Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
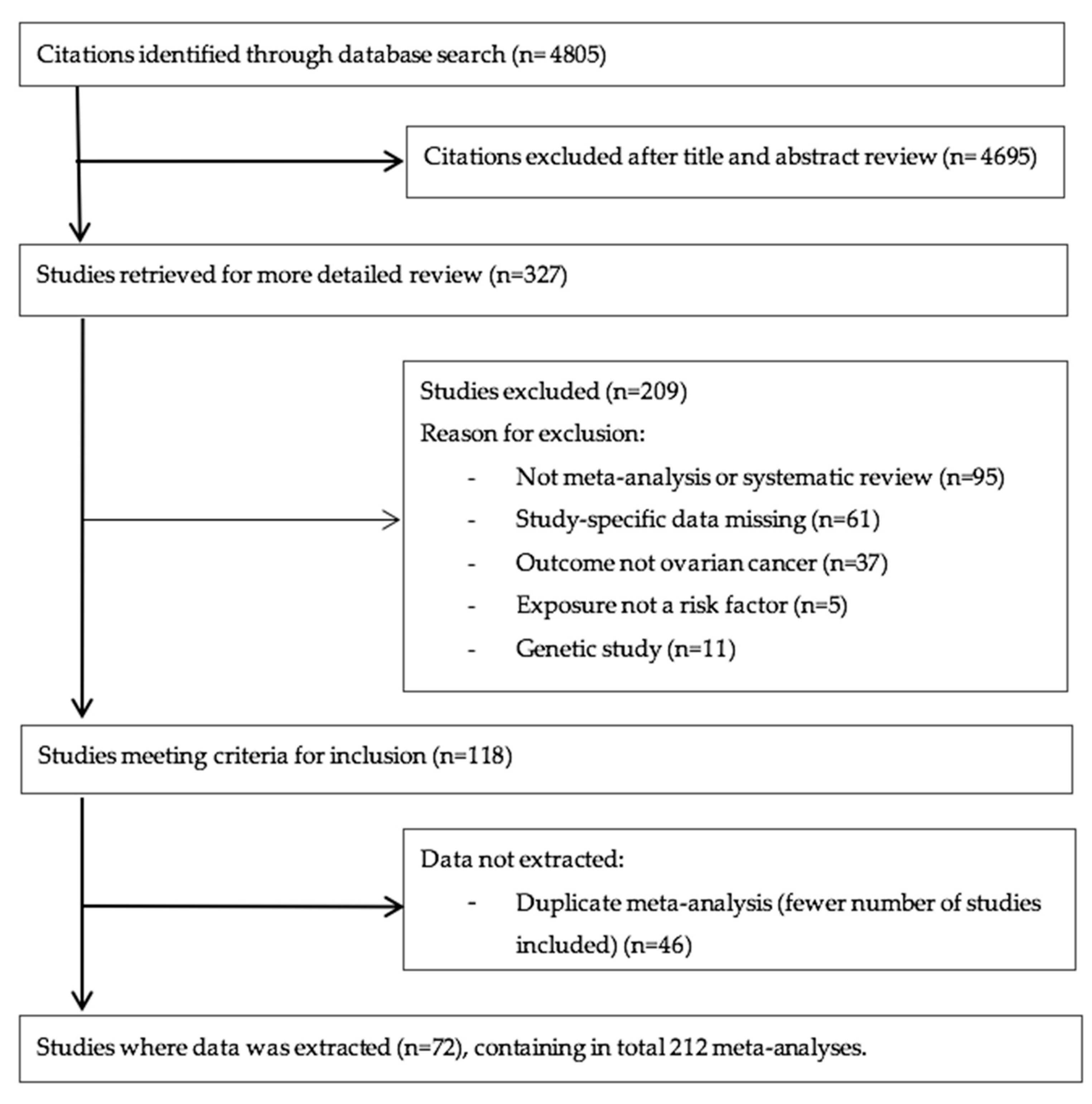
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Evaluation of the Strength of Evidence
2.4. Evaluation of the Quality of Included Meta-Analyses
2.5. Data Analysis
3. Results
3.1. Characteristics of Meta-Analyses
3.2. Summary Effect Size
3.3. Heterogeneity between Studies
3.4. Small Study Effects
3.5. Excess Significance
3.6. Grading the Evidence
3.7. Quality Assessment
3.8. Sensitivity Analyses
4. Discussion
4.1. Main Findings and Interpretation
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2018. Available online: https://gco.iarc.fr/tomorrow (accessed on 16 March 2020).
- Cancer Research UK. Ovarian Cancer Statistics; Cancer Research UK: London, UK, 2018. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer#heading-One (accessed on 2 February 2018).
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; et al. European Society of Gynaecological Oncology (ESGO) Guidelines for Ovarian Cancer Surgery. Int. J. Gynecol. Cancer 2017, 27, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009, 10, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L.; et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef] [Green Version]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.A. Why Most Discovered True Associations Are Inflated. Epidemiology 2008, 19, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Dwan, K.; Gamble, C.; Williamson, P.R.; Kirkham, J.J. Systematic Review of the Empirical Evidence of Study Publication Bias and Outcome Reporting Bias—An Updated Review. PLoS ONE 2013, 8, e66844. [Google Scholar] [CrossRef] [Green Version]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [Green Version]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef] [Green Version]
- Rezende, L.F.M.; Sa, T.H.; Markozannes, G.; Rey-Lopez, J.P.; Lee, I.M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770,000 cancer cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Mitra, A.; Terzidou, V.; Bennett, P.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Obesity and gynaecological and obstetric conditions: Umbrella review of the literature. BMJ 2017, 359, j4511. [Google Scholar] [CrossRef] [Green Version]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [Green Version]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chlebowski, R.; LaMonte, M.J.; Bea, J.W.; Qi, L.; Wallace, R.; Lavasani, S.; Walsh, B.W.; Anderson, G.; Vitolins, M.; et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: Results from the Women’s Health Initiative. Gynecol. Oncol. 2014, 133, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Davey Smith, G. Sifting the evidence. Proc. Natl. Acad Sci. USA 2001, 322, 226–231. [Google Scholar]
- Johnson, V.E. Revised standards for statistical evidence. Proc. Natl. Acad Sci. USA 2013, 110, 19313–19317. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.; Tarone, R.; McLaughlin, J.K. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology 2011, 22, 450–456. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanti, G.; Ioannidis, J.P. Synthesis of observational studies should consider credibility ceilings. J. Clin. Epidemiol. 2009, 62, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Statistical Software, Release 15.0; StataCorp LLC: College Station, TX, USA, 2015.
