PET-CT in Clinical Adult Oncology—V. Head and Neck and Neuro Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Head and Neck Cancer
2.1. Technical Notes, Nuances and Overview
2.2. Squamous Cell Carcinoma of the Head and Neck (SCCHN)
- Oral cavity squamous cell carcinoma
- 2.
- Oropharyngeal squamous cell carcinoma
- 3.
- Hypopharyngeal squamous cell carcinoma
- 4.
- Cervical esophageal squamous cell carcinoma
- 5.
- Laryngeal squamous cell carcinoma
- a.
- Supraglottic larynx
- b.
- Glottic larynx
- c.
- Subglottic larynx
2.3. Nasopharyngeal Carcinoma
2.4. Sinonasal Tumors
- Sinonasal squamous cell carcinoma
- 2.
- Sinonasal adenocarcinoma
- 3.
- Sinonasal undifferentiated carcinoma
- 4.
- Esthesioneuroblastoma
2.5. Major and Minor Salivary Gland, Lacrimal Gland Tumors
- Pleomorphic adenoma and carcinoma ex pleomorphic adenoma
- 2.
- Warthin tumor
- 3.
- Mucoepidermoid carcinoma
- 4.
- Adenoid cystic carcinoma
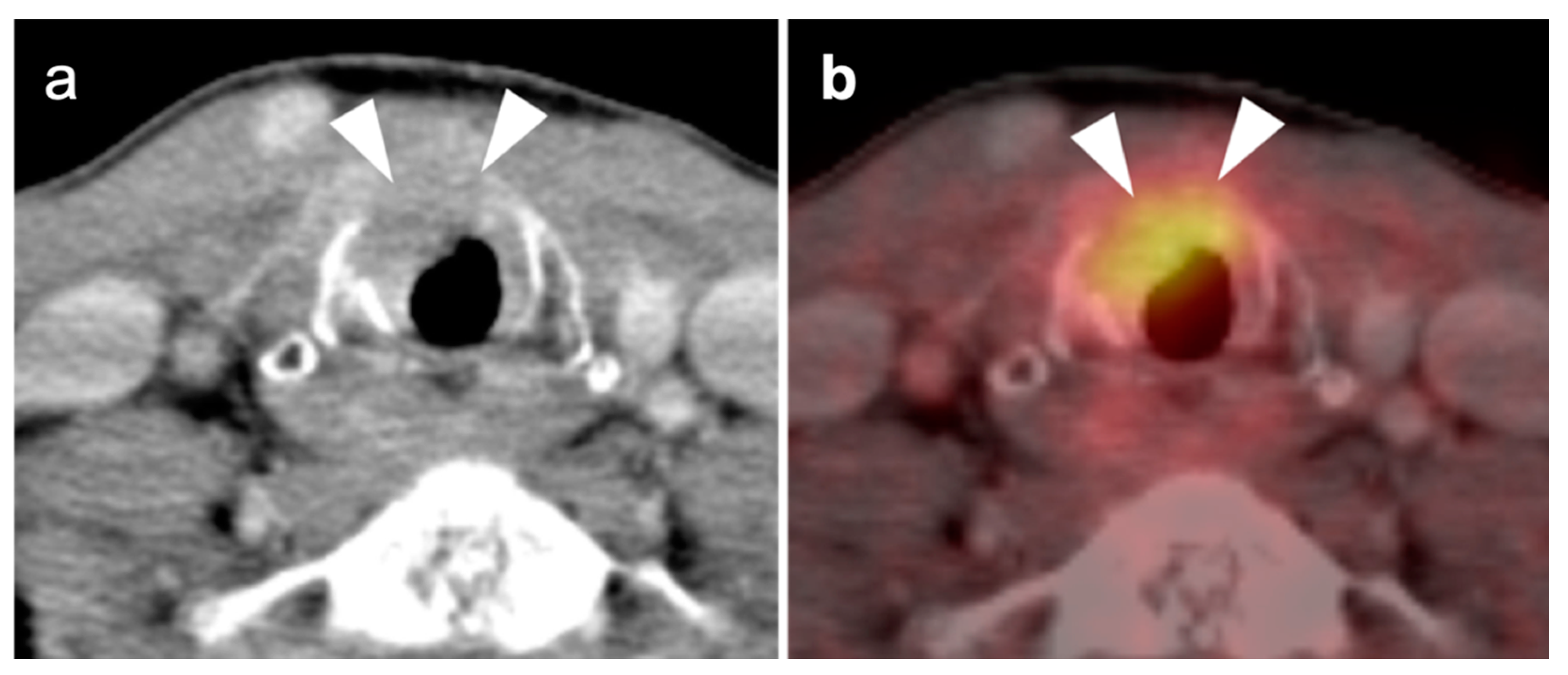
2.6. Thyroid Cancer
- Thyroid Incidentaloma
- 2.
- Differentiated thyroid cancer
- 3.
- Anaplastic thyroid cancer
- 4.
