Expression Profile and Diagnostic Significance of MicroRNAs in Papillary Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Detection of the miRNA Profiles
2.3. Validation of the NanoString Results by Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
2.4. Data Analysis
2.5. miRNA Target Prediction and Functional Annotation of the Selected miRNA Targets
3. Results
3.1. miRNA Profile in Papillary Thyroid Cancer
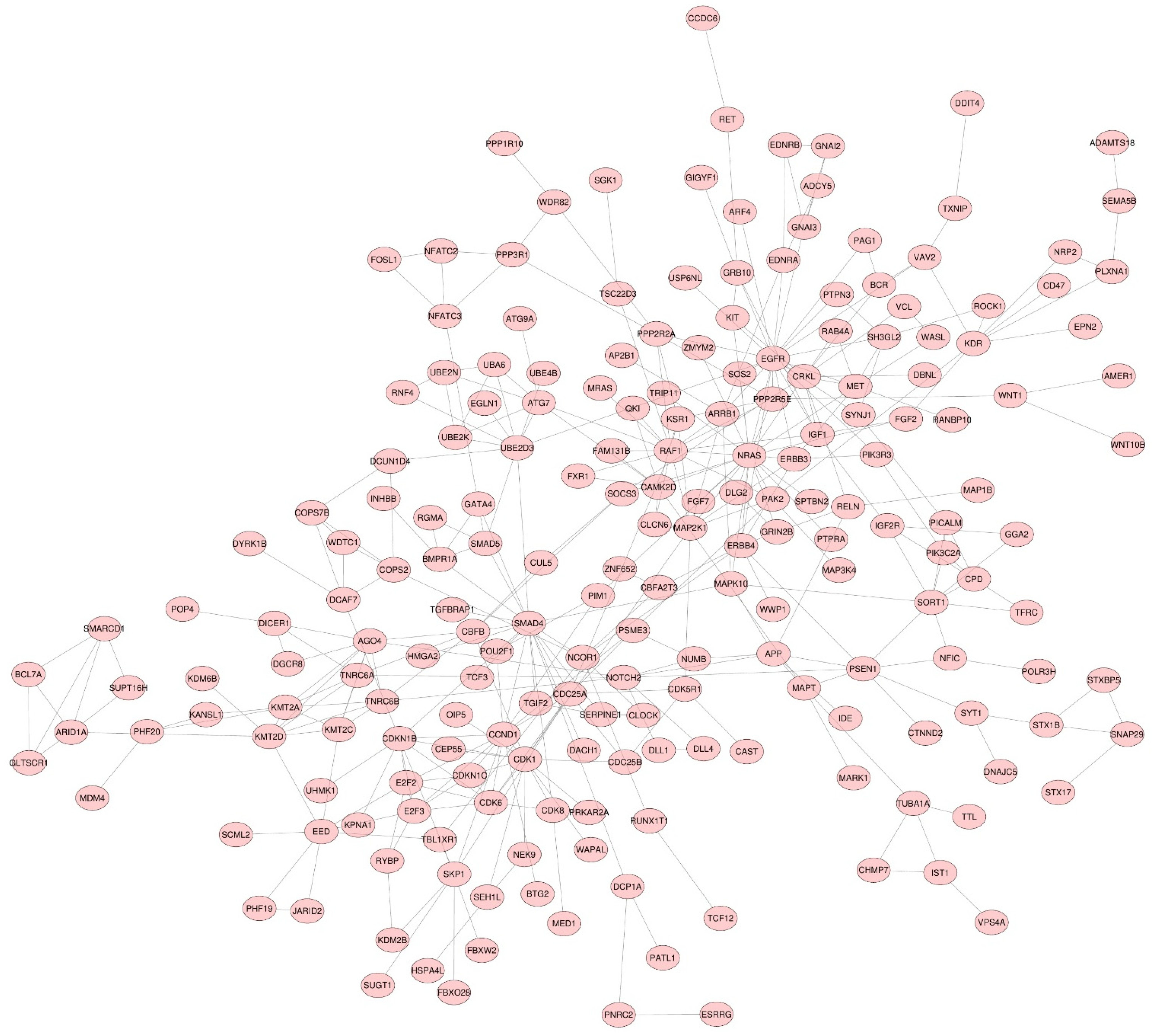
3.2. miRNA Target Genes
3.3. Functional Enrichment Analysis
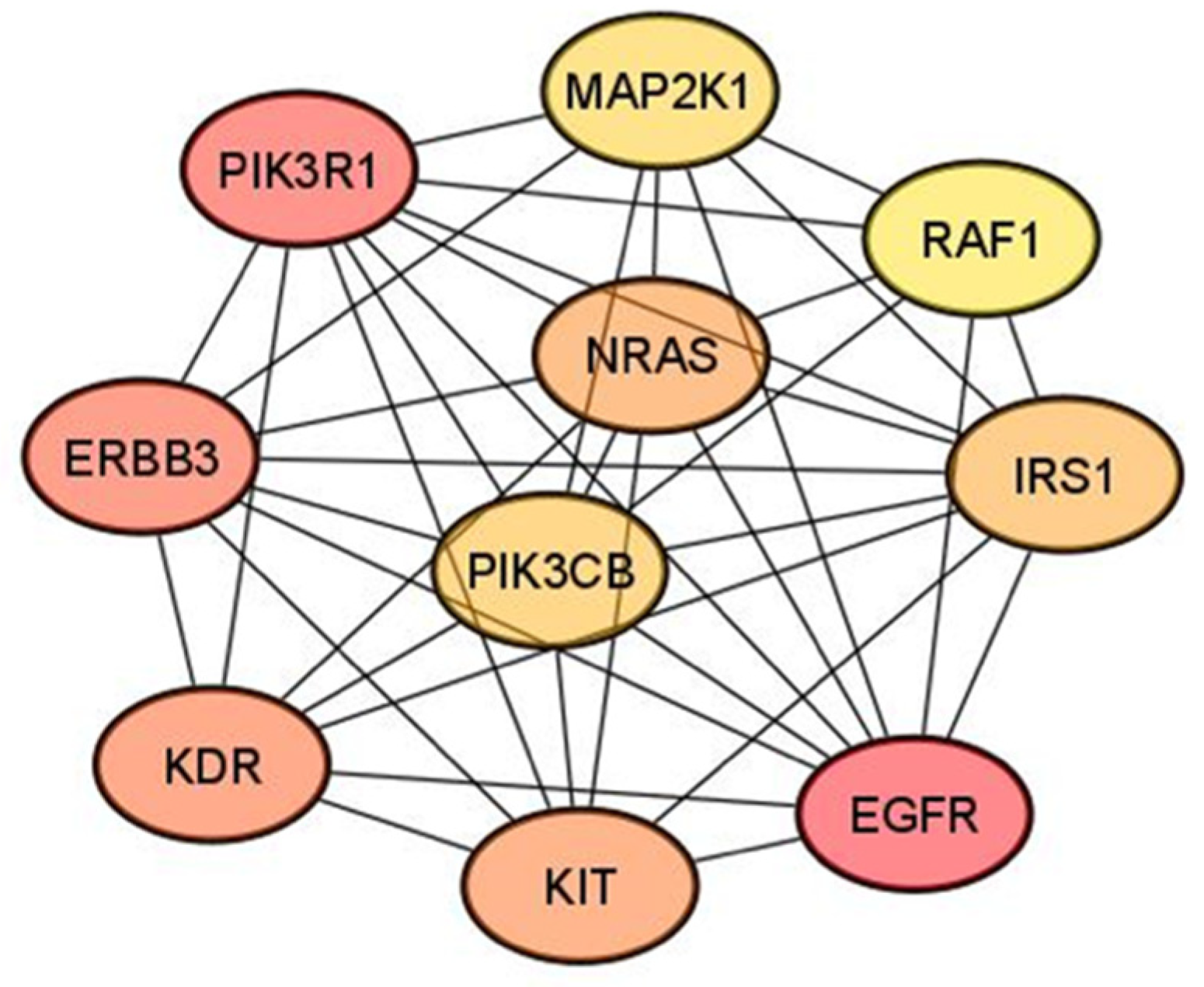
3.4. Hub Gene Identification
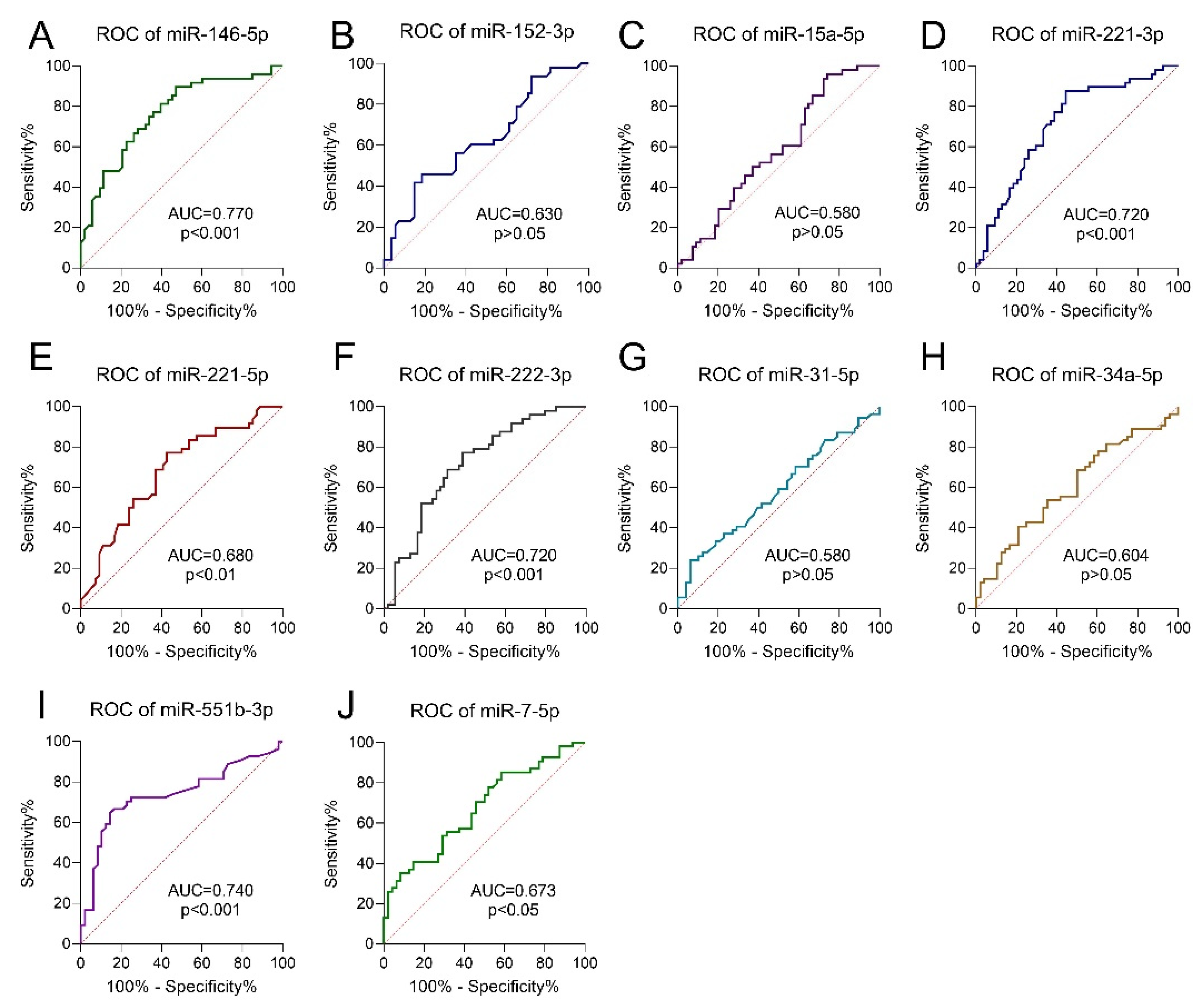
3.5. Receiver Operating Characteristic Curve Analysis
3.6. Logistic Regression Model
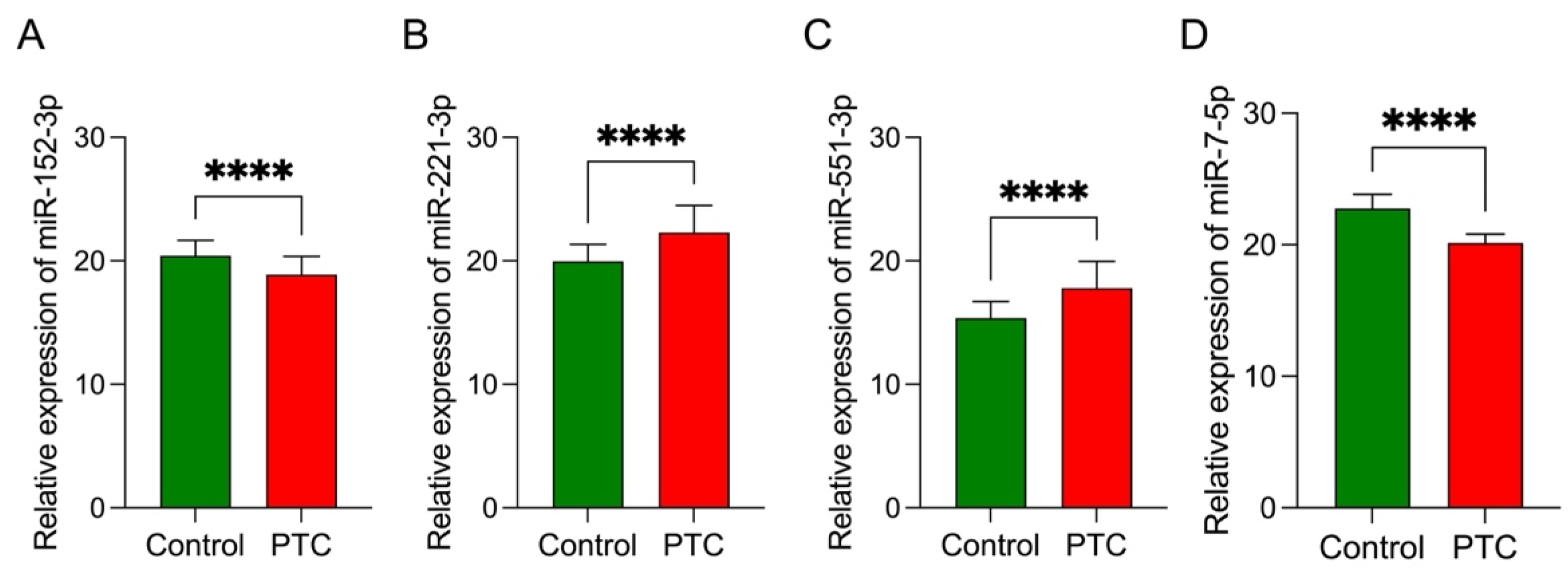
3.7. Data Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, L.; Morris, L.G.; Haymart, M.; Chen, A.Y.; Goldenberg, D.; Morris, J.; Ogilvie, J.B.; Terris, D.J.; Netterville, J.; Wong, R.J.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocr. Pract. 2015, 21, 686–696. [Google Scholar] [CrossRef] [Green Version]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 2307. [Google Scholar] [CrossRef] [Green Version]
- Grogan, R.H.; Kaplan, S.P.; Cao, H.; Weiss, R.E.; Degroot, L.J.; Simon, C.A.; Embia, O.M.; Angelos, P.; Kaplan, E.L.; Schechter, R.B. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 2013, 154, 1436–1446, discussion 1446–1437. [Google Scholar] [CrossRef]
- Sun, J.-H.; Li, Y.-R.; Chang, K.-H.; Liou, M.-J.; Lin, S.-F.; Tsai, S.-S.; Yu, M.-C.; Hsueh, C.; Chen, S.-T. Evaluation of recurrence risk in patients with papillary thyroid cancer through tumor-node-metastasis staging: A single-center observational study in Taiwan. Biomed. J. 2021. [Google Scholar] [CrossRef]
- Sippel, R.S.; Chen, H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid 2009, 19, 1373–1380. [Google Scholar] [CrossRef]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Gimm, O.; Ivansson, K.; Beka, E.; Rossitti, H.M.; Garvin, S.; Söderkvist, P. Increased diagnostic sensitivity of palpation-guided thyroid nodule fine-needle aspiration cytology by BRAF V600E-mutation analysis. J. Pathol. Clin. Res. 2021, 7, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Feletti, F.; Mellini, L.; Pironi, F.; Carnevale, A.; Parenti, G.C. Role of the cytopathologist during the procedure of fine-needle aspiration biopsy of thyroid nodules. Insights Imaging 2021, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, D.-h.; He, X.-m.; Deng, X.; Ji, Y.-c.; Yang, H.; Cheng, L.; Zhou, X.-y. Aberrant expression of five miRNAs in papillary thyroid carcinomas. J. Clin. Lab. Anal. 2021, 35, e23907. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, M.; Wang, Y.; Gong, B.; Zhuang, T.; Liang, L.; Qiao, H. Identification of Biomarkers Based on Differentially Expressed Genes in Papillary Thyroid Carcinoma. Sci. Rep. 2018, 8, 9912. [Google Scholar] [CrossRef] [PubMed]