- Aune, D.; Navarro Rosenblatt, D.A.; Chan, D.S.; Abar, L.; Vingeliene, S.; Vieira, A.R.; Greenwood, D.C.; Norat, T. Anthropometric factors and ovarian cancer risk: A systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J. Cancer 2015, 136, 1888–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baandrup, L.; Faber, M.T.; Christensen, J.; Jensen, A.; Andersen, K.K.; Friis, S.; Kjaer, S.K. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: Systematic review and meta-analysis of observational studies. Acta Obstet. Gynecol. Scand. 2013, 92, 245–255. [Google Scholar] [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Berge, W.; Mundt, K.; Luu, H.; Boffetta, P. Genital use of talc and risk of ovarian cancer: A meta-analysis. Eur. J. Cancer Prev. 2018, 27, 248–257. [Google Scholar] [CrossRef]
- Bernatsky, S.; Ramsey-Goldman, R.; Foulkes, W.D.; Gordon, C.; Clarke, A.E. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: A meta-analysis. Br. J. Cancer 2011, 104, 1478–1481. [Google Scholar] [CrossRef] [Green Version]
- Bonovas, S.; Filioussi, K.; Sitaras, N.M. Paracetamol use and risk of ovarian cancer: A meta-analysis. Br. J. Clin. Pharmacol. 2006, 62, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Butler, L.M.; Wu, A.H. Green and black tea in relation to gynecologic cancers. Mol. Nutr. Food Res. 2011, 55, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Camargo, M.C.; Stayner, L.T.; Straif, K.; Reina, M.; Al-Alem, U.; Demers, P.A.; Landrigan, P.J. Occupational exposure to asbestos and ovarian cancer: A meta-analysis. Environ. Health Perspect. 2011, 119, 1211–1217. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Doll, R.; Hermon, C.; Peto, R.; Reeves, G. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008, 371, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Ma, W.; Chen, X.B.; Chang, Z.W.; Zhang, X.D.; Zhang, M.Z. Meta-analysis of green tea drinking and the prevalence of gynecological tumors in women. Asia Pac. J. Public Health 2013, 25, 43S–48S. [Google Scholar] [CrossRef]
- Garg, P.P.; Kerlikowske, K.; Subak, L.; Grady, D. Hormone replacement therapy and the risk of epithelial ovarian carcinoma: A meta-analysis. Obstet. Gynecol. 1998, 92, 472–479. [Google Scholar] [CrossRef]
- Gong, T.T.; Wu, Q.J.; Vogtmann, E.; Lin, B.; Wang, Y.L. Age at menarche and risk of ovarian cancer: A meta-analysis of epidemiological studies. Int. J. Cancer 2013, 132, 2894–2900. [Google Scholar] [CrossRef]
- Han, B.; Li, X.; Yu, T. Cruciferous vegetables consumption and the risk of ovarian cancer: A meta-analysis of observational studies. Diagn. Pathol. 2014, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Hankinson, S.E.; Colditz, G.A.; Hunter, D.J.; Spencer, T.L.; Rosner, B.; Stampfer, M.J. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet. Gynecol. 1992, 80, 708–714. [Google Scholar] [CrossRef]
- Huncharek, M.; Klassen, H.; Kupelnick, B. Dietary beta-carotene intake and the risk of epithelial ovarian cancer: A meta-analysis of 3,782 subjects from five observational studies. In Vivo 2001, 15, 339–343. [Google Scholar]
- Huncharek, M.; Muscat, J.; Onitilo, A.; Kupelnick, B. Use of cosmetic talc on contraceptive diaphragms and risk of ovarian cancer: A meta-analysis of nine observational studies. Eur. J. Cancer Prev. 2007, 16, 422–429. [Google Scholar] [CrossRef]
- Huo, Y.L.; Qiao, J.M.; Gao, S. Association between antidepressant medication use and epithelial ovarian cancer risk: A systematic review and meta-analysis of observational studies. Br. J. Clin. Pharmacol. 2018, 84, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep./Technol. Assess. 2007, 153, 17764214. [Google Scholar]
- Jiang, P.Y.; Jiang, Z.B.; Shen, K.X.; Yue, Y. Fish intake and ovarian cancer risk: A meta-analysis of 15 case-control and cohort studies. PLoS ONE 2014, 9, e94601. [Google Scholar] [CrossRef] [Green Version]
- Keum, N.; Greenwood, D.C.; Lee, D.H.; Kim, R.; Aune, D.; Ju, W.; Hu, F.B.; Giovannucci, E.L. Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. J. Natl. Cancer Inst. 2015, 107, djv088. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Marchand, N.; Oh, H.; Liu, H.; Aune, D.; Greenwood, D.C.; Giovannucci, E.L. Egg intake and cancers of the breast, ovary and prostate: A dose-response meta-analysis of prospective observational studies. Br. J. Nutr. 2015, 114, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, J.W.; Shouten, L.J.; Larsson, S.C.; Chung, H.H.; Kim, Y.B.; Ju, W.; Park, N.H.; Song, Y.S.; Kim, S.C.; et al. Wine drinking and epithelial ovarian cancer risk: A meta-analysis. J. Gynecol. Oncol. 2010, 21, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef]