- Medullary thyroid cancer
3. Neuro Oncology
3.1. Technical Notes and Overview
3.2. Paraneoplastic CNS Manifestations and Sources of False Positive FDG PET Scans That Can Mimic Brain Tumors
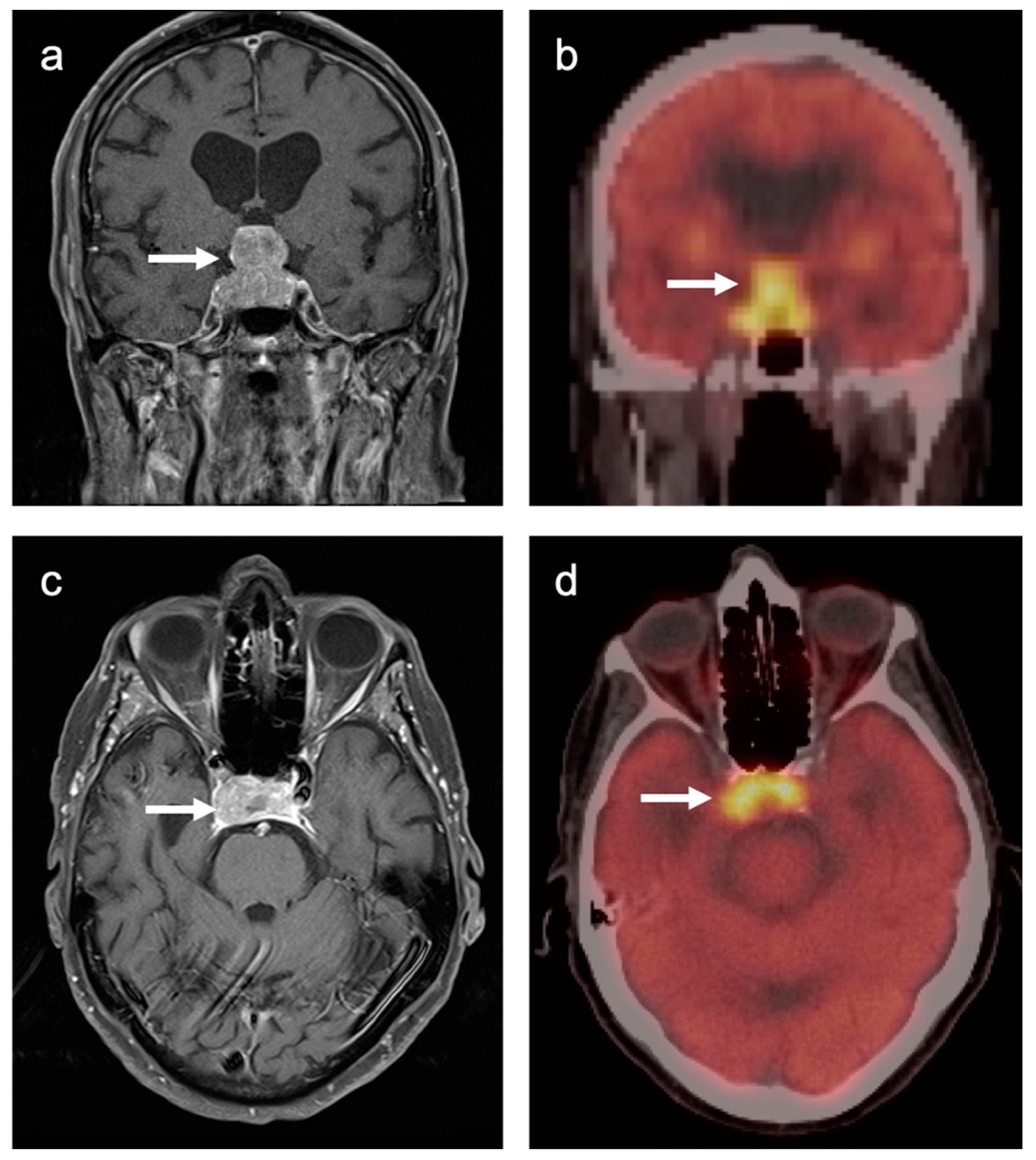
3.3. Primary Brain Tumors
3.4. CNS Lymphoma
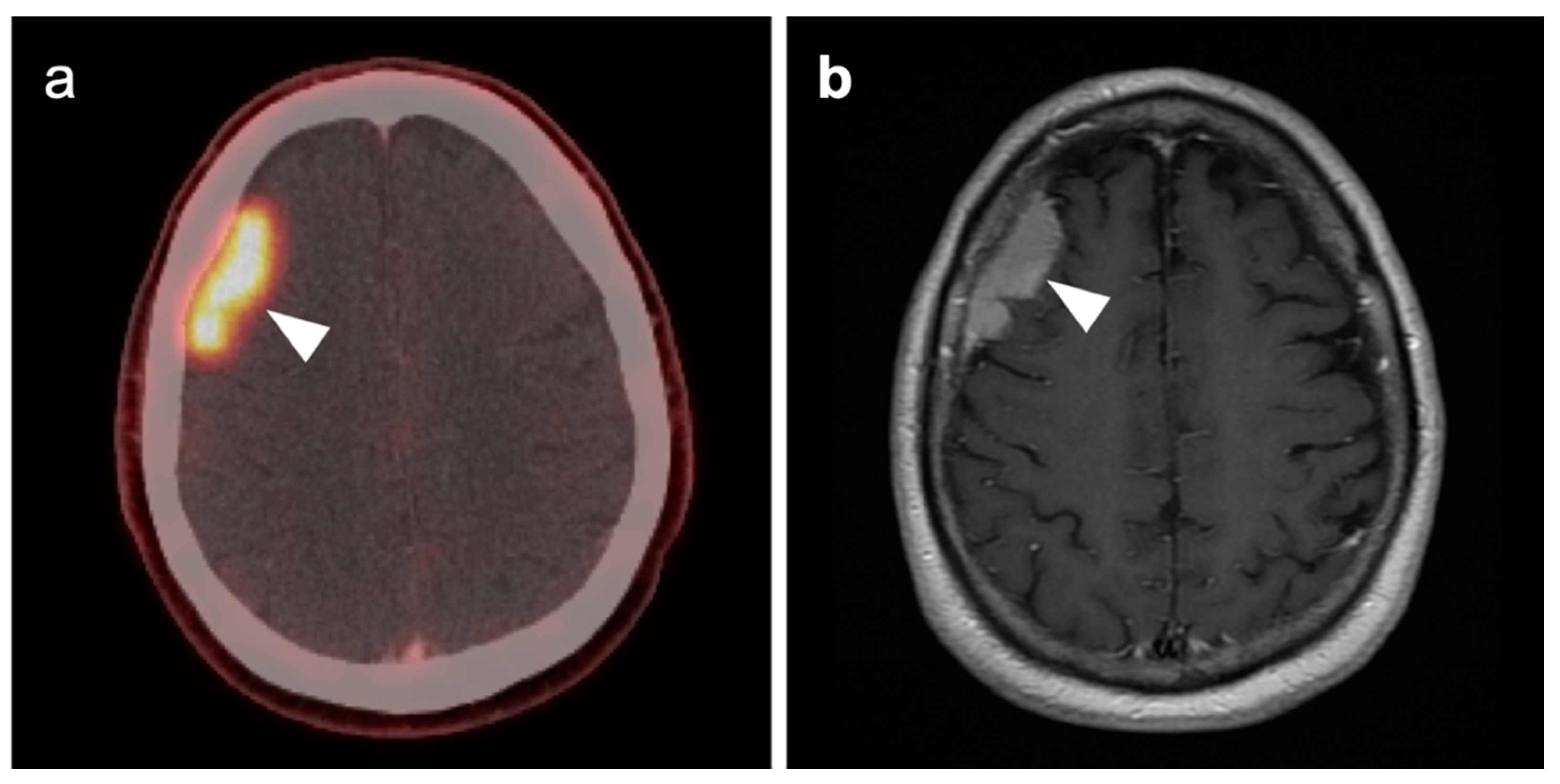
3.5. Brain Metastases
3.6. Response to Therapy
3.7. Meningioma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Wong, W.L. PET-CT for Staging and Detection of Recurrence of Head and Neck Cancer. Semin. Nucl Med. 2021, 51, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Wong, T.Z.; Turkington, T.G.; Hawk, T.C.; Coleman, R.E. Head and neck cancer: Dedicated FDG PET/CT protocol for detection--phantom and initial clinical studies. Radiology 2007, 244, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Bozza, F.A.; Christian, P.E.; Hoffman, J.M.; Butterfield, R.I.; Christensen, C.R.; Heilbrun, M.; Wiggins, R.H.; Hunt, J.P.; Bentz, B.G.; et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J. Nucl. Med. 2009, 50, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helsen, N.; Roothans, D.; Heuvel, B.V.D.; Wyngaert, T.V.D.; Weyngaert, D.V.D.; Carp, L.; Stroobants, S. 18F-FDG-PET/CT for the detection of disease in patients with head and neck cancer treated with radiotherapy. PLoS ONE 2017, 12, e0182350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacicedo, J.; Navarro, A.; Del Hoyo, O.; Gomez-Iturriaga, A.; Alongi, F.; Medina, J.A.; Elicin, O.; Skanjeti, A.; Giammarile, F.; Bilbao, P.; et al. Role of fluorine-18 fluorodeoxyglucose PET/CT in head and neck oncology: The point of view of the radiation oncologist. Br. J. Radiol. 2016, 89, 20160217. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Ciarallo, A.; Tahari, A.K.; Mena, E.; Koch, W.; Wahl, R.L.; Kiess, A.P.; Kang, H.; Subramaniam, R.M. Head and neck PET/CT: Therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J. Nucl. Med. 2014, 55, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Aiken, A.H.; Rath, T.J.; Anzai, Y.; Branstetter, B.F.; Hoang, J.K.; Wiggins, R.H.; Juliano, A.F.; Glastonbury, C.; Phillips, C.D.; Brown, R.; et al. ACR Neck Imaging Reporting and Data Systems (NI-RADS): A White Paper of the ACR NI-RADS Committee. J. Am. Coll. Radiol. 2018, 15, 1097–1108. [Google Scholar] [CrossRef]
- Hsu, D.; Rath, T.J.; Branstetter, B.F.; Anzai, Y.; Phillips, C.D.; Juliano, A.F.; Mosier, K.M.; Bazylewicz, M.P.; Poliashenko, S.M.; Kulzer, M.H.; et al. Interrater Reliability of NI-RADS on Posttreatment PET/Contrast-enhanced CT Scans in Head and Neck Squamous Cell Carcinoma. Radiol. Imaging Cancer 2021, 3, e200131. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Branstetter, B.; Hughes, M.; Clump, D.; Heron, D.; Rath, T. Negative Predictive Value of NI-RADS Category 2 in the First Posttreatment FDG-PET/CT in Head and Neck Squamous Cell Carcinoma. AJNR Am. J. Neuroradiol. 2018, 39, 1884–1888. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Version 1. 2022–December 8, 2021. Head and Neck Cancer. NCCN. Available online: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 2 April 2022).
