammarota, G.; et al. Association between hypothyroidism and small intestinal bacterial overgrowth. J. Clin. Endocrinol. Metab. 2007, 92, 4180–4184. [Google Scholar] [CrossRef] [Green Version]
- Hiromatsu, Y.; Satoh, H.; Amino, N. Hashimoto’s thyroiditis: History and future outlook. Hormones 2013, 12, 12–18. [Google Scholar] [CrossRef]
- Caturegli, P.; de Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Chiovato, L.; Magri, F.; Carle, A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Morshed, S.A.; Latif, R.; Davies, T.F. Delineating the autoimmune mechanisms in Graves’ disease. Immunol. Res. 2012, 54, 191–203. [Google Scholar] [CrossRef]
- Subekti, I.; Pramono, L.A. Current Diagnosis and Management of Graves’ Disease. Acta Med. Indones 2018, 50, 177–182. [Google Scholar] [PubMed]
- Yu, X.; Jiang, W.; Kosik, R.O.; Song, Y.; Luo, Q.; Qiao, T.; Tong, J.; Liu, S.; Deng, C.; Qin, S.; et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J. Adv. Res. 2022, 35, 61–70. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Silva, M.; Brunner, V.; Tschurtschenthaler, M. Microbiota and Colorectal Cancer: From Gut to Bedside. Front. Pharmacol. 2021, 12, 760280. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Hiergeist, A.; Glasner, J.; Reischl, U.; Gessner, A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015, 56, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Muhlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Nesic, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429–433. [Google Scholar] [CrossRef]
- Wang, X.; Huycke, M.M. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 2007, 132, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Lochhead, P.; Giovannucci, E.; Meyerhardt, J.A.; Fuchs, C.S.; Chan, A.T. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: Power and promise of molecular pathological epidemiology. Oncogene 2014, 33, 2949–2955. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Bai, J.; Ma, C.; Wei, J.; Du, X. The Role of Gut Microbiota in Tumor Immunotherapy. J. Immunol. Res. 2021, 2021, 5061570. [Google Scholar] [CrossRef] [PubMed]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophy. Acta Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docimo, G.; Cangiano, A.; Romano, R.M.; Pignatelli, M.F.; Offi, C.; Paglionico, V.A.; Galdiero, M.; Donnarumma, G.; Nigro, V.; Esposito, D.; et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020, 11, 586529. [Google Scholar] [CrossRef]
- Yu, A.I.; Zhao, L.; Eaton, K.A.; Ho, S.; Chen, J.; Poe, S.; Becker, J.; Gonzalez, A.; McKinstry, D.; Hasso, M.; et al. Gut Microbiota Modulate CD8 T Cell Responses to Influence Colitis-Associated Tumorigenesis. Cell Rep. 2020, 31, 107471. [Google Scholar] [CrossRef]
- Guedj, A.; Geiger-Maor, A.; Galun, E.; Benyamini, H.; Nevo, Y.; Elgavish, S.; Amsalem, H.; Rachmilewitz, J. Early age decline in DNA repair capacity in the liver: In depth profile of differential gene expression. Aging 2016, 8, 3131–3146. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef] [Green Version]
- Valiathan, R.; Ashman, M.; Asthana, D. Effects of Ageing on the Immune System: Infants to Elderly. Scand J. Immunol. 2016, 83, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, E.; Fuentes, M.; Alarcon, M.; Palomo, I. Immune System Dysfunction in the Elderly. An. Acad. Bras. Ciências 2017, 89, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huck, O.; Al-Hashemi, J.; Poidevin, L.; Poch, O.; Davideau, J.L.; Tenenbaum, H.; Amar, S. Identification and Characterization of MicroRNA Differentially Expressed in Macrophages Exposed to Porphyromonas gingivalis Infection. Infect. Immun. 2017, 85, e00771-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pere-Vedrenne, C.; Prochazkova-Carlotti, M.; Rousseau, B.; He, W.; Chambonnier, L.; Sifre, E.; Buissonniere, A.; Dubus, P.; Megraud, F.; Varon, C.; et al. The Cytolethal Distending Toxin Subunit CdtB of Helicobacter hepaticus Promotes Senescence and Endoreplication in Xenograft Mouse Models of Hepatic and Intestinal Cell Lines. Front. Cell Infect. Microbiol. 2017, 7, 268. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Elmen, L.; Segota, I.; Xian, Y.; Tinoco, R.; Feng, Y.; Fujita, Y.; Munoz, R.R.S.; Schmaltz, R.; Bradley, L.M.; et al. Prebiotic-Induced Anti-tumor Immunity Attenuates Tumor Growth. Cell Rep. 2020, 30, 1753–1766.e1756. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S. Potential Impacts of Prebiotics and Probiotics on Cancer Prevention. Anticancer Agents Med. Chem. 2022, 22, 605–628. [Google Scholar] [CrossRef]