- Kolahdooz, F.; van der Pols, J.C.; Bain, C.J.; Marks, G.C.; Hughes, M.C.; Whiteman, D.C.; Webb, P.M.; Australian Cancer Study (Ovarian Cancer) and the Australian Ovarian Cancer Study Group. Meat, fish, and ovarian cancer risk: Results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Koushik, A.; Hunter, D.J.; Spiegelman, D.; Anderson, K.E.; Buring, J.E.; Freudenheim, J.L.; Goldbohm, R.A.; Hankinson, S.E.; Larsson, S.C.; Leitzmann, M.; et al. Intake of the major carotenoids and the risk of epithelial ovarian cancer in a pooled analysis of 10 cohort studies. Int. J. Cancer 2006, 119, 2148–2154. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Milk, milk products and lactose intake and ovarian cancer risk: A meta-analysis of epidemiological studies. Int. J. Cancer 2006, 118, 431–441. [Google Scholar] [CrossRef]
- Lee, P.N.; Thornton, A.J.; Hamling, J.S. Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul. Toxicol. Pharmacol. 2016, 80, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Li, D.P.; Du, C.; Zhang, Z.M.; Li, G.X.; Yu, Z.F.; Wang, X.; Li, P.F.; Cheng, C.; Liu, Y.P.; Zhao, Y.S. Breastfeeding and ovarian cancer risk: A systematic review and meta-analysis of 40 epidemiological studies. Asian Pac. J. Cancer Prev. 2014, 15, 4829–4837. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Sci. Rep. 2014, 4, 4885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Jiao, X.; Yuan, Z.; Qiu, H.; Guo, R. C-reactive protein and risk of ovarian cancer: A systematic review and meta-analysis. Medicine 2017, 96, e7822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, A.; Li, T.; Qin, X.; Li, S. Effect of statin on risk of gynecologic cancers: A meta-analysis of observational studies and randomized controlled trials. Gynecol. Oncol. 2014, 133, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.T.; Zhao, J.J.; Qi, S.F.; Du, P.; Liu, D.W.; Tian, Q.B. The association between overweight, obesity and ovarian cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2015, 45, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Gao, X.P.; Zhu, S.; Liu, Y.H.; Wang, L.J.; Jing, C.X.; Zeng, F.F. Dietary inflammatory index and risk of gynecological cancers: A systematic review and meta-analysis of observational studies. J. Gynecol. Oncol. 2019, 30, e23. [Google Scholar] [CrossRef]
- Luan, N.N.; Wu, Q.J.; Gong, T.T.; Vogtmann, E.; Wang, Y.L.; Lin, B. Breastfeeding and ovarian cancer risk: A meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2013, 98, 1020–1031. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.A.; Trabert, B.; Yang, H.P.; Park, Y.; Brinton, L.A.; Hartge, P.; Sherman, M.E.; Hollenbeck, A.; Wentzensen, N. Non-steroidal anti-inflammatory drug use and ovarian cancer risk: Findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control 2012, 23, 1839–1852. [Google Scholar] [CrossRef]
- Negri, E.; Franceschi, S.; Tzonou, A.; Booth, M.; La Vecchia, C.; Parazzini, F.; Beral, V.; Boyle, P.; Trichopoulos, D. Pooled analysis of 3 European case-control studies: I. Reproductive factors and risk of epithelial ovarian cancer. Int. J. Cancer 1991, 49, 50–56. [Google Scholar] [CrossRef]
- Olsen, C.M.; Green, A.C.; Whiteman, D.C.; Sadeghi, S.; Kolahdooz, F.; Webb, P.M. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2007, 43, 690–709. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, W. Dietary protein intake and risk of ovarian cancer: Evidence from a meta-analysis of observational studies. Biosci. Rep. 2018, 38, BSR20181857. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Shin, H.R.; Shin, A.; Yeo, Y.; Choi, J.Y.; Jung, K.W.; Kim, B.G.; Kim, Y.M.; Noh, D.Y.; et al. Population attributable risks of modifiable reproductive factors for breast and ovarian cancers in Korea. BMC Cancer 2016, 16, 5. [Google Scholar] [CrossRef] [Green Version]
- Pelucchi, C.; Bosetti, C.; Galeone, C.; La Vecchia, C. Dietary acrylamide and cancer risk: An updated meta-analysis. Int. J. Cancer 2015, 136, 2912–2922. [Google Scholar] [CrossRef]
- Penninkilampi, R.; Eslick, G.D. Perineal Talc Use and Ovarian Cancer: A Systematic Review and Meta-Analysis. Epidemiology 2018, 29, 41–49. [Google Scholar] [CrossRef]
- Poorolajal, J.; Jenabi, E.; Masoumi, S.Z. Body mass index effects on risk of ovarian cancer: A meta- analysis. Asian Pac. J. Cancer Prev. 2014, 15, 7665–7671. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Lu, H.; Qi, Y.; Wang, X. Dietary fat intake and ovarian cancer risk: A meta-analysis of epidemiological studies. Oncotarget 2016, 7, 37390–37406. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.L.; Fang, Y.; Zhang, M.; Zhang, Y.Z. Phytoestrogen intake and risk of ovarian cancer: A meta-analysis of 10 observational studies. Asian Pac. J. Cancer Prev. 2014, 15, 9085–9091. [Google Scholar] [CrossRef] [Green Version]