- Arunsingh, M.; Vaidyanathan, S.; Dyker, K.; Sen, M.; Scarsbrook, A.; Prestwich, R. Accuracy of Response Assessment Positron Emission Tomography-Computed Tomography Following Definitive Radiotherapy Without Chemotherapy for Head and Neck Squamous Cell Carcinoma. Clin. Oncol. 2019, 31, 212–218. [Google Scholar] [CrossRef]
- Dos Anjos, R.F.; Dos Anjos, D.A.; Vieira, D.L.; Leite, A.; Figueiredo, P.T.D.S.; De Melo, N.S. Effectiveness of FDG-PET/CT for evaluating early response to induction chemotherapy in head and neck squamous cell carcinoma: A systematic review. Medicine 2016, 95, e4450. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.D.; Wong, L.S.; Crowther, J.; Taylor, W.M.; McManners, J.; Devine, J.C.; Wales, C.; MacIver, C. Patterns of local recurrence after primary resection of cancers that arise in the sinonasal region and the maxillary alveolus. Br. J. Oral Maxillofac. Surg. 2013, 51, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Linge, A.; Lohaus, F.; Löck, S.; Nowak, A.; Gudziol, V.; Valentini, C.; von Neubeck, C.; Jütz, M.; Tinhofer, I.; Budach, V.; et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 2016, 121, 364–373. [Google Scholar] [CrossRef]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Shah, J.P.; Candela, F.C.; Poddar, A.K. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer 1990, 66, 109–113. [Google Scholar] [CrossRef]
- Vogel, D.W.T.; Zbaeren, P.; Thoeny, H.C. Cancer of the oral cavity and oropharynx. Cancer Imaging 2010, 10, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Golusinski, P.; Di Maio, P.; Pehlivan, B.; Colley, S.; Nankivell, P.; Kong, A.; Hartley, A.; Mehanna, H. Evidence for the approach to the diagnostic evaluation of squamous cell carcinoma occult primary tumors of the head and neck. Oral Oncol. 2019, 88, 145–152. [Google Scholar] [CrossRef]
- Rassy, E.; Nicolai, P.; Pavlidis, N. Comprehensive management of HPV-related squamous cell carcinoma of the head and neck of unknown primary. Head Neck 2019, 41, 3700–3711. [Google Scholar] [CrossRef]
- Kuo, P.; Ba, M.M.C.; Decker, R.H.; Yarbrough, W.G.; Judson, B.L. Hypopharyngeal cancer incidence, treatment, and survival: Temporal trends in the United States. Laryngoscope 2014, 124, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.G.; MacLennan, K. Cervical node metastases in laryngeal and hypopharyngeal cancer: A prospective analysis of prevalence and distribution. Head Neck 2000, 22, 380–385. [Google Scholar] [CrossRef]
- Siddiqui, N.; Branstetter, B.; Hamilton, B.; Ginsberg, L.; Glastonbury, C.; Harnsberger, H.; Barnes, E.; Myers, E. Imaging characteristics of primary laryngeal lymphoma. AJNR Am. J. Neuroradiol. 2010, 31, 1261–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, C.-Y.; Yang, C.-F.; Gau, J.-P.; Kuan, E.C.; Ho, C.-Y.; Chiou, T.-J.; Hsiao, L.-T.; Lin, T.-A.; Lan, M.-Y. Nasopharyngeal Lymphoma: A 22-Year Review of 35 Cases. J. Clin. Med. 2019, 8, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Cancer Observatory. International Agency for Research on Cancer. World Health Organization. Available online: https://gco.iarc.fr/ (accessed on 16 February 2022).