- Rogucki, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 2021, 10, 4738. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, S.; Liu, Y.; Wang, F.; Liu, Y.; Xiao, H. Expression of miRNAs in Papillary Thyroid Carcinomas Is Associated with BRAF Mutation and Clinicopathological Features in Chinese Patients. Int. J. Endocrinol. 2013, 2013, 128735. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Wang, Y.; Chen, L.; Zheng, J.; Li, J.; Wu, X. MiR-221, a potential prognostic biomarker for recurrence in papillary thyroid cancer. World J. Surg. Oncol. 2017, 15, 11. [Google Scholar] [CrossRef] [Green Version]
- Sondermann, A.; Andreghetto, F.M.; Moulatlet, A.C.; da Silva Victor, E.; de Castro, M.G.; Nunes, F.D.; Brandão, L.G.; Severino, P. MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin. Exp. Metastasis 2015, 32, 521–530. [Google Scholar] [CrossRef]
- Hitu, L.; Gabora, K.; Bonci, E.-A.; Piciu, A.; Hitu, A.-C.; Ștefan, P.-A.; Piciu, D. MicroRNA in Papillary Thyroid Carcinoma: A Systematic Review from 2018 to June 2020. Cancers 2020, 12, 3118. [Google Scholar] [CrossRef]
- Fugazzola, L.; Muzza, M.; Pogliaghi, G.; Vitale, M. Intratumoral Genetic Heterogeneity in Papillary Thyroid Cancer: Occurrence and Clinical Significance. Cancers 2020, 12, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, K.; Kang, W.; Yu, J.; Wang, X.; Zhang, Z.; Sun, J.; Ouyang, S. Changing patterns of thyroid cancer in different stages of Universal Salt Iodization in Peking Union Medical College Hospital, 1986-2018. Gland Surg. 2020, 9, 1338–1345. [Google Scholar] [CrossRef]
- Glynn, N.; Hannon, M.J.; Lewis, S.; Hillery, P.; Al-Mousa, M.; Hill, A.D.; Keeling, F.; Morrin, M.; Thompson, C.J.; Smith, D.; et al. Utility of repeat cytological assessment of thyroid nodules initially classified as benign: Clinical insights from multidisciplinary care in an Irish tertiary referral centre. BMC Endocr. Disord. 2016, 16, 45. [Google Scholar] [CrossRef] [Green Version]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018, 319, 914–924. [Google Scholar] [CrossRef]
- Ito, Y.; Hirokawa, M. Complications of Fine Needle Aspiration Biopsy. In Thyroid FNA Cytology: Differential Diagnoses and Pitfalls; Kakudo, K., Ed.; Springer: Singapore, 2019; pp. 425–428. [Google Scholar]