- Reid, A.; de Klerk, N.; Musk, A.W. Does exposure to asbestos cause ovarian cancer? A systematic literature review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, F.; Salari-Moghaddam, A.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Coffee and caffeine intake and risk of ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2019, 29, 579–584. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Television viewing and time spent sedentary in relation to cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju098. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.F.; Wu, Y.; Li, C.Y. Hormone therapy and risk of ovarian cancer in postmenopausal women: A systematic review and meta-analysis. Menopause 2016, 23, 417–424. [Google Scholar] [CrossRef]
- Siristatidis, C.; Sergentanis, T.N.; Kanavidis, P.; Trivella, M.; Sotiraki, M.; Mavromatis, I.; Psaltopoulou, T.; Skalkidou, A.; Petridou, E.T. Controlled ovarian hyperstimulation for IVF: Impact on ovarian, endometrial and cervical cancer—A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 105–123. [Google Scholar] [CrossRef]
- Song, X.; Li, Z.; Ji, X.; Zhang, D. Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Nutrients 2017, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Wang, Y.; Zhang, J.; Song, N.; Xu, X.; Lu, Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Arthritis Res. Ther. 2018, 20, 270. [Google Scholar] [CrossRef] [Green Version]
- Wallin, A.; Orsini, N.; Wolk, A. Red and processed meat consumption and risk of ovarian cancer: A dose-response meta-analysis of prospective studies. Br. J. Cancer 2011, 104, 1196–1201. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liang, Z.; Liu, X.; Zhang, Q.; Li, S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. Int. J. Environ. Res. Public Health 2016, 13, 1138. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.F.; Yao, A.L.; Sun, Y.Y.; Zhang, A.H. Empirically derived dietary patterns and ovarian cancer risk: A meta-analysis. Eur. J. Cancer Prev. 2018, 27, 493–501. [Google Scholar] [CrossRef]
- WCRF/AICR. The Associations between Food, Nutrition, and Physical Activity and the Risk of Ovarian Cancer Continuous Update Project Report; WCRF/AICR: London, UK, 2013. [Google Scholar]
- Wen, Q.; Zhao, Z.; Wen, J.; Zhou, J.; Wu, J.; Lei, S.; Miao, Y. The association between metformin therapy and risk of gynecological cancer in patients: Two meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 33–41. [Google Scholar] [CrossRef]
- Xu, H.; Ding, Y.; Xin, X.; Wang, W.; Zhang, D. Dietary fiber intake is associated with a reduced risk of ovarian cancer: A dose-response meta-analysis. Nutr. Res. 2018, 57, 1–11. [Google Scholar] [CrossRef]
- Yan-Hong, H.; Jing, L.; Hong, L.; Shan-Shan, H.; Yan, L.; Ju, L. Association between alcohol consumption and the risk of ovarian cancer: A meta-analysis of prospective observational studies. BMC Public Health 2015, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Circulating vitamin D and ovarian cancer risk. Gynecol. Oncol. 2011, 121, 369–375. [Google Scholar] [CrossRef]
- Zeng, S.T.; Guo, L.; Liu, S.K.; Wang, D.H.; Xi, J.; Huang, P.; Liu, D.T.; Gao, J.F.; Feng, J.; Zhang, L. Egg consumption is associated with increased risk of ovarian cancer: Evidence from a meta-analysis of observational studies. Clin. Nutr. 2015, 34, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wei, H.; Yeoh, E.; Zhang, Z.; Ren, Z.F.; Colditz, G.A.; Tworoger, S.S.; Su, X. Inflammatory Markers of CRP, IL6, TNFalpha, and Soluble TNFR2 and the Risk of Ovarian Cancer: A Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, X.; Wang, J.; Pan, S.; Lu, C. Tea consumption and the risk of ovarian cancer: A meta-analysis of epidemiological studies. Oncotarget 2017, 8, 37796–37806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, N.; Xi, Y.; Zhao, Y.; Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017, 130, 43–52. [Google Scholar] [CrossRef]
- Zhang, D.; Kaushiva, A.; Xi, Y.; Wang, T.; Li, N. Non-herbal tea consumption and ovarian cancer risk: A systematic review and meta-analysis of observational epidemiologic studies with indirect comparison and dose-response analysis. Carcinogenesis 2018, 39, 808–818. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.S.; Zhang, Y.M.; Li, B.; Fan, B.; Zhao, Y.; Yang, S.J. Risk reduction of endometrial and ovarian cancer after bisphosphonates use: A meta-analysis. Gynecol. Oncol. 2018, 150, 509–514. [Google Scholar] [CrossRef]