- Knezevic, J.; Starchl, C.; Berisha, A.T.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Leoni, S.G.; Sastre-Perona, A.; de la Vieja, A.; Santisteban, P. Selenium Increases Thyroid-Stimulating Hormone-Induced Sodium/Iodide Symporter Expression Through Thioredoxin/Apurinic/Apyrimidinic Endonuclease 1-Dependent Regulation of Paired Box 8 Binding Activity. Antioxid. Redox Signal. 2016, 24, 855–866. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Y.; Chen, Y.; Chen, D. The Role of the Microbiota in Graves’ Disease and Graves’ Orbitopathy. Front. Cell. Infect. Microbiol. 2021, 11, 739707. [Google Scholar] [CrossRef]
- Ishaq, H.M.; Mohammad, I.S.; Shahzad, M.; Ma, C.; Raza, M.A.; Wu, X.; Guo, H.; Shi, P.; Xu, J. Molecular Alteration Analysis of Human Gut Microbial Composition in Graves’ disease Patients. Int. J. Biol. Sci. 2018, 14, 1558–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayres, L.C.F.; de Salis, L.V.V.; Rodrigues, G.S.P.; Lengert, A.V.H.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhao, Y.; Li, Y.; Ma, S.; Wang, Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin. Sci. 2020, 134, 1521–1535. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef] [PubMed]
- Ludmir, E.B.; Stephens, S.J.; Palta, M.; Willett, C.G.; Czito, B.G. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J. Gastrointest. Oncol. 2015, 6, 287–295. [Google Scholar]
- Mohiuddin, M.K.; Chava, S.; Upendrum, P.; Latha, M.; Zubeda, S.; Kumar, A.; Ahuja, Y.R.; Hasan, Q.; Mohan, V. Role of Human papilloma virus infection and altered methylation of specific genes in esophageal cancer. Asian Pac. J. Cancer Prev. 2013, 14, 4187–4193. [Google Scholar] [CrossRef] [Green Version]
- Tomasello, G.; Tralongo, P.; Damiani, P.; Sinagra, E.; di Trapani, B.; Zeenny, M.N.; Hussein, I.H.; Jurjus, A.; Leone, A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J. Gastroenterol. 2014, 20, 18121–18130. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Jurjus, A.; Eid, A.; Al Kattar, S.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Rashtak, S.; Rego, R.; Sweetser, S.R.; Sinicrope, F.A. Sessile Serrated Polyps and Colon Cancer Prevention. Cancer Prev. Res. 2017, 10, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett-Hartman, A.N.; Newcomb, P.A.; Phipps, A.I.; Passarelli, M.N.; Grady, W.M.; Upton, M.P.; Zhu, L.C.; Potter, J.D. Colorectal endoscopy, advanced adenomas, and sessile serrated polyps: Implications for proximal colon cancer. Am. J. Gastroenterol. 2012, 107, 1213–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejea, C.M.; Sears, C.L. Do biofilms confer a pro-carcinogenic state? Gut Microbes 2016, 7, 54–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Powell, J.; Mathioudakis, N.; Kane, S.; Fernandez, E.; Sears, C.L. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect. Immun. 2004, 72, 5832–5839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Trapani, K.M.; Boghossian, L.J.; Caskey, E. Clostridium subterminale Septicemia in a Patient with Metastatic Gastrointestinal Adenocarcinoma. Case Rep. Infect. Dis. 2018, 2018, 6031510. [Google Scholar] [CrossRef] [Green Version]
- Dahmus, J.D.; Kotler, D.L.; Kastenberg, D.M.; Kistler, C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. J. Gastrointest. Oncol. 2018, 9, 769–777. [Google Scholar] [CrossRef]
- Doorakkers, E.; Lagergren, J.; Engstrand, L.; Brusselaers, N. Eradication of Helicobacter pylori and Gastric Cancer: A Systematic Review and Meta-analysis of Cohort Studies. J. Natl. Cancer Inst. 2016, 108, djw132. [Google Scholar] [CrossRef] [Green Version]
- Sato, F.; Meltzer, S.J. CpG island hypermethylation in progression of esophageal and gastric cancer. Cancer 2006, 106, 483–493. [Google Scholar] [CrossRef]