- Anderson, T.J.T.; Atalay, M.K.; Grand, D.J.; Baird, G.L.; Cronan, J.J.; Beland, M.D. Management of Nodules with Initially Nondiagnostic Results of Thyroid Fine-Needle Aspiration: Can We Avoid Repeat Biopsy? Radiology 2014, 272, 777–784. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Farina, N.H.; Wood, M.E.; Perrapato, S.D.; Francklyn, C.S.; Stein, G.S.; Stein, J.L.; Lian, J.B. Standardizing analysis of circulating microRNA: Clinical and biological relevance. J. Cell. Biochem. 2014, 115, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiselet, M.; Gacquer, D.; Spinette, A.; Craciun, L.; Decaussin-Petrucci, M.; Andry, G.; Detours, V.; Maenhaut, C. New global analysis of the microRNA transcriptome of primary tumors and lymph node metastases of papillary thyroid cancer. BMC Genom. 2015, 16, 828. [Google Scholar] [CrossRef] [PubMed]
- Rosignolo, F.; Memeo, L.; Monzani, F.; Colarossi, C.; Pecce, V.; Verrienti, A.; Durante, C.; Grani, G.; Lamartina, L.; Forte, S.; et al. MicroRNA-based molecular classification of papillary thyroid carcinoma. Int. J. Oncol. 2017, 50, 1767–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galuppini, F.; Censi, S.; Merante Boschin, I.; Fassan, M.; Sbaraglia, M.; Valeri, N.; Hahne, J.C.; Bertazza, L.; Munari, G.; Galasso, M.; et al. Papillary Thyroid Carcinoma: Molecular Distinction by MicroRNA Profiling. Front. Endocrinol. 2022, 13, 834075. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.; Chorti, A.G.; Chatzikyriakidou, A.; Giannoulis, K.; Bakkar, S.; Papavramidis, T.S. MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 2022, 11, 755097. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Laukiene, R.; Jakubkevicius, V.; Ambrozaityte, L.; Cimbalistiene, L.; Utkus, A. Dysregulation of microRNAs as the risk factor of lymph node metastasis in papillary thyroid carcinoma: Systematic review. Endokrynol. Pol. 2021, 72, 145–152. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010, 14, 236–240. [Google Scholar]
- Li, D.; Jian, W.; Wei, C.; Song, H.; Gu, Y.; Luo, Y.; Fang, L. Down-regulation of miR-181b promotes apoptosis by targeting CYLD in thyroid papillary cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7672–7680. [Google Scholar]
- Xiang, D.; Tian, B.; Yang, T.; Li, Z. miR-222 expression is correlated with the ATA risk stratifications in papillary thyroid carcinomas. Medicine 2019, 98, e16050. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, H.; Wang, J.; Sun, C. miR-146a and miR-146b in the diagnosis and prognosis of papillary thyroid carcinoma. Oncol. Rep. 2017, 38, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.L.; Gao, A.B.; Wang, Q.; Lou, X.E.; Zhao, J.; Lu, Q.J. MicroRNA-221 promotes papillary thyroid carcinoma cell migration and invasion via targeting RECK and regulating epithelial-mesenchymal transition. Onco Targets Ther. 2019, 12, 2323–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, M.L.C.; Beasley, N.J.P.; Ezzat, S.; Freeman, J.L.; Asa, S.L. Overexpression of Cyclin D1 and Underexpression of p27 Predict Lymph Node Metastases in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2002, 87, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, D.; Keutgen, X.M.; Crowley, M.; Filicori, F.; Aldailami, H.; Hoda, R.; Liu, Y.