- Zheng, B.; Shen, H.; Han, H.; Han, T.; Qin, Y. Dietary fiber intake and reduced risk of ovarian cancer: A meta-analysis. Nutr. J. 2018, 17, 99. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, L.; Lv, M.; Ma, T.; Zhang, X.; Zhao, J. Nonoccupational physical activity and risk of ovarian cancer: A meta-analysis. Tumour Biol. 2014, 35, 11065–11073. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Q.; Cong, R.; Gu, H.; Tang, N.; Yang, L.; Wang, B. Hormone replacement therapy and ovarian cancer risk: A meta-analysis. Gynecol. Oncol. 2008, 108, 641–651. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, F.; Yuan, J.; Tang, J.; Colditz, G.A.; Tworoger, S.S.; Trabert, B.; Su, X. Pelvic inflammatory disease and the risk of ovarian cancer: A meta-analysis. Cancer Causes Control 2017, 28, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Sun, W.; Xin, X.; Wang, W.; Zhang, D. Age at last birth and risk of developing epithelial ovarian cancer: A meta-analysis. Biosci. Rep. 2019, 39, BSR20182035. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Milajerdi, A.; Surkan, P.J.; Larijani, B.; Esmaillzadeh, A. Caffeine, Type of Coffee, and Risk of Ovarian Cancer: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2019, 104, 5349–5359. [Google Scholar] [CrossRef]
- Santucci, C.; Bosetti, C.; Peveri, G.; Liu, X.; Bagnardi, V.; Specchia, C.; Gallus, S.; Lugo, A. Dose-risk relationships between cigarette smoking and ovarian cancer histotypes: A comprehensive meta-analysis. Cancer Causes Control 2019, 30, 1023–1032. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Gasevic, D.; Brunt, E.; McLachlan, F.; Millenson, M.; Timofeeva, M.; Ioannidis, J.P.A.; Campbell, H.; Theodoratou, E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann. Intern. Med. 2018, 169, 543–553. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009, 181, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, R.; Tang, S.; Feng, F.; Liu, C.; Wang, L.; Zhao, W.; Zhang, T.; Yao, Y.; Wang, X.; et al. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 35–46. [Google Scholar] [CrossRef]
- Giovannucci, E. A growing link-what is the role of height in cancer risk? Br. J. Cancer 2019, 120, 575–576. [Google Scholar] [CrossRef] [Green Version]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Relton, C.L.; Lophatananon, A.; Muir, K.; Menon, U.; Gentry-Maharaj, A.; Walther, A.; Zheng, J.; Fasching, P.; Zheng, W.; et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: A Mendelian randomization analysis. PLoS Med. 2019, 16, e1002893. [Google Scholar] [CrossRef] [Green Version]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Olsen, C.M.; Nagle, C.M.; Whiteman, D.C.; Ness, R.; Pearce, C.L.; Pike, M.C.; Rossing, M.A.; Terry, K.L.; Wu, A.H.; Australian Cancer Study; et al. Obesity and risk of ovarian cancer subtypes: Evidence from the Ovarian Cancer Association Consortium. Endocr. Relat. Cancer 2013, 20, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Charlton, B.M.; Rich-Edwards, J.W.; Colditz, G.A.; Missmer, S.A.; Rosner, B.A.; Hankinson, S.E.; Speizer, F.E.; Michels, K.B. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: Prospective cohort study. BMJ 2014, 349, g6356. [Google Scholar] [CrossRef] [Green Version]
- Iversen, L.; Sivasubramaniam, S.; Lee, A.J.; Fielding, S.; Hannaford, P.C. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners’ Oral Contraception Study. Am. J. Obstet. Gynecol. 2017, 216, 580.e1–580.e9. [Google Scholar] [CrossRef]
- McGuire, V.; Hartge, P.; Liao, L.M.; Sinha, R.; Bernstein, L.; Canchola, A.J.; Anderson, G.L.; Stefanick, M.L.; Whittemore, A.S. Parity and Oral Contraceptive Use in Relation to Ovarian Cancer Risk in Older Women. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Michels, K.A.; Trabert, B. Oral Contraceptive Progestin and Estrogen Use and Increases in Breast, Ovarian, and Endometrial Cancers-Reply. JAMA Oncol. 2018, 4, 1623–1624. [Google Scholar] [CrossRef]
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V.; Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289. [Google Scholar] [CrossRef]
- Cunat, S.; Hoffmann, P.; Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Mungenast, F.; Thalhammer, T. Estrogen biosynthesis and action in ovarian cancer. Front. Endocrinol. (Lausanne) 2014, 5, 192. [Google Scholar] [CrossRef] [Green Version]