- Chen, Y.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Ho, F.C.H.; Tham, I.W.K.; Earnest, A.; Lee, K.M.; Lu, J.J. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: A meta-analysis of clinical evidence. BMC Cancer 2012, 12, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.J.; Leung, S.W.; Lian, S.L.; Wang, C.J.; Fang, F.M.; Ho, Y.H. Patterns of distant metastases in nasopharyngeal carcinoma. Kaohsiung J. Med. Sci. 1996, 12, 229–234. [Google Scholar]
- Chang, M.-C.; Chen, J.-H.; Liang, J.-A.; Yang, K.-T.; Cheng, K.-Y.; Kao, C.-H. Accuracy of whole-body FDG-PET and FDG-PET/CT in M staging of nasopharyngeal carcinoma: A systematic review and meta-analysis. Eur. J. Radiol. 2013, 82, 366–373. [Google Scholar] [CrossRef]
- Mohandas, A.; Marcus, C.; Kang, H.; Truong, M.-T.; Subramaniam, R.M. FDG PET/CT in the management of nasopharyngeal carcinoma. AJR Am. J. Roentgenol. 2014, 203, W146–W157. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.J.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Xu, W.; Yan, W.-L.; Ye, M.; Bai, Y.-R.; Huang, G. FDG-PET, CT, MRI for diagnosis of local residual or recurrent nasopharyngeal carcinoma, which one is the best? A systematic review. Radiother. Oncol. 2007, 85, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, H.B. Imaging of sinonasal tumours. Cancer Imaging 2012, 12, 136–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Mizuta, K.; Aoki, M.; Hara, A.; Matsuo, M. Imaging Characteristics of Malignant Sinonasal Tumors. J. Clin. Med. 2017, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturk, K.; Gencturk, M.; Caicedo-Granados, E.; Li, F.; Cayci, Z. Utility of FDG PET/CT in the Characterization of Sinonasal Neoplasms: Analysis of Standardized Uptake Value Parameters. AJR Am. J. Roentgenol. 2018, 211, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Gencturk, M.; Caicedo-Granados, E.; Li, F.; Cayci, Z. Appropriate timing of surveillance intervals with whole-body 18F-FDG PET/CT following treatment for sinonasal malignancies. Eur. J. Radiol. 2019, 118, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.S.; Brooks, S.G.; Stubbs, V.; Ghosh, A.; Tajudeen, B.A.; Khalili, S.; Palmer, J.N.; Lee, J.Y.; Nabavizadeh, S.A.; Learned, K.O.; et al. Temporal patterns of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography sinonasal uptake after treatment of sinonasal malignancy. Int. Forum Allergy Rhinol 2016, 6, 1301–1307. [Google Scholar] [CrossRef]
- Salfrant, M.; Garcia, G.C.T.E.; Guichard, J.-P.; Bidault, F.; Reizine, D.; Aupérin, A.; Bresson, D.; Verillaud, B.; Herman, P.; Moya-Plana, A. Imaging of Skull Base and Orbital Invasion in Sinonasal Cancer: Correlation with Histopathology. Cancers 2021, 13, 4963. [Google Scholar] [CrossRef]
- Ozturk, K.; Gencturk, M.; Rischall, M.; Caicedo-Granados, E.; Li, F.; Cayci, Z. Role of Whole-Body 18F-FDG PET/CT in Screening for Metastases in Newly Diagnosed Sinonasal Malignancies. AJR Am. J. Roentgenol. 2019, 212, 1327–1334. [Google Scholar] [CrossRef]
- Maurer, A.; Meerwein, C.M.; Soyka, M.B.; Grünig, H.; Skawran, S.; Mühlematter, U.J.; Messerli, M.; Mader, C.E.; Husmann, L.; Rupp, N.J.; et al. Whole-body hybrid positron emission tomography imaging yields clinically relevant information in the staging and restaging of sinonasal tumors. Head Neck 2021, 43, 3572–3585. [Google Scholar] [CrossRef]
- Ozturk, K.; Gencturk, M.; Caicedo-Granados, E.; Li, F.; Cayci, Z. Performance of whole-body 18F-FDG PET/CT as a posttreatment surveillance tool for sinonasal malignancies. Eur. Arch. Otorhinolaryngol. 2019, 276, 847–855. [Google Scholar] [CrossRef]
- Lewis, J.S.J. Sinonasal Squamous Cell Carcinoma: A Review with Emphasis on Emerging Histologic Subtypes and the Role of Human Papillomavirus. Head Neck Pathol. 2016, 10, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaku, H.; Fujisaka, M.; Yasumura, S.; Ishida, M.; Tsubota, M.; Nishida, H.; Watanabe, Y.; Kawano, M.; Shimizu, M.; Fukuoka, J. Positron emission tomography for predicting malignancy of sinonasal inverted papilloma. Clin. Nucl. Med. 2007, 32, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.G.; Baredes, S.; Zuckier, L.S.; Mirani, N.M.; Liu, Y.; Ghesani, N.V. 18F-FDG PET evaluation of sinonasal papilloma. AJR Am. J. Roentgenol. 2009, 193, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Bhayani, M.K.; Yilmaz, T.; Sweeney, A.; Calzada, G.; Roberts, D.B.; Levine, N.B.; DeMonte, F.; Hanna, E.Y.; Kupferman, M.E. Sinonasal adenocarcinoma: A 16-year experience at a single institution. Head Neck 2014, 36, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Maina, I.W.; Kuan, E.C.; Triantafillou, V.; Trope, M.A.; Carey, R.M.; Workman, A.D.; Tong, C.C.; Kohanski, M.A.; Palmer, J.N.; et al. Adenocarcinoma of the Sinonasal Tract: A Review of the National Cancer Database. J. Neurol. Surg. Part B Skull Base 2020, 81, 701–708. [Google Scholar] [CrossRef]
- Lehmann, A.E.; Remenschneider, A.; Dedmon, M.; Meier, J.; Gray, S.T.; Lin, D.T.; Chambers, K.J. Incidence and survival patterns of sinonasal undifferentiated carcinoma in the United States. J. Neurol. Surg. Part B Skull Base 2015, 76, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Amit, M.; Abdelmeguid, A.S.; Watcherporn, T.; Takahashi, H.; Tam, S.; Bell, D.; Ferrarotto, R.; Glisson, B.; Kupferman, M.E.; Roberts, D.B.; et al. Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma. J. Clin. Oncol. 2019, 37, 504–512. [Google Scholar] [CrossRef]
- Kadish, S.; Goodman, M.; Wang, C.C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 1976, 37, 1571–1576. [Google Scholar] [CrossRef]
- Hyams, V.J.; Batsakis, J.G.; Michaels, L. Tumors of the upper respiratory tract and ear. In Atlas of Tumor Pathology; Armed Forces Institute of Pathology: Dhaka, Bangladesh, 1988. [Google Scholar]
- Leivo, I. Sinonasal Adenocarcinoma: Update on Classification, Immunophenotype and Molecular Features. Head Neck Pathol. 2016, 10, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Broski, S.M.; Hunt, C.H.; Johnson, G.B.; Subramaniam, R.M.; Peller, P.J. The added value of 18F-FDG PET/CT for evaluation of patients with esthesioneuroblastoma. J. Nucl. Med. 2012, 53, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Dadgar, H.; Norouzbeigi, N.; Ahmadzadehfar, H.; Assadi, M. 68Ga-DOTATATE and 18F-FDG PET/CT for the Management of Esthesioneuroblastoma of the Sphenoclival Region. Clin. Nucl. Med. 2020, 45, e363–e364. [Google Scholar] [CrossRef] [PubMed]
- Sabongi, J.G.; Gonçalves, M.C.P.; Alves, C.D.C.; Alves, J.; Scapulatempo-Neto, C.; Moriguchi, S.M. Lutetium 177-DOTA-TATE therapy for esthesioneuroblastoma: A case report. Exp. Ther. Med. 2016, 12, 3078–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, C.R.; Wiggins, R.H. FDG-PET Imaging of Salivary Gland Tumors. Semin. Ultrasound CT MRI 2019, 40, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Avey, G.D.; Wieland, A.M.; Harari, P.M.; Glazer, T.A.; McCulloch, T.M.; Hartig, G.K. Auriculotemporal Nerve Involvement in Parotid Bed Malignancy. Ann. Otol. Rhinol. Laryngol. 2019, 128, 647–653. [Google Scholar] [CrossRef]
