Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Postoperative Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pathological Findings and Surgical Outcomes
3.3. Predictive Risk Factors of Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, J.D.; Fu, P.; Harth, K.C.; Margevicius, S.; Wilhelm, S.M. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 2010, 148, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Clark, O.H. Thyroid cancer and lymph node metastases. J. Surg. Oncol. 2011, 103, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Morris, L.G.; Shaha, A.R.; Tuttle, R.M.; Sikora, A.G.; Ganly, I. Tall-cell variant of papillary thyroid carcinoma: A matched-pair analysis of survival. Thyroid 2010, 20, 153–158. [Google Scholar] [CrossRef]
- Kazaure, H.S.; Roman, S.A.; Sosa, J.A. Aggressive variants of papillary thyroid cancer: Incidence, characteristics and predictors of survival among 43,738 patients. Ann. Surg. Oncol. 2012, 19, 1874–1880. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, W.; Chen, T.; Guo, Y.; Zhang, C.; Liu, C.; Huang, T. Correction: A comparison of the clinicopathological features and prognoses of the classical and the tall cell variant of papillary thyroid cancer: A meta-analysis. Oncotarget 2018, 9, 16271. [Google Scholar] [CrossRef]
- Wong, K.S.; Higgins, S.E.; Marqusee, E.; Nehs, M.A.; Angell, T.; Barletta, J.A. Tall cell variant of papillary thyroid carcinoma: Impact of change in WHO definition and molecular analysis. Endocr. Pathol. 2019, 30, 43–48. [Google Scholar] [CrossRef]
- Longheu, A.; Canu, G.L.; Cappellacci, F.; Erdas, E.; Medas, F.; Calò, P.G. Tall cell variant versus conventional papillary thyroid carcinoma: A retrospective analysis in 351 consecutive patients. J. Clin. Med. 2020, 10, 70. [Google Scholar] [CrossRef]
- Asioli, S.; Erickson, L.A.; Sebo, T.J.; Zhang, J.; Jin, L.; Thompson, G.B.; Lloyd, R.V. Papillary thyroid carcinoma with prominent hobnail features: A new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am. J. Surg. Pathol. 2010, 34, 44–52. [Google Scholar] [CrossRef]
- Chen, J.-H.; Faquin, W.C.; Lloyd, R.V.; Nosé, V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod. Pathol. 2011, 24, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Spyroglou, A.; Kostopoulos, G.; Bramis, K.; Tseleni, S.; Toulis, K.; Mastorakos, G.; Konstadoulakis, M.; Vamvakidis, K.; Alexandraki, K. Hobnail variant of papillary thyroid carcinoma, a systematic review and meta-analysis. Endocrine 2022, 72, 27–39. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.S.; Hong, S.; Park, C.S. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid 2009, 19, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Regalbuto, C.; Malandrino, P.; Tumminia, A.; Le Moli, R.; Vigneri, R.; Pezzino, V. A diffuse sclerosing variant of papillary thyroid carcinoma: Clinical and pathologic features and outcomes of 34 consecutive cases. Thyroid 2011, 21, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Gopalan, V.; Smith, R.A.; Lam, A.K.-Y. Diffuse sclerosing variant of papillary thyroid carcinoma—an update of its clinicopathological features and molecular biology. Crit. Rev. Oncol./Hematol. 2015, 94, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Shen, F.; Cai, W.; Gan, X.; Deng, X.; Xu, B. Survival of aggressive variants of papillary thyroid carcinoma in patients under 55 years old: A SEER population-based retrospective analysis. Endocrine 2018, 61, 499–505. [Google Scholar] [CrossRef]
- Cavaco, D.; Martins, A.F.; Cabrera, R.; Vilar, H.; Leite, V. Diffuse sclerosing variant of papillary thyroid carcinoma: Outcomes of 33 cases. Eur. Thyroid. J. 2022, 11, e210020. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Erickson, L.A.; Nikiforova, M.N.; Caudill, C.M.; Lloyd, R.V. Solid variant of papillary thyroid carcinoma: Incidence, clinical–pathologic characteristics, molecular analysis, and biologic behavior. Am. J. Surg. Pathol. 2001, 25, 1478–1484. [Google Scholar] [CrossRef]