- Lau, K.M.; Mok, S.C.; Ho, S.M. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc. Natl. Acad Sci. USA 1999, 96, 5722–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilidis, K.K.; Allen, N.E.; Key, T.J.; Dossus, L.; Kaaks, R.; Bakken, K.; Lund, E.; Fournier, A.; Dahm, C.C.; Overvad, K.; et al. Menopausal hormone therapy and risk of ovarian cancer in the European prospective investigation into cancer and nutrition. Cancer Causes Control 2011, 22, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.S.; Gapstur, S.M.; Feigelson, H.S.; Teras, L.R.; Thun, M.J.; Patel, A.V. Postmenopausal hormone use and incident ovarian cancer: Associations differ by regimen. Int. J. Cancer 2010, 127, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Fielding, S.; Lidegaard, O.; Morch, L.S.; Skovlund, C.W.; Hannaford, P.C. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: Prospective, nationwide cohort study. BMJ 2018, 362, k3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vessey, M.; Yeates, D. Oral contraceptive use and cancer: Final report from the Oxford-Family Planning Association contraceptive study. Contraception 2013, 88, 678–683. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Allen, N.E.; Key, T.J.; Dossus, L.; Lukanova, A.; Bakken, K.; Lund, E.; Fournier, A.; Overvad, K.; Hansen, L.; et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2011, 105, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Fathalla, M.F. Incessant ovulation and ovarian cancer-a hypothesis re-visited. Facts Views Vis. Obgyn 2013, 5, 292–297. [Google Scholar]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar] [CrossRef]
- Yang-Hartwich, Y.; Gurrea-Soteras, M.; Sumi, N.; Joo, W.D.; Holmberg, J.C.; Craveiro, V.; Alvero, A.B.; Mor, G. Ovulation and extra-ovarian origin of ovarian cancer. Sci. Rep. 2014, 4, 6116. [Google Scholar] [CrossRef] [Green Version]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Pavone, M.E.; Lyttle, B.M. Endometriosis and ovarian cancer: Links, risks, and challenges faced. Int. J. Womens Health 2015, 7, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Dawson, A.; Fernandez, M.L.; Anglesio, M.; Yong, P.J.; Carey, M.S. Endometriosis and endometriosis-associated cancers: New insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018, 12, 803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhai, Z.; Yang, Y.; Wan, J.; Xie, W.; Zhu, J.; Shen, Y.H.; Wang, C. Diabetes mellitus is associated with increased bleeding in pulmonary embolism receiving conventional anticoagulant therapy: Findings from a “real-world study”. J. Thromb. Thrombolysis 2017, 43, 540–549. [Google Scholar] [CrossRef]
- Narod, S.A. Talc and ovarian cancer. Gynecol. Oncol. 2016, 141, 410–412. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Tworoger, S.S.; Harris, H.R.; Anderson, G.L.; Weinberg, C.R.; Trabert, B.; Kaunitz, A.M.; D’Aloisio, A.A.; Sandler, D.P.; Wentzensen, N. Association of Powder Use in the Genital Area With Risk of Ovarian Cancer. JAMA 2020, 323, 49–59. [Google Scholar] [CrossRef]
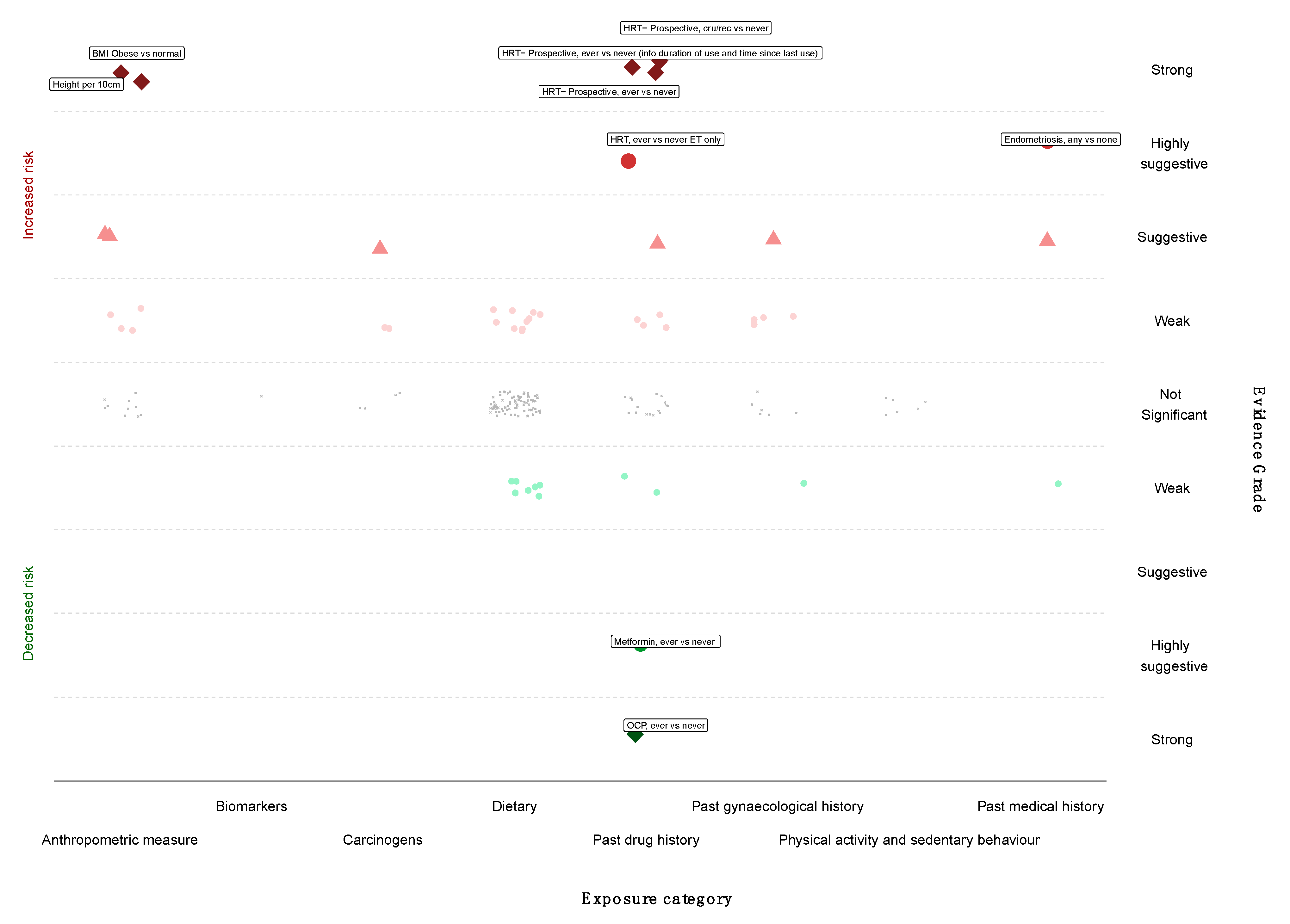

| Evidence | Criteria Used | Decreased Risk | Increased Risk |
|---|---|---|---|
| Strong | p < 10−6 ||; >1000 cases; I2 < 50%; no small study effects ¶; prediction interval excludes the null value; no excess significance bias † n = 6 | Past drug history OCP inc Ever vs. never ** | Anthropometric measure Height inc Per 10 cm BMI; ≥30 kg/m2 vs. normal |
| Past drug history HRT inc Ever vs. never (prospective studies) HRT inc Current/recent vs. never (prospective studies) HRT inc Ever vs. never (prospective studies; info duration of use and time since last use known) | |||
| Highly Suggestive | p < 10−6 ||; >1000 cases; p < 0.05 of the largest study in a meta-analysis n = 3 | Past drug history Metformin inc Ever vs. never | Past drug history HRT inc Ever vs. never ET only |
| Medical history Endometriosis inc Any vs. none | |||
| Suggestive | p < 10−3 ||; >1000 cases n = 5 | None | Anthropometric measures BMI inc iya per 5 kg/m2 increase BMI inc per 5 kg/m2 increase |
| Asbestos Any vs. none MO | |||
| Medical history Diabetes inc; Yes vs. no | |||
| Past drug history HRT inc; Current vs. ever | |||
| Weak | p < 0.05 n = 26 | Reproductive factors Breastfeeding inc; Per 5 mo increase in duration | Anthropometric measures BMI inc PrMP Obese vs. normal BMI inc PoMP; Obese vs. normal Per 5 kg weight inc WG per 5 kg increase PoMP, HRT, inc |
| Past drug history NSAIDS inc, Non aspirin; Ever vs. never OCP inc; Ever vs. never | |||
| Medical history SLE inc; observed vs. expected | Asbestos Total exposed vs. non-exposed, MO High exposed vs. non-exposed, MO | ||
| Dietary Intake Tea (black) inc; Highest vs. lowest Non herbal tea inc; Highest vs. lowest Calcium inc; Highest vs. lowest Non-starchy vegetables inc; Per 100 g/day | |||