- Graham, M.M.; Otsuka, H.; Kogame, M.; Nishitani, H. The impact of FDG-PET in the management of patients with salivary gland malignancy. Ann. Nucl. Med. 2005, 19, 691–694. [Google Scholar] [CrossRef]
- Khalesi, S. A Review of Carcinoma Ex-Pleomorphic Adenoma of the Salivary Glands. Int. J. Pathol. Clin. Res. 2016, 2. [Google Scholar] [CrossRef]
- Antony, J.; Gopalan, V.; Smith, R.A.; Lam, A.K.Y. Carcinoma ex pleomorphic adenoma: A comprehensive review of clinical, pathological and molecular data. Head Neck Pathol. 2012, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Raut, R.P.; Vaibhav, N.; Ghosh, A.; Keerthi, R. Carcinoma ex pleomorphic adenoma: Diagnostic dilemma and treatment protocol. Indian J. Dent. 2014, 5, 157–160. [Google Scholar] [CrossRef]
- Harrison, W.; Pittman, P.; Cummings, T. Pleomorphic adenoma of the lacrimal gland: A review with updates on malignant transformation and molecular genetics. Saudi J. Ophthalmol. 2018, 32, 13–16. [Google Scholar] [CrossRef]
- Avdagic, E.; Farber, N.; Katabi, N.; Shinder, R. Carcinoma Ex Pleomorphic Adenoma of the Lacrimal Gland with Epithelial-Myoepithelial Carcinoma Histologic Type. Ophthalmic Plast. Reconstr. Surg. 2017, 33, S136–S138. [Google Scholar] [CrossRef]
- Wei, P.; Shao, C.; Huan, T.; Wang, H.; Ding, Z.; Han, Z. Diagnostic value of maximum signal intensity on T1-weighted MRI images for differentiating parotid gland tumours along with pathological correlation. Clin. Radiol. 2021, 76, 472.e19–472.e25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, E.-K.; Park, C.S.; Choi, Y.-S.; Kim, Y.H.; Choi, E.C. Characteristic sonographic findings of Warthin’s tumor in the parotid gland. J. Clin. Ultrasound 2004, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Reny, D.C.; Ranasinghe, V.J.; Magana, L.C.; Kaufman, A.C.; Chalian, A.A.; O’Malley, B.W.J.; Weinstein, G.S.; Brody, R.M. Predictors of Nodal Metastasis in Mucoepidermoid Carcinoma of the Oral Cavity and Oropharynx. ORL 2020, 82, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Devaraju, R.; Gantala, R.; Aitha, H.; Gotoor, S.G. Mucoepidermoid carcinoma. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Adams, A.L. Mucoepidermoid carcinoma of the bronchus: A review. Arch. Pathol. Lab. Med. 2007, 131, 1400–1404. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Ramshankar, V.; Majhi, U. Role of fluorine-18-fluorodeoxyglucose positron emission tomography-computed tomography in management of pulmonary mucoepidermoid carcinomas and review of literature. Indian J. Nucl. Med. 2016, 31, 128–130. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.M.; Garcia, J.; Granchi, P.J.; Bs, J.J.; Eisele, D.W. Late recurrence from salivary gland cancer: When does “cure” mean cure? Cancer 2008, 112, 340–344. [Google Scholar] [CrossRef]
- Shum, J.W.; Chatzistefanou, I.; Qaisi, M.; Lubek, J.E.; Ord, R.A. Adenoid cystic carcinoma of the minor salivary glands: A retrospective series of 29 cases and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 210–214. [Google Scholar] [CrossRef]
- Li, W.; Hua, W.; Yan, F.-G.; Shen, H.-H.; Xu, H. Adenoid cystic carcinoma of trachea: A case report and review of literature. Chin. Med. J. 2012, 125, 2238–2239. [Google Scholar]
- Ide, F.; Mishima, K.; Yamada, H.; Saito, I. Adenoid cystic carcinoma ex pleomorphic adenoma of the parotid gland. Head Neck Pathol. 2009, 3, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Castelnuovo, P.; Turri-Zanoni, M. Adenoid Cystic Carcinoma. Adv. Otorhinolaryngol. 2020, 84, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Ruhlmann, V.; Poeppel, T.D.; Veit, J.; Nagarajah, J.; Umutlu, L.; Hoffmann, T.K.; Bockisch, A.; Herrmann, K.; Sauerwein, W. Diagnostic accuracy of 18F-FDG PET/CT and MR imaging in patients with adenoid cystic carcinoma. BMC Cancer 2017, 17, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Boxtel, W.; Lütje, S.; Grunsven, I.C.V.E.-V.; Verhaegh, G.W.; Schalken, J.A.; Jonker, M.A.; Nagarajah, J.; Gotthardt, M.; van Herpen, C.M. 68Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics 2020, 10, 2273–2283. [Google Scholar] [CrossRef]
- Barrio, M.; Czernin, J.; Yeh, M.W.; Diaz, M.F.P.; Gupta, P.; Allen-Auerbach, M.; Schiepers, C.; Herrmann, K. The incidence of thyroid cancer in focal hypermetabolic thyroid lesions: An 18F-FDG PET/CT study in more than 6000 patients. Nucl. Med. Commun. 2016, 37, 1290–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soelberg, K.K.; Bonnema, S.J.; Brix, T.H.; Hegedüs, L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Thyroid 2012, 22, 918–925. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Zampella, E.; Klain, M.; Pace, L.; Cuocolo, A. PET/CT in the management of differentiated thyroid cancer. Diagn. Interv. Imaging 2021, 102, 515–523. [Google Scholar] [CrossRef]
- Stokkel, M.P.M.; Duchateau, C.S.J.; Dragoiescu, C. The value of FDG-PET in the follow-up of differentiated thyroid cancer: A review of the literature. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 78–87. [Google Scholar]
- Kim, S.-J.; Lee, S.-W.; Pak, K.; Shim, S.R. Diagnostic performance of PET in thyroid cancer with elevated anti-Tg Ab. Endocr. Relat. Cancer. 2018, 25, 643–652. [Google Scholar] [CrossRef]
- Kolodziej, M.; Saracyn, M.; Lubas, A.; Brodowska-Kania, D.; Mazurek, A.; Dziuk, M.; Dymus, J.; Kaminski, G. Evaluation of the usefulness of positron emission tomography with (18F)fluorodeoxylglucose performed to detect non-radioiodine avid recurrence and/or metastasis of differentiated thyroid cancer—A preliminary study. Nucl. Med. Rev. 2021, 24, 63–69. [Google Scholar] [CrossRef]
- Rosenbaum-Krumme, S.J.; Görges, R.; Bockisch, A.; Binse, I. ¹⁸F-FDG PET/CT changes therapy management in high-risk DTC after first radioiodine therapy. Eur. J. Nucl. Med. Mol. Imaging. 2012, 39, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Russell, J.; Rooper, L.M.; Ladenson, P.W.; Pomper, M.G.; Rowe, S.P. The prostate-specific membrane antigen (PSMA)-targeted radiotracer 18F-DCFPyL detects tumor neovasculature in metastatic, advanced, radioiodine-refractory, differentiated thyroid cancer. Med. Oncol. 2020, 37, 98. [Google Scholar] [CrossRef] [PubMed]
- Wächter, S.; Di Fazio, P.; Maurer, E.; Manoharan, J.; Keber, C.; Pfestroff, A.; Librizzi, D.; Bartsch, D.K.; Luster, M.; Eilsberger, F. Prostate-Specific Membrane Antigen in Anaplastic and Poorly Differentiated Thyroid Cancer-A New Diagnostic and Therapeutic Target? Cancers 2021, 13, 5688. [Google Scholar] [CrossRef] [PubMed]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Wagle, N.; Grabiner, B.C.; Van Allen, E.M.; Amin-Mansour, A.; Taylor-Weiner, A.; Rosenberg, M.; Gray, N.S.; Barletta, J.A.; Guo, Y.; Swanson, S.J.; et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med. 2014, 371, 1426–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulcahy, N. FDA OKs Targeted Therapy Combo for Anaplastic Thyroid Cancer. Medscape Medical News. 4 May 2018. Available online: https://www.medscape.com/viewarticle/896190 (accessed on 8 February 2018).