-F.; Hoda, R.S.; Scognamiglio, T.; Jin, M.; et al. Intercellular Adhesion Molecule-1 (ICAM-1) is Upregulated in Aggressive Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 2012, 19, 973–980. [Google Scholar] [CrossRef]
- Park, J.L.; Kim, S.K.; Jeon, S.; Jung, C.K.; Kim, Y.S. MicroRNA Profile for Diagnostic and Prognostic Biomarkers in Thyroid Cancer. Cancers 2021, 13, 632. [Google Scholar] [CrossRef]
- Mei, Z.; Zhou, L.; Zhu, Y.; Jie, K.; Fan, D.; Chen, J.; Liu, X.; Jiang, L.; Jia, Q.; Li, W. Interleukin-22 promotes papillary thyroid cancer cell migration and invasion through microRNA-595/Sox17 axis. Tumour. Biol. 2016, 37, 11753–11762. [Google Scholar] [CrossRef]
- Perdas, E.; Stawski, R.; Kaczka, K.; Zubrzycka, M. Analysis of Let-7 Family miRNA in Plasma as Potential Predictive Biomarkers of Diagnosis for Papillary Thyroid Cancer. Diagnostics 2020, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Geraldo, M.V.; Fuziwara, C.S.; Friguglieti, C.U.M.; Costa, R.B.; Kulcsar, M.A.V.; Yamashita, A.S.; Kimura, E.T. MicroRNAs miR-146-5p and let-7f as prognostic tools for aggressive papillary thyroid carcinoma: A case report. Arq. Bras. Endocrinol. Metabol. 2012, 56, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Jia, W.; Ni, S. miR-199a-5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochem. Biophys. Res. Commun. 2018, 497, 181–186. [Google Scholar] [CrossRef]
- Dong, S.; Xue, S.; Sun, Y.; Han, Z.; Sun, L.; Xu, J.; Liu, J. MicroRNA-363-3p downregulation in papillary thyroid cancer inhibits tumor progression by targeting NOB1. J. Investig. Med. 2021, 69, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-P.; Tang, X.-Z.; Liang, Q.-K.; Zeng, X.-J.; Yang, J.-B.; Xu, J. microRNA-599 promotes apoptosis and represses proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells via downregulation of Hey2-depentent Notch signaling pathway. J. Cell. Physiol. 2020, 235, 2492–2505. [Google Scholar] [CrossRef]
- Reda El Sayed, S.; Cristante, J.; Guyon, L.; Denis, J.; Chabre, O.; Cherradi, N. MicroRNA Therapeutics in Cancer: Current Advances and Challenges. Cancers 2021, 13, 2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, D.; Pan, J.; Yang, Z.; Chen, M.; Han, J.; Zhang, S.; Sun, L.; Qiao, H. Dynamic monitoring of circulating microRNAs as a predictive biomarker for the diagnosis and recurrence of papillary thyroid carcinoma. Oncol. Lett. 2017, 13, 4252–4266. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H. MicroRNA as a diagnostic tool in fine-needle aspiration biopsy of thyroid nodules. Oncologist 2012, 17, 1032–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.; Gundara, J.S.; Glover, A.; Serpell, J.; Sidhu, S.B. MicroRNA expression profiles in the management of papillary thyroid cancer. Oncologist 2014, 19, 1141–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, C.R.; Geraldo, M.V.; Fuziwara, C.S.; Kimura, E.T.; Santos, M.F. MiRNA-146b-5p upregulates migration and invasion of different Papillary Thyroid Carcinoma cells. BMC Cancer 2016, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Wu, B.; Xiao, K.; Kang, J.; Xie, J.; Zhang, X.; Fan, Y. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell. Physiol. Biochem. 2015, 35, 71–82. [Google Scholar] [CrossRef]
- Ye, T.; Zhong, L.; Ye, X.; Liu, J.; Li, L.; Yi, H. miR-221-3p and miR-222-3p regulate the SOCS3/STAT3 signaling pathway to downregulate the expression of NIS and reduce radiosensitivity in thyroid cancer. Exp. Ther. Med. 2021, 21, 652. [Google Scholar] [CrossRef]
- Kalfert, D.; Ludvikova, M.; Pesta, M.; Ludvik, J.; Dostalova, L.; Kholová, I. Multifunctional Roles of miR-34a in Cancer: A Review with the Emphasis on Head and Neck Squamous Cell Carcinoma and Thyroid Cancer with Clinical Implications. Diagnostics 2020, 10, 563. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Feng, J.; Liu, X.; Tian, Y.; Zhao, L.; Xie, R.; Liu, C.; Zhao, S.; Sun, H. Clinical significance of miR-34a expression in thyroid diseases-An (18)F-FDG PET-CT study. Cancer Manag. Res. 2017, 9, 903–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.Y.; Liu, Y.; Wang, M.L.; Guo, M.; Wang, Y.; Cheng, Z.F. Construction and analyses of the microRNA-target gene differential regulatory network in thyroid carcinoma. PLoS ONE 2017, 12, e0178331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Cai, Y.; Zhou, Y.; Jiang, J.; Ning, Y.; Shui, C.; Sun, R.; Wang, Y.; Li, C. Circ-NUP214 Promotes Papillary Thyroid Carcinoma Tumorigenesis by Regulating HK2 Expression Through miR-15a-5p. Biochem. Genet. 2022. [Google Scholar] [CrossRef] [PubMed]
- Augenlicht, A.; Saiselet, M.; Decaussin-Petrucci, M.; Andry, G.; Dumont, J.E.; Maenhaut, C. MiR-7-5p inhibits thyroid cell proliferation by targeting the EGFR/MAPK and IRS2/PI3K signaling pathways. Oncotarget 2021, 12, 1587–1599. [Google Scholar] [CrossRef]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. microRNAs in cancer management. Lancet Oncol. 2012, 13, e249–e258. [Google Scholar] [CrossRef]
- Bu, R.; Siraj, A.K.; Masoodi, T.; Parvathareddy, S.K.; Iqbal, K.; Al-Rasheed, M.; Haqawi, W.; Diaz, M.; Victoria, I.G.; Aldughaither, S.M.; et al. Recurrent Somatic MAP2K1 Mutations in Papillary Thyroid Cancer and Colorectal Cancer. Front. Oncol. 2021, 11, 670423. [Google Scholar] [CrossRef]