- Sitaraman, R. Helicobacter pylori DNA methyltransferases and the epigenetic field effect in cancerization. Front. Microbiol. 2014, 5, 115. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Sarrias, A.; Gimenez-Bastida, J.A.; Nunez-Sanchez, M.A.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J. Agric. Food Chem. 2010, 58, 3965–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grat, M.; Wronka, K.M.; Krasnodebski, M.; Masior, L.; Lewandowski, Z.; Kosinska, I.; Grat, K.; Stypulkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis. Transpl. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Feng, Y.; Theve, E.J.; Raczynski, A.R.; Fiala, J.L.; Doernte, A.L.; Williams, M.; McFaline, J.L.; Essigmann, J.M.; Schauer, D.B.; et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010, 59, 88–97. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Wormann, S.M.; Diakopoulos, K.N.; Lesina, M.; Algul, H. The immune network in pancreatic cancer development and progression. Oncogene 2014, 33, 2956–2967. [Google Scholar] [CrossRef] [Green Version]
- Michaud, D.S.; Izard, J. Microbiota oral microbiome, and pancreatic cancer. Cancer J. 2014, 20, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Ghannoum, M.; Gallogly, M.; de Lima, M.; Malek, E. Influence of gut microbiome on multiple myeloma: Friend or foe? J. ImmunoTherapy Cancer 2020, 8, e000576. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Dinh, P.; He, K. Association of Iodine and Iron with Thyroid Function. Biol. Trace Elem. Res. 2017, 179, 38–44. [Google Scholar] [CrossRef]
- Kunc, M.; Gabrych, A.; Witkowski, J.M. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochim. Pol. 2016, 63, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X.; et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonklaas, J.; Danielsen, M.; Wang, H. A pilot study of serum selenium, vitamin D, and thyrotropin concentrations in patients with thyroid cancer. Thyroid 2013, 23, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, X.; Li, H.; Li, X.; Lin, Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PLoS ONE 2017, 12, e0179664. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Shi, Y.; Liang, B.; Cai, H.; Cai, Q. Iodinated TG in Thyroid Follicles Regulate TSH/TSHR Signaling for NIS Expression. Biol. Trace Elem. Res. 2017, 180, 206–213. [Google Scholar] [CrossRef]
- Samimi, H.; Haghpanah, V. Gut Microbiome and Radioiodine-Refractory Papillary Thyroid Carcinoma Pathophysiology. Trends Endocrinol. Metab. 2020, 31, 627–630. [Google Scholar] [CrossRef]
- Bizhanova, A.; Kopp, P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology 2009, 150, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.T.; Zhang, Y.; Liu, Y.M.; Yin, S.; Zhang, X.Y.; Wei, W.J.; Sun, Z.K.; Song, H.J.; Qiu, Z.L.; Wang, C.R.; et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin. Endocrinol. 2017, 87, 844–852. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2019, 144, 2728–2745. [Google Scholar] [CrossRef]
- Liu, C.J.; Chen, S.Q.; Zhang, S.Y.; Wang, J.L.; Tang, X.D.; Yang, K.X.; Li, X.R. The comparison of microbial communities in thyroid tissues from thyroid carcinoma patients. J. Microbiol. 2021, 59, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, T.; Gao, X.; Yan, H.; Chen, J.; Huang, M.; Wang, L.; Yin, D.; Li, H.; Ma, R.; et al. Gut Microbiome Alterations in Patients With Thyroid Nodules. Front. Cell Infect. Microbiol. 2021, 11, 643968. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, A.; Castaneda, G.; Iyangar, A.; Magesh, S.; Perez, D.; Chakladar, J.; Li, W.T.; Bouvet, M.; Chang, E.Y.; Ongkeko, W.M. The intratumor microbiome predicts prognosis across gender and subtypes in papillary thyroid carcinoma. Comput. Struct. Biotechnol. J. 2021, 19, 1986–1997. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Gastrointes. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, M. Microbiome. Microbes aid cancer drugs. Science 2015, 350, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Grenda, A.; Krawczyk, P. Cancer trigger or remedy: Two faces of the human microbiome. Appl. Microbiol. Biotechnol. 2021, 105, 1395–1405. [Google Scholar] [CrossRef]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef]
- Koulouridi, A.; Messaritakis, I.; Gouvas, N.; Tsiaoussis, J.; Souglakos, J. Immunotherapy in Solid Tumors and Gut Microbiota: The Correlation-A Special Reference to Colorectal Cancer. Cancers 2020, 13, 43. [Google Scholar] [CrossRef]