- Wiseman, S.M.; Masoudi, H.; Niblock, P.; Turbin, D.; Rajput, A.; Hay, J.; Bugis, S.; Filipenko, D.; Huntsman, D.; Gilks, B. Anaplastic thyroid carcinoma: Expression profile of targets for therapy offers new insights for disease treatment. Ann. Surg. Oncol. 2007, 14, 719–729. [Google Scholar] [CrossRef]
- Bogsrud, T.V.; Karantanis, D.; Nathan, M.A.; Mullan, B.P.; Wiseman, G.A.; Kasperbauer, J.L.; Reading, C.C.; Hay, I.D.; Lowe, V.J. 18F-FDG PET in the management of patients with anaplastic thyroid carcinoma. Thyroid 2008, 18, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A.J. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.E.; Sherman, S.; O’Dorisio, T.; Howe, J. Medical management of metastatic medullary thyroid cancer. Cancer 2014, 120, 3287–3301. [Google Scholar] [CrossRef] [Green Version]
- Treglia, G.; Villani, M.F.; Giordano, A.; Rufini, V. Detection rate of recurrent medullary thyroid carcinoma using fluorine-18 fluorodeoxyglucose positron emission tomography: A meta-analysis. Endocrine 2012, 42, 535–545. [Google Scholar] [CrossRef]
- Rodríguez-Bel, L.; Sabaté-Llobera, A.; Rossi-Seoane, S.; Reynes-Llompart, G.; Conejero, J.L.V.; Cos-Domingo, M.; Moreno-Llorente, P.; Pérez-Maraver, M.; Cortés-Romera, M.; Gamez-Cenzano, C. Diagnostic accuracy of 18F-FDG PET/CT in patients with biochemical evidence of recurrent, residual, or metastatic medullary thyroid carcinoma. Clin. Nucl. Med. 2019, 44, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Damle, N.A.; Parida, G.K.; Singhal, A.; Nalli, H.; Dattagupta, S.; Bal, C. Recurrent Medullary Thyroid Carcinoma on 68Ga-Prostate-Specific Membrane Antigen PET/CT: Exploring New Theranostic Avenues. Clin. Nucl. Med. 2018, 43, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Conry, B.G.; Papathanasiou, N.D.; Prakash, V.; Kayani, I.; Caplin, M.; Mahmood, S.; Bomanji, J.B. Comparison of (68)Ga-DOTATATE and (18)F-fluorodeoxyglucose PET/CT in the detection of recurrent medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.E.; Uslu-Beşli, L.; Asa, S.; Sağer, S.; Sönmezoğlu, K. The role of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT in the follow-up of patients with medullary thyroid cancer. Hell. J. Nucl. Med. 2020, 23, 321–329. [Google Scholar] [CrossRef]
- Holzgreve, A.; Albert, N.; Galldiks, N.; Suchorska, B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers 2021, 13, 2093. [Google Scholar] [CrossRef]
- Zhang, J.; Traylor, K.S.; Mountz, J.M. PET and SPECT Imaging of Brain Tumors. Semin. Ultrasound CT MRI 2020, 41, 530–540. [Google Scholar] [CrossRef]
- Shooli, H.; Dadgar, H.; Wáng, Y.-X.J.; Vafaee, M.S.; Kashuk, S.R.; Nemati, R.; Jafari, E.; Nabipour, I.; Gholamrezanezhad, A.; Assadi, M.; et al. An update on PET-based molecular imaging in neuro-oncology: Challenges and implementation for a precision medicine approach in cancer care. Quant. Imaging Med. Surg. 2019, 9, 1597–1610. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; McConathy, J. Overview of PET Tracers for Brain Tumor Imaging. PET Clin. 2013, 8, 129–146. [Google Scholar] [CrossRef]
- Basu, S.; Alavi, A. Molecular imaging (PET) of brain tumors. Neuroimaging Clin. N. Am. 2009, 19, 625–646. [Google Scholar] [CrossRef]
- Verger, A.; Kas, A.; Darcourt, J.; Guedj, E. PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers 2022, 14, 1103. [Google Scholar] [CrossRef]
- Zhang-Yin, J.T.; Girard, A.; Bertaux, M. What Does PET Imaging Bring to Neuro-Oncology in 2022? A Review. Cancers 2022, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Krex, D.; Klink, B.; Hartmann, C.; von Deimling, A.; Pietsch, T.; Simon, M.; Sabel, M.; Steinbach, J.P.; Heese, O.; Reifenberger, G.; et al. Long-term survival with glioblastoma multiforme. Brain 2007, 130, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, A.M.; Zadeh, G.; et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro-Oncology 2017, 19, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Nandu, H.; Wen, P.Y.; Huang, R.Y. Imaging in neuro-oncology. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418759865. [Google Scholar] [CrossRef] [Green Version]
- Greenlee, J.E.; Carlson, N.G.; Abbatemarco, J.R.; Herdlevær, I.; Clardy, S.L.; Vedeler, C.A. Paraneoplastic and Other Autoimmune Encephalitides: Antineuronal Antibodies, T Lymphocytes, and Questions of Pathogenesis. Front. Neurol. 2022, 12, 744653. [Google Scholar] [CrossRef]
- Nandu, H.; Berkowitz, F.; Esposito, G. False-positive finding of brain metastasis on (18)F-FDG PET imaging due to early subacute ischemic stroke. Clin. Nucl. Med. 2013, 38, 465–466. [Google Scholar] [CrossRef]
- Gandy, N.; Arshad, A.M.; Wallitt, K.L.; Dubash, S.; Khan, S.; Barwick, T.D. Immunotherapy-related adverse effects on 18F-FDG PET/CT imaging. Br. J. Radiol. 2020, 93, 20190832. [Google Scholar] [CrossRef]
- Di Chiro, G.; DeLaPaz, R.L.; Brooks, R.A.; Sokoloff, L.; Kornblith, P.L.; Smith, B.H.; Patronas, N.J.; Kufta, C.V.; Kessler, R.M.; Johnston, G.S.; et al. Glucose utilization of cerebral gliomas measured by (18)F fluorodeoxyglucose and positron emission tomography. Neurology 1982, 32, 1323–1329. [Google Scholar] [CrossRef]
- Di Chiro, G.; Brooks, A.R. PET-FDG of untreated and treated cerebral gliomas. J. Nucl. Med. 1988, 29, 421–423. [Google Scholar]