- Castro, L.; Alves, S.; Chaves, S.R.; Costa, J.L.; Soares, P.; Preto, A. RAF-1 promotes survival of thyroid cancer cells harboring RET/PTC1 rearrangement independently of ERK activation. Mol. Cell. Endocrinol. 2015, 415, 64–75. [Google Scholar] [CrossRef]
- Li, G.; Kong, Q. LncRNA LINC00460 promotes the papillary thyroid cancer progression by regulating the LINC00460/miR-485-5p/Raf1 axis. Biol. Res. 2019, 52, 61. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hao, S.A.; Jiang, Y.; Gao, B.; Tian, W.G.; Zhang, S.; Guo, L.J.; Wang, L.L.; Luo, D.L. MicroRNA-1271 inhibits the progression of papillary thyroid carcinoma by targeting IRS1 and inactivating AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7989–7999. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.K.; Dong, J.; Xie, J.; Xing, M. Uncommon GNAQ, MMP8, AKT3, EGFR, and PIK3R1 mutations in thyroid cancers. Endocr. Pathol. 2011, 22, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Qin, H.; Cui, Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem. Biophys. Res. Commun. 2013, 441, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Masago, K.; Asato, R.; Fujita, S.; Hirano, S.; Tamura, Y.; Kanda, T.; Mio, T.; Katakami, N.; Mishima, M.; Ito, J. Epidermal growth factor receptor gene mutations in papillary thyroid carcinoma. Int. J. Cancer 2009, 124, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, X.; Yin, Y.; Lv, Z.; Jia, C.; Liao, Y.; Sun, H.; Liu, T.; Cong, L.; Fei, Z.; et al. Identification of Potential BRAF Inhibitor Joint Therapy Targets in PTC based on WGCAN and DCGA. J. Cancer 2021, 12, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sousa, L.P.; Mandel-Bausch, E.M.; Tome, F.; Reshetnyak, A.V.; Hadari, Y.; Schlessinger, J.; Lax, I. Distinct cellular properties of oncogenic KIT receptor tyrosine kinase mutants enable alternative courses of cancer cell inhibition. Proc. Natl. Acad. Sci. USA 2016, 113, E4784–E4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Jazdzewski, K.; Li, W.; Liyanarachchi, S.; Nagy, R.; Volinia, S.; Calin, G.A.; Liu, C.G.; Franssila, K.; Suster, S.; et al. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 19075–19080. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, Y.J.; Shi, Y.; Wu, T. NRP-1 and KDR polymorphisms are associated with survival time in patients with advanced gastric cancer. Oncol. Lett. 2019, 18, 4629–4638. [Google Scholar] [CrossRef]
- Fakhruddin, N.; Jabbour, M.; Novy, M.; Tamim, H.; Bahmad, H.; Farhat, F.; Zaatari, G.; Aridi, T.; Kriegshauser, G.; Oberkanins, C.; et al. BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases. Sci. Rep. 2017, 7, 4666. [Google Scholar] [CrossRef] [Green Version]