| Dietary intake Dairy total inc; Highest vs. lowest Dairy skim/low fat inc; Highest vs. lowest Dairy lactose inc; Highest vs. lowest Meat (processed) inc; Highest vs. lowest Meat (red and processed) MO; Per 100 g/week increment | |||
| Past drug history HRT inc; Ever vs. never (continuous E + P) HRT inc; Ever vs. never (sequential E + P) HRT inc; Ever vs. never (E + P) HRT inc; Ever vs. never (E + E/P) | |||
| Reproductive Factors PID inc; Ever vs never IVF inc; Ever vs. never (reference group general population) IVF inc; Ever vs. never (reference group IVF population) |
| Exposure | Exposure Contrast | N ˆ | Sample Size Cases/Cohort | Largest Study # | Random Effects Summary RR (95% CI) ¥ | Random p-Value || | 95% Prediction Interval | Egger’s p ∞ | I2 (%) | Excess Significance § | Evidence Grading **,†,¶ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E α | p-Value φ | |||||||||||
| Strong evidence | ||||||||||||
| Anthropometric measure | ||||||||||||
| Height | Per 10 cm | 16 | 18663/13600000 | 1.14 (1.10–1.18) | 1.16 (1.11–1.20) | 2.2 × 10−13 | 1.06–1.26 | 0.18 | 27 | 9/6.22 | 0.15 | Strong |
| BMI | Obese vs. normal | 13 | 6947/20560388 τ | 1.27 (1.19–1.36) | 1.27 (1.17–1.38) | 2.6 × 10−8 | 1.09–1.47 | 0.88 | 12 | 3/5.30 | NP | Strong |
| Past drug history | ||||||||||||
| HRT-Prospective | Current/recent vs. never | 12 | 11664/948390 | 1.28 (1.14–1.44) | 1.37 (1.27–1.48) | 1.3 × 10−15 | 1.26–1.50 | 0.68 | 0 | 3/7.20 | NP | Strong |
| HRT-Prospective | Ever vs. never | 17 | 12110/950663 | 1.15 (1.06–1.26) | 1.20 (1.13–1.28) | 2.1 × 10−9 | 1.13–1.28 | 0.71 | 0 | 4/4.90 | NP | Strong |
| HRT-Prospective | Ever vs. never (info duration of use and time since last use) | 14 | 11866/949657 | 1.16 (1.05–1.28) | 1.24 (1.16–1.32) | 6.0 × 10−10 | 1.15–1.33 | 0.97 | 0 | 2/4.87 | NP | Strong |
| OCP | Ever vs. never | 45 | 7726/32201 | 0.74 (0.67–0.82) | 0.74 (0.69–0.80) | 5.8 × 10−16 | 0.68–0.81 | 0.61 | 0 | 3/6.68 | NP | Strong |
| Highly suggestive evidence | ||||||||||||
| Past drug history | ||||||||||||
| HRT | Ever vs. never ET only | 11 | 7512/2302683 | 1.31 (1.11–1.54) | 1.44 (1.25–1.66) | 7.1 × 10−7 | 0.99–2.09 | 0.71 | 48 | 6/7.01 | NP | Highly suggestive |
| Metformin | Ever vs. never | 3 | 3288/513702 | 0.16 (0.14–0.17) | 0.18 (0.12–0.25) | 2.5 × 10−23 | 0.01–4.31 | 0.38 | 14 | 2/2.42 | NP | Highly suggestive |
| Anthropometric measure | ||||||||||||
| Height | per 5 cm increase | 13 | 16198/3514114 | 1.07 (1.05–1.09) | 1.07 (1.05–1.10) | 1.2 × 10−9 | 1.02–1.14 | 0.31 | 32 | 8/2.77 | <0.01 | Highly suggestive |
| Medical history | ||||||||||||
| Metformin | Ever vs. never | 3 | 3288/513702 | 0.16 (0.14–0.17) | 0.18 (0.12–0.25) | 2.5 × 10−23 | 0.01–4.31 | 0.38 | 14 | 2/2.42 | NP | Highly suggestive |
| Suggestive evidence | ||||||||||||
| Environmental factors | ||||||||||||
| Asbestos | Any vs. none | 14 | 5165/906145 | 1.30 (0.90–1.80) | 1.86 (1.46–2.36) | 5.0 × 10−7 | 1.05–3.29 | 0.64 | 28 | 4/1.87 | 0.10 | Suggestive |
| Anthropometric measures | ||||||||||||
| BMI | per 5 kg/m2 increase | 24 | 17734/16300000 | 0.97 (0.93–1.01) | 1.07 (1.04–1.11) | <0.01 | 0.96–1.21 | 0.07 | 48 | 6/1.80 | <0.01 | Suggestive |
| BMI | iya per 5 kg/m2 increase | 6 | 9452/11100000 | 1.16 (1.04–1.29) | 1.12 (1.05–1.19) | <0.01 | 1.03–1.23 | 0.60 | 0 | 0/1.00 | NP | Suggestive |
| Medical history | ||||||||||||
| Diabetes Mellitus | DM vs. no DM | 17 | 5036/2868215 | 1.23 (1.15–1.32) | 1.32 (1.14–1.52) | <0.01 | 0.81–2.15 | 0.30 | 80 | 6/6.71 | NP | Suggestive |
| Past drug history | ||||||||||||
| HRT | Current vs. ever | 5 | 3958/1342899 | 1.20 (1.09–1.32) | 1.28 (1.15–1.42) | 6.5 × 10−6 | 1.01–1.62 | 0.08 | 14 | 3/2.91 | 0.93 | Suggestive |
| Weak evidence | ||||||||||||
| Asbestos | ||||||||||||
| Asbestos | Total exp vs. nonexp | 20 | 126/21973 | 1.12 (0.66–1.80) | 1.77 (1.37–2.27) | 9.7 × 10−6 | 0.85–3.66 | 0.72 | 35 | 6/1.06 | <0.01 | Weak |
| Asbestos | High exp vs. nonexp | 6 | 20/6149 | 1.10 (0.37–2.21) | 2.78 (1.36–5.66) | 0.01 | 0.42–18.44 | 0.78 | 45 | 2/0.31 | <0.01 | Weak |
| Anthropometric measure | ||||||||||||
| BMI | PrMP Obese vs. normal | 3 | 71/350211 | 1.56 (1.14–2.16) | 1.57 (1.20–2.06) | <0.01 | 0.27–9.02 | 0.61 | 0 | 1/0.66 | 0.64 | Weak |
| BMI | PoMP Obese vs. normal | 5 | 350/546195 | 1.02 (0.82–1.26) | 1.23 (1.03–1.47) | 2.6 × 10−1 | 0.72–2.09 | 0.52 | 46 | 1/ 0.25 | 0.13 | Weak |
| Weight | Per 5 kg weight | 4 | 1006/297350 | 1.02 (1.00–1.05) | 1.03 (1.01–1.05) | <0.01 | 0.98–1.08 | 0.42 | 7 | 1/0.21 | 0.08 | Weak |