- Huang, C.; Li, M.; Liu, B.; Zhu, H.; Dai, Q.; Fan, X.; Mehta, K.; Huang, C.; Neupane, P.; Wang, F.; et al. Relating Gut Microbiome and Its Modulating Factors to Immunotherapy in Solid Tumors: A Systematic Review. Front. Oncol. 2021, 11, 642110. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.D.; Hong, H.; Ahmad, S.; Holloway, R.W. The gut microbiome and cancer immunotherapeutics: A review of emerging data and implications for future gynecologic cancer research. Crit. Rev. Oncol. Hematol. 2021, 157, 103165. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Nandi, D.; Nag, A. The pint-sized powerhouse: Illuminating the mighty role of the gut microbiome in improving the outcome of anti-cancer therapy. Semin. Cancer Biol. 2021, 70, 98–111. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Liu, X.; Min, J.J.; Tan, W.; Zheng, J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020, 469, 102–110. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56 (bright) perforin (low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, A.; Yan, H.; Ohashi, S.; Gotoh, Y.; Sato, A.; Tsutsui, H.; Kaisho, T.; Toda, T.; Tsuji, N.M. Lactococcus lactis subsp. cremoris FC triggers IFN-gamma production from NK and T cells via IL-12 and IL-18. Int. Immunopharmacol. 2012, 14, 729–733. [Google Scholar] [CrossRef]

| Samples | Microbes Associated | Roles | Mechanisms | Ref. |
|---|---|---|---|---|
| TC vs. Peritumor tissue | Sphingomonas and Aeromonas ↑ in TC Comamonas, Acinetobacter, and Peptostreptococcus ↑ in Peritumor Sphingomonas ↑ in N1-stage of TC | Distinguish tumor and peritumor tissues Sphingomonas is a marker of lymph node metastasis | N/A | [6] |
| HH vs. Healthy control | Bifidobacterium and Lactobacillus↓ in HH Enterococcus ↑ in HH | N/A | N/A | [13] |
| TC vs. Healthy control | Proteobacteria ↑ in TC | N/A | Decline in aminoacyl—tRNA biosynthesis, homologous recombination, mismatch repair, DNA replication, and nucleotide excision repair | [22] |
| GD vs. Healthy control | Prevotellaceae and Pasteurellaceae ↑ in GD Enterobacteriaceae, Veillonellaceae and Rikenellaceae↓ in GD | N/A | N/A | [51] |
| HT vs. Healthy control | Bacteroides ↑ and Bifidobacterium↓ in HT | Zonulin ↑ Alterations in the microbiota and intestinal permeability | N/A | [52] |
| HM vs. Healthy control | Veillonella, Paraprevotella, Neisseria, and Rheinheimera↓ in HM | Positively associated with FT3 and FT4, and negatively associated with TSH | Increased serum LPS levels | [53] |
| TC, TN vs. Healthy control | Neisseria ↑ and Streptococcus ↑ in TC and TN Butyricimonas↓ and Lactobacillus↓ in TC and TN | Identify thyroid nodules and thyroid cancer | N/A | [82] |
| TC vs. Healthy control | Enrichment of 19 and depletion of 8 genera in TC | Lipoprotein A ↑ and apolipoprotein B ↑ | Necroptosis Glycerolipid metabolism Fc-gamma R-mediated phagocytosis | [90] |
| TC vs. Peritumor tissue | Proteobacteria ↑ in TC Firmicutes ↑ in stool of TC | Closely related to TSH and T3 | Pyruvate, fatty acid metabolism and glycolysis or gluconeogenesis | [91] |
| TN vs. Healthy control | Multiple butyrate producing microbes↓ | Greater amino acid degradation and lower butyrate production | L-histidine metabolism | [92] |
| TC in male vs. female | Micrococcus luteus ↑ in TC Chroococcidiopsis sp. ↑ in female M. luteus and Bradyrhizobium sp. BTAi1 ↑ in male | Highly correlated with immune-associated genes Strong positive correlation to MACIS score | DNA checkpoint and damage-related group BRAF V600E mutation p53 instability | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Sun, W.; Zhang, H. Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer. Cancers 2022, 14, 2656. https://doi.org/10.3390/cancers14112656
Liu Q, Sun W, Zhang H. Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer. Cancers. 2022; 14(11):2656. https://doi.org/10.3390/cancers14112656
Chicago/Turabian StyleLiu, Qi, Wei Sun, and Hao Zhang. 2022. "Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer" Cancers 14, no. 11: 2656. https://doi.org/10.3390/cancers14112656
APA StyleLiu, Q., Sun, W., & Zhang, H. (2022). Interaction of Gut Microbiota with Endocrine Homeostasis and Thyroid Cancer. Cancers, 14(11), 2656. https://doi.org/10.3390/cancers14112656