- Faustino, A.; Couto, J.P.; Pópulo, H.; Rocha, A.S.; Pardal, F.; Cameselle-Teijeiro, J.M.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P. mTOR pathway overactivation in BRAF mutated papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, E1139–E1149. [Google Scholar] [CrossRef]
- Tavares, C.; Eloy, C.; Melo, M.; Gaspar da Rocha, A.; Pestana, A.; Batista, R.; Bueno Ferreira, L.; Rios, E.; Sobrinho Simões, M.; Soares, P. mTOR Pathway in Papillary Thyroid Carcinoma: Different Contributions of mTORC1 and mTORC2 Complexes for Tumor Behavior and SLC5A5 mRNA Expression. Int. J. Mol. Sci. 2018, 19, 1448. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Hussain, A.R.; Bavi, P.; Ahmed, S.O.; AlSobhi, S.S.; Al-Dayel, F.; Uddin, S.; Al-Kuraya, K.S. High prevalence of mTOR complex activity can be targeted using Torin2 in papillary thyroid carcinoma. Carcinogenesis 2014, 35, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.K.; Williams, W.K.; Pascuzzi, P.E.; Balanis, N.G.; Schiemann, B.J.; Carlin, C.R.; Schiemann, W.P. The antitumorigenic function of EGFR in metastatic breast cancer is regulated by expression of Mig6. Neoplasia 2015, 17, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers (Basel) 2021, 13, 2748. [Google Scholar] [CrossRef]
- Ma, J.; Lyu, H.; Huang, J.; Liu, B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol. Cancer 2014, 13, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Gupta, P.; Parashar, D.; Nair, G.G.; George, J.; Geethadevi, A.; Wang, W.; Tsaih, S.-W.; Bradley, W.; Ramchandran, R.; et al. ERBB3-induced furin promotes the progression and metastasis of ovarian cancer via the IGF1R/STAT3 signaling axis. Oncogene 2020, 39, 2921–2933. [Google Scholar] [CrossRef]
- Baraldi, J.H.; Martyn, G.V.; Shurin, G.V.; Shurin, M.R. Tumor Innervation: History, Methodologies, and Significance. Cancers 2022, 14, 1979. [Google Scholar] [CrossRef]
- Restaino, A.C.; Vermeer, P.D. Neural regulations of the tumor microenvironment. FASEB BioAdvances 2022, 4, 29–42. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Peng, W.; Jiang, P.; Deng, Z.; Wu, C. MiR-7-5pand miR-451 as diagnostic biomarkers for papillary thyroid carcinoma in formalin-fixed paraffin-embedded tissues. Pharmazie 2020, 75, 266–270. [Google Scholar] [CrossRef]
- Chou, C.K.; Liu, R.T.; Kang, H.Y. MicroRNA-146b: A Novel Biomarker and Therapeutic Target for Human Papillary Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 636. [Google Scholar] [CrossRef]
- Cai, S.; Ma, J.; Wang, Y.; Cai, Y.; Xie, L.; Chen, X.; Yang, Y.; Peng, Q. Biomarker Value of miR-221 and miR-222 as Potential Substrates in the Differential Diagnosis of Papillary Thyroid Cancer Based on Data Synthesis and Bioinformatics Approach. Front. Endocrinol. 2022, 12, 794490. [Google Scholar] [CrossRef]