| Weight gain | per 5 kg increase PoMP, HRT | 2 | 217/23984 | 1.16 (1.03–1.31) | 1.13 (1.03–1.24) | 0.01 | NA | NA | NA | 1/0.27 | 0.13 | Weak |
| Dietary intake | ||||||||||||
| Dairy, total products | Highest vs. lowest | 2 | 427/90001 | 1.61 (1.07–2.42) | 1.66 (1.19–2.31) | <0.01 | NA | NA | NA | 1/1.87 | NP | Weak |
| Dairy, skim/low fat | Highest vs. lowest | 3 | 728/170327 | 1.32 (0.97–1.82) | 1.35 (1.09–1.68) | <0.01 | 0.35–5.43 | 0.21 | 0 | 0/1.93 | NP | Weak |
| Dairy, lactose | Highest vs. lowest | 3 | 728/170327 | 1.48 (1.05–2.09) | 1.47 (1.17–1.84) | <0.01 | 0.34–6.29 | 0.42 | 0 | 1/2.64 | NP | Weak |
| Meat; processed | Highest vs. lowest | 3 | 1018/696100 | 1.23 (0.92–1.63) | 1.26 (1.02–1.56) | 3.5 × 10−2 | 0.31–5.07 | 0.37 | 0 | 0/1.56 | NP | Weak |
| Meat; red and processed | Per 100 g/week increment | 21 | 6536/2140286 | 1.02 (0.98–1.06) | 1.01 (1.00–1.04) | 3.4 × 10−2 | 1.00–1.04 | 0.12 | 0 | 0/1.14 | NP | Weak |
| Non starchy vegetables | Per 100 g/day | 6 | 2053/641079 | 1.00 (0.93–1.07) | 0.94 (0.89–1.00) | 4.0 × 10−2 | 0.82–1.08 | 0.21 | 28 | 1/0.30 | 0.19 | Weak |
| Tea; black | Highest vs. lowest | 5 | 1299/203998 | 0.63 (0.40–0.99) | 0.73 (0.56–0.93) | 1.2 × 10−2 | 0.42–1.24 | 0.44 | 15 | 2/4.71 | NP | Weak |
| Calcium | Highest vs. lowest | 5 | 1726/351192 | 0.86 (0.68–1.10) | 0.86 (0.74–1.00) | 4.0 × 10−2 | 0.67–1.09 | 0.62 | 0 | 0/1.66 | NP | Weak |
| Non herbal tea | Highest vs. lowest | 3 | 734/164882 | 0.63 (0.40–0.99) | 0.69 (0.52–0.93) | 1.4 × 10−2 | 0.11–4.57 | 0.03 | 0 | 1/2.74 | NP | Weak |
| Past drug history | ||||||||||||
| HRT | Ever vs. never (continuous E + P) | 4 | 3337/1265735 | 1.13 (0.96–1.34) | 1.22 (1.06–1.40) | <0.01 | 0.90–1.65 | 0.07 | 0 | 1/1.63 | NP | Weak |
| HRT | Ever vs. never (sequential E + P) | 4 | 3337/1265736 | 1.14 (0.98–1.32) | 1.35 (1.06–1.72) | 1.5 × 10−2 | 0.54–3.35 | 0.18 | 50 | 2/1.75 | 0.80 | Weak |
| HRT | Ever vs. never (ET + PT) | 9 | 7512/2302683 | 1.50 (1.34–1.68) | 1.23 (1.08–1.14) | 2.3 × 10−3 | 0.87–1.75 | 0.40 | 53 | 3/8.65 | NP | Weak |
| HRT | Ever vs. never (ET+ E/PT) | 2 | 543/141880 | 1.50 (0.92–2.44) | 1.55 (1.05–2.30) | 2.7 × 10−2 | NA | NA | NA | 0/1.89 | NP | Weak |
| NSAIDS Non aspirin | Ever vs. never | 6 | 1782/505136 | 0.90 (0.75–1.08) | 0.90 (0.81–1.00) | 4.4 × 10−2 | 0.78–1.04 | 0.55 | 0 | 0/1.02 | NP | Weak |
| OCP | Ever vs. never | 3 | 60/80670 | 0.60 (0.30–1.40) | 0.43 (0.25–0.75) | 3.0 × 10−3 | 0.01–15.16 | 0.41 | NA | 1/0.73 | 0.71 | Weak |
| Reproductive factors | ||||||||||||
| PID | Ever vs. never | 6 | 8285/2929284 | 1.05 (0.92–1.20) | 1.32 (1.05–1.66) | 1.6 × 10−2 | 0.71–2.47 | 0.35 | 65 | 2/1.01 | 0.28 | Weak |
| IVF | Ever vs. never (reference group general population excluding OC diagnosis < 1yr post treatment) | 6 | 31606/1438001 | 1.30 (0.90–1.88) | 1.47 (1.06–2.03) | 2.0 × 10−2 | 0.73–2.96 | 0.64 | 23 | 1/1.55 | NP | Weak |
| IVF | Ever vs. never reference group IVF population; total follow up | 6 | 31606/1438002 | 1.35 (0.93–1.96) | 1.66 (1.08–2.55) | 2.2 × 10−2 | 0.52–5.28 | 0.91 | 52 | 2/1.64 | 0.74 | Weak |
| Breastfeeding | Per 5 mo increase in duration | 3 | 1180/447386 | 0.98 (0.92–1.05) | 0.94 (0.89–1.00) | 0.03 | 0.80–1.49 | 0.59 | 22 | 1/0.17 | 0.04 | Weak |
| Medical history | ||||||||||||
| SLE | Observed vs. expected | 4 | 44/40855 | 0.82 (0.54–1.20) | 0.73 (0.53–1.00) | 4.9 × 10−2 | 0.36–1.46 | 0.97 | 0 | 0/0.26 | NP | Weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whelan, E.; Kalliala, I.; Semertzidou, A.; Raglan, O.; Bowden, S.; Kechagias, K.; Markozannes, G.; Cividini, S.; McNeish, I.; Marchesi, J.; et al. Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature. Cancers 2022, 14, 2708. https://doi.org/10.3390/cancers14112708
Whelan E, Kalliala I, Semertzidou A, Raglan O, Bowden S, Kechagias K, Markozannes G, Cividini S, McNeish I, Marchesi J, et al. Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature. Cancers. 2022; 14(11):2708. https://doi.org/10.3390/cancers14112708
Chicago/Turabian StyleWhelan, Eilbhe, Ilkka Kalliala, Anysia Semertzidou, Olivia Raglan, Sarah Bowden, Konstantinos Kechagias, Georgios Markozannes, Sofia Cividini, Iain McNeish, Julian Marchesi, and et al. 2022. "Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature" Cancers 14, no. 11: 2708. https://doi.org/10.3390/cancers14112708
APA StyleWhelan, E., Kalliala, I., Semertzidou, A., Raglan, O., Bowden, S., Kechagias, K., Markozannes, G., Cividini, S., McNeish, I., Marchesi, J., MacIntyre, D., Bennett, P., Tsilidis, K., & Kyrgiou, M. (2022). Risk Factors for Ovarian Cancer: An Umbrella Review of the Literature. Cancers, 14(11), 2708. https://doi.org/10.3390/cancers14112708