- Parent, E.E.; Johnson, D.R.; Gleason, T.; Villanueva-Meyer, J.E. Neuro-Oncology Practice Clinical Debate: FDG PET to differentiate glioblastoma recurrence from treatment-related changes. Neurooncol. Pract. 2021, 8, 518–525. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Bailo, M.; Cicone, F.; Carideo, L.; Quartuccio, N.; Mortini, P.; Falini, A.; Cascini, G.; Minniti, G. Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers 2021, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Lohmann, P.; Albert, N.L.; Tonn, J.C.; Langen, K.-J. Current status of PET imaging in neuro-oncology. Neurooncol Adv. 2019, 1, vdz010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parent, E.E.; Benayoun, M.; Ibeanu, I.; Olson, J.J.; Hadjipanayis, C.G.; Brat, D.J.; Adhikarla, V.; Nye, J.; Schuster, D.M.; Goodman, M.M. (18F)Fluciclovine PET discrimination between high- and low-grade gliomas. EJNMMI Res. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Enslow, M.S.; Zollinger, L.V.; Morton, K.A.; Butterfield, R.I.; Kadrmas, D.J.; Christian, P.E.; Boucher, K.; Heilbrun, M.E.; Jensen, R.L.; Hoffman, J.M. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin. Nucl. Med. 2012, 37, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Binneboese, A.; Covington, M.F.; Horn, K.P.; Archibald, Z.G.; Boucher, K.M.; Morton, A.K.; Hoffman, J.M. Correlation between FDG-PET uptake and survival in patients with primary brain tumors. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 196–206. [Google Scholar]
- De Witte, O.; Levivier, M.; Violon, P.; Salmon, I.; Damhaut, P.; Wikler, D.J.; Hildebrand, J.; Brotchi, J.; Goldman, S. Prognostic value positron emission tomography with (18F)fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery 1996, 39, 470–476. [Google Scholar] [CrossRef]
- Kosaka, N.; Tsuchida, T.; Uematsu, H.; Kimura, H.; Okazawa, H.; Itoh, H. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am. J. Roentgenol. 2008, 190, W365–W369. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshiura, T.; Hiwatashi, A.; Togao, O.; Yoshimoto, K.; Suzuki, S.O.; Abe, K.; Kikuchi, K.; Maruoka, Y.; Mizoguchi, M.; et al. Differentiating primary CNS lymphoma from glioblastoma multiforme: Assessment using arterial spin labeling, diffusion-weighted imaging, and 18F-fluorodeoxyglucose positron emission tomography. Neuroradiology 2013, 55, 135–143. [Google Scholar] [CrossRef]
- Tralins, K.S.; Douglas, J.G.; Stelzer, K.J.; Mankoff, A.D.; Silbergeld, D.L.; Rostomily, R.C.; Hummel, S.; Scharnhorst, J.; Krohn, A.K.; Spence, A.M.; et al. Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: Prognostic information and possible role in definition of target volumes in radiation dose escalation. J. Nucl. Med. 2002, 43, 1667–1673. [Google Scholar] [PubMed]
- Kawai, N.; Miyake, K.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. 18F-FDG PET in the diagnosis and treatment of primary central nervous system lymphoma. Biomed Res. Int. 2013, 2013, 247152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, S.S.; Hoffman, J.M.; Coleman, R.E.; Glantz, M.J.; Hanson, M.W.; Schold, S.C. Studies of primary central nervous system lymphoma with fluorine-18-fluorodeoxyglucose positron emission tomography. J. Nucl. Med. 1992, 33, 532–536. [Google Scholar] [PubMed]
- Park, H.Y.; Suh, C.H.; Huang, R.Y.; Guenette, J.P.; Kim, H.S. Diagnostic Yield of Body CT and Whole-Body FDG PET/CT for Initial Systemic Staging in Patients With Suspected Primary CNS Lymphoma: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2021, 216, 1172–1182. [Google Scholar] [CrossRef]
- Marcus, C.; Feizi, P.; Hogg, J.; Summerfield, H.; Castellani, R.; Sriwastava, S.; Marano, G.D. Imaging in Differentiating Cerebral Toxoplasmosis and Primary CNS Lymphoma With Special Focus on FDG PET/CT. AJR Am. J. Roentgenol. 2021, 216, 157–164. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bal, C.; Tripathi, M.; Das, C.J.; Shamim, S.A. Cerebral Toxoplasmosis Masquerading CNS Lymphoma on FDG PET-CT in Post Renal Transplant Patient. Indian J. Nucl. Med. 2017, 32, 148–149. [Google Scholar] [CrossRef] [Green Version]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response Assessment in Neuro-Oncology (RANO) group. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Krüger, S.; Mottaghy, F.M.; Buck, A.K.; Maschke, S.; Kley, H.; Frechen, D.; Wibmer, T.; Reske, S.N.; Pauls, S. Brain metastasis in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for diagnosis in the initial staging. Nuklearmedizin 2011, 50, 101–106. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.C.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.J.; Albert, N.L. 18F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2016, 58, 584–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicone, F.; Carideo, L.; Scaringi, C.; Romano, A.; Mamede, M.; Papa, A.; Tofani, A.; Cascini, G.L.; Bozzao, A.; Scopinaro, F.; et al. Long-term metabolic evolution of brain metastases with suspected radiation necrosis following stereotactic radiosurgery: Longitudinal assessment by F-DOPA PET. Neuro-Oncology 2021, 23, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, I.E.; Waguespack, S.G.; Fuller, G.; Couldwell, W.T. Metastatic melanoma to the pituitary gland. Can. J. Neurol. Sci. 2007, 34, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Mehta, P.; Bartscht, T.; Schmid, S.M.; Fahlbusch, F.B.; Rades, D. Prevalence of metastases within the hypothalamic-pituitary area in patients with brain metastases. Radiat. Oncol. 2019, 14, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, S.T.; Suh, J.H.; Raja, S.; Lee, S.-Y.; Barnett, G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int. J. Cancer 2001, 96, 191–197. [Google Scholar] [CrossRef]
- Horky, L.L.; Hsiao, E.M.; Weiss, S.E.; Drappatz, J.; Gerbaudo, V.H. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J. Neurooncol. 2011, 103, 137–146. [Google Scholar] [CrossRef]