| Characteristic | Samples of PTC |
|---|---|
| Total | 41 |
| Men/women | 8/33 |
| pT1 | 31 |
| pT2 | 5 |
| pT3 | 2 |
| pT4 | 3 |
| Men: Age at diagnosis—mean (range) | 59.7 (41–77) |
| Women: Age at diagnosis—mean (range) | 53.1 (30–77) |
| Diameter of the tumor—mean (mm) | 10.5 |
| Number of samples with features of angioinvasion | 12 |
| Number of samples with multifocal features | 27 |
| miRNA | FC | p-Value | FDR |
|---|---|---|---|
| miR-146b-5p | 6.62 | 0.00000001 | 0.00 |
| miR-221-3p | 3.23 | 0.00000001 | 0.00 |
| miR-221-5p | 2.36 | 0.00000016 | 0.00 |
| miR-222-3p | 2.94 | 0.00000001 | 0.00 |
| miR-34a-5p | 1.65 | 0.00002568 | 0.00 |
| miR-551b-3p | 2.63 | 0.00000003 | 0.00 |
| miR-152-3p | −1.53 | 0.00040006 | 0.01 |
| miR-15a-5p | 1.57 | 0.00031352 | 0.01 |
| miR-31-5p | 1.60 | 0.00051338 | 0.02 |
| miR-7-5p | −2.57 | 0.00108612 | 0.03 |
| Hub Gene | DEMs Targeting Hub Gene |
|---|---|
| MAP2K1 | miR-152-3p; miR-15a-5p; miR-34a-5p |
| RAF1 | miR-15a-5p; miR-7-5p |
| IRS1 | miR-15a-5p; miR-7-5p |
| EGFR | miR-152-3p; miR-221-3p; miR-222-3p; miR-7-5p |
| KIT | miR-152-3p; miR-221-3p; miR-222-3p |
| PIK3CB | miR-146b-5p; miR-7-5p |
| NRAS | miR-146b-5p; miR-152-3p |
| KDR | miR-15a-5p; miR-221-3p; miR-222-3p |
| ERBB3 | miR-152-3p; miR-221-3p; miR-222-3p |
| PIK3R1 | miR-15a-5p; miR-221-3p; miR-222-3p |
| Model | TP Rate | FP Rate | Precision | AUC | Intercept | Coefficient |
|---|---|---|---|---|---|---|
| x1 = miR-152-3p | 0.769 | 0.227 | 0.774 | 0.841 | −1.8033 | a1 = −1.2104 |
| x2 = miR-221-3p | a2 = 0.8173 | |||||
| x3 = miR-551b-3p | a3 = 0.5172 | |||||
| x4 = miR-7-5p | a4 = −0.0178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogucki, M.; Sidorkiewicz, I.; Niemira, M.; Dzięcioł, J.B.; Buczyńska, A.; Adamska, A.; Siewko, K.; Kościuszko, M.; Maliszewska, K.; Wójcicka, A.; et al. Expression Profile and Diagnostic Significance of MicroRNAs in Papillary Thyroid Cancer. Cancers 2022, 14, 2679. https://doi.org/10.3390/cancers14112679
Rogucki M, Sidorkiewicz I, Niemira M, Dzięcioł JB, Buczyńska A, Adamska A, Siewko K, Kościuszko M, Maliszewska K, Wójcicka A, et al. Expression Profile and Diagnostic Significance of MicroRNAs in Papillary Thyroid Cancer. Cancers. 2022; 14(11):2679. https://doi.org/10.3390/cancers14112679
Chicago/Turabian StyleRogucki, Mariusz, Iwona Sidorkiewicz, Magdalena Niemira, Janusz Bogdan Dzięcioł, Angelika Buczyńska, Agnieszka Adamska, Katarzyna Siewko, Maria Kościuszko, Katarzyna Maliszewska, Anna Wójcicka, and et al. 2022. "Expression Profile and Diagnostic Significance of MicroRNAs in Papillary Thyroid Cancer" Cancers 14, no. 11: 2679. https://doi.org/10.3390/cancers14112679