- Maza, S.; Buchert, R.; Brenner, W.; Munz, D.L.; Thiel, E.; Korfel, A.; Kiewe, P. Brain and whole-body FDG-PET in diagnosis, treatment monitoring and long-term follow-up of primary CNS lymphoma. Radiol. Oncol. 2013, 47, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Rozental, J.M.; Levine, R.L.; Mehta, M.P.; Kinsella, T.J.; Levin, A.B.; Allan, O.; Mendoza, M.; Hanson, J.M.; Schrader, D.A.; Nickles, R.J. Early changes in tumor metabolism after treatment: The effects of stereotactic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 1053–1060. [Google Scholar] [CrossRef]
- Rozental, J.M.; Levine, R.L.; Nickles, R.J.; Dobkin, J.A. Glucose uptake by gliomas after treatment. A positron emission tomographic study. Arch. Neurol. 1989, 46, 1302–1307. [Google Scholar] [CrossRef]
- Glantz, M.J.; Hoffman, J.M.; Coleman, R.E.; Friedman, A.H.; Hanson, M.W.; Burger, P.C.; Herndon, J.E.; Meisler, W.J.; Schold, S.C.J. Identification of early recurrence of primary central nervous system tumors by (18-F)fluorodeoxyglucose positron emission tomography. Ann. Neurol. 1991, 29, 347–355. [Google Scholar] [CrossRef]
- Buchpiguel, C.A.; Alavi, J.B.; Alavi, A.; Kenyon, L.C. PET versus SPECT in distinguishing radiation necrosis from tumor recurrence in the brain. J. Nucl. Med. 1995, 36, 159–164. [Google Scholar] [PubMed]
- De Witte, O.; Lefranc, F.; Levivier, M.; Salmon, I.; Brotchi, J.; Goldman, S. FDG-PET as a prognostic factor in high-grade astrocytoma. J. Neurooncol. 2000, 49, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Patronas, N.J.; Di Chiro, G.; Brooks, A.R.; Delapaz, R.L.; Kornblith, P.L.; Smith, B.H.; Rizzoli, H.V.; Kessler, R.M.; Manning, R.G.; Channing, M.; et al. Work in progress: (18F) fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 1982, 144, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.; Alfonso, J.M.; Ossola, G.; Pozo, M.A.; Santos, M.; Rodrìguez, S. The Role of PET-FDG in resolving diagnostic doubt: Recurrence vs. radionecrosis in brain tumors: Experience in 94 patients. Riv. Di Neuroradiol. 2003, 16, 887–890. [Google Scholar] [CrossRef]
- Alavi, J.B.; Alavi, A.; Chawluk, J.; Kushner, M.; Powe, J.; Hickey, W.; Reivich, M. Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 1988, 62, 1074–1078. [Google Scholar] [CrossRef]
- Brock, C.S.; Young, H.; O’Reilly, S.M.; Matthews, J.; Osman, S.; Evans, H.; Newlands, E.S.; Price, P.M. Early evaluation of tumor metabolic response using (18F)fluorodeoxyglucose and positron emission tomography: A pilot study following the phase II chemotherapy schedule for temozolomide in recurrent high-grade gliomas. Br. J. Cancer 2000, 82, 608–615. [Google Scholar] [CrossRef]
- Paulus, W.; Peiffer, J. Intratumoral histological heterogeneity of gliomas. A quantitative study. Cancer 1989, 64, 442–447. [Google Scholar] [CrossRef]
- Burger, P.C.; Kleihues, P. Cytologic composition of the untreated glioblastoma with implications for evaluation of needle biopsies. Cancer 1989, 63, 2014–2023. [Google Scholar] [CrossRef]
- Hua, L.; Hua, F.; Zhu, H.; Deng, J.; Wang, D.; Luan, S.; Tang, H.; Guan, Y.; Xie, Q.; Gong, Y. The Diagnostic Value Of Using 18F-Fluorodeoxyglucose Positron Emission Tomography To Differentiate Between Low- And High-Grade Meningioma. Cancer Manag. Res. 2019, 11, 9185–9193. [Google Scholar] [CrossRef] [Green Version]
- Seystahl, K.; Stoecklein, V.; Schüller, U.; Rushing, E.; Nicolas, G.; Schäfer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.-C.; et al. Somatostatin-receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro-Oncology 2016, 18, 1538–1547. [Google Scholar] [CrossRef] [Green Version]

















































| Hopkins Score | FDG Uptake | Interpretation |
|---|---|---|
| 1 | Focal uptake < IJV | Complete metabolic response |
| 2 | Focal uptake > IJV but <liver | Likely complete metabolic response |
| 3 | Diffuse uptake > IJV and <liver | Likely post-treatment inflammation |
| 4 | Focal uptake > liver | Likely residual tumor |
| 5 | Focal uptake markedly > liver | Residual tumor |
| Category | Interpretation | Imaging Findings | Management Recommendation |
|---|---|---|---|
| 0 | Incomplete | New baseline without prior comparisons available | Compare to previous imaging |
| 1 | No evidence of recurrence/persistence | Expected post-treatment changes, no abnormal FDG uptake | Routine surveillance |
| 2a | Low suspicion of recurrence/persistence (endoscopically superficial lesion) | Focal mucosal enhancement, mild to moderate mucosal FDG uptake | Direct visual inspection |
| 2b | Low suspicion of recurrence/persistence (deep lesion) | No discrete mass and either no, mild or moderate FDG uptake | Short imaging follow-up |
| 3 | High suspicion of recurrence/persistence | New or enlarged mass or node, enhancement and intense FDG uptake | Biopsy |
| 4 | Definite recurrence/persistence | Histologically proven or definite progression | Clinical management |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiggins, R.H.; Hoffman, J.M.; Fine, G.C.; Covington, M.F.; Salem, A.E.; Koppula, B.R.; Morton, K.A. PET-CT in Clinical Adult Oncology—V. Head and Neck and Neuro Oncology. Cancers 2022, 14, 2726. https://doi.org/10.3390/cancers14112726
Wiggins RH, Hoffman JM, Fine GC, Covington MF, Salem AE, Koppula BR, Morton KA. PET-CT in Clinical Adult Oncology—V. Head and Neck and Neuro Oncology. Cancers. 2022; 14(11):2726. https://doi.org/10.3390/cancers14112726
Chicago/Turabian StyleWiggins, Richard H., John M. Hoffman, Gabriel C. Fine, Matthew F. Covington, Ahmed Ebada Salem, Bhasker R. Koppula, and Kathryn A. Morton. 2022. "PET-CT in Clinical Adult Oncology—V. Head and Neck and Neuro Oncology" Cancers 14, no. 11: 2726. https://doi.org/10.3390/cancers14112726