- Vuong, H.G.; Odate, T.; Duong, U.N.; Mochizuki, K.; Nakazawa, T.; Katoh, R.; Kondo, T. Prognostic importance of solid variant papillary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2018, 40, 1588–1597. [Google Scholar] [CrossRef]
- Kim, B.W.; Yousman, W.; Wong, W.X.; Cheng, C.; McAninch, E.A. Less is more: Comparing the 2015 and 2009 American Thyroid Association guidelines for thyroid nodules and cancer. Thyroid 2016, 26, 759–764. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contal, C.; O’Quigley, J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput. Stat. Data Anal. 1999, 30, 253–270. [Google Scholar] [CrossRef]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef]
- Vaccarella, S.; Dal Maso, L.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef]
- Silver, C.E.; Owen, R.P.; Rodrigo, J.P.; Rinaldo, A.; Devaney, K.O.; Ferlito, A. Aggressive variants of papillary thyroid carcinoma. Head Neck 2011, 33, 1052–1059. [Google Scholar] [CrossRef]
- Limberg, J.; Ullmann, T.M.; Stefanova, D.; Buicko, J.L.; Finnerty, B.M.; Zarnegar, R.; Fahey, T.J., III; Beninato, T. Does aggressive variant histology without invasive features predict overall survival in papillary thyroid cancer? A national cancer database analysis. Ann. Surg. 2021, 274, e276–e281. [Google Scholar] [CrossRef]
- Wenig, B.M.; Thompson, L.D.; Adair, C.F.; Shmookler, B.; Heffess, C.S. Thyroid papillary carcinoma of columnar cell type: A clinicopathologic study of 16 cases. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 82, 740–753. [Google Scholar] [CrossRef]
- Ganly, I.; Ibrahimpasic, T.; Rivera, M.; Nixon, I.; Palmer, F.; Patel, S.G.; Tuttle, R.M.; Shah, J.P.; Ghossein, R. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid 2014, 24, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Jeon, M.J.; Oh, H.-S.; Han, M.; Lee, Y.-M.; Kim, T.Y.; Chung, K.-W.; Kim, W.B.; Shong, Y.K.; Song, D.E. Do aggressive variants of papillary thyroid carcinoma have worse clinical outcome than classic papillary thyroid carcinoma? Eur. J. Endocrinol. 2018, 179, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C. Papillary thyroid cancer—Aggressive variants and impact on management: A narrative review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef]
- Chan, S.; Karamali, K.; Kolodziejczyk, A.; Oikonomou, G.; Watkinson, J.; Paleri, V.; Nixon, I.; Kim, D. Systematic review of recurrence rate after hemithyroidectomy for low-risk well-differentiated thyroid cancer. Eur. Thyroid. J. 2020, 9, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.; Fingeret, A. Contemporary evaluation and management of tall cell variant of papillary thyroid carcinoma. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 351–357. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Zhao, Q.; Song, H.; Yi, P.; Liu, C. Columnar cell papillary thyroid carcinoma prognosis: Findings from the SEER database using propensity score matching analysis. Am. J. Transl. Res. 2019, 11, 6262. [Google Scholar] [PubMed]
| HT Group (n = 46) | BTT Group (n = 203) | p-Value | |
|---|---|---|---|
| Sex, male:female | 10:36 | 55:148 | 0.455 |
| Age (years) | 38.0 ± 12.3 (16–66) | 35.7 ± 13.1 (7–79) | 0.195 |
| Diagnostic Sx | |||
| Incidentaloma | 43 (93.5) | 152 (74.9) | 0.006 |
| Anterior neck mass | 3 (6.5) | 38 (18.7) | 0.044 |
| Lateral neck mass | 0 (0.0) | 6 (3.0) | 0.238 |
| Hoarseness | 0 (0.0) | 5 (2.5) | 0.282 |
| Others | 0 (0.0) | 2 (1.0) | 0.499 |
| Hypothyroidism hx | 0 (0.0) | 5 (2.5) | 0.282 |
| Family hx | 12 (26.1) | 27 (13.3) | 0.031 |
| Thyroid cancer of first degree | 7 (15.2) | 18 (8.9) | |
| Thyroid cancer of second degree | 0 (0.0) | 1 (0.5) | |
| Others (other organ cancers, hypothyroidism) | 5 (10.7) | 8 (4.0) |
| HT Group (n = 46) | BTT Group (n = 203) | p-Value | |
|---|---|---|---|
| Clinical T stage | <0.001 | ||
| T1 | 31 (67.4) | 47 (23.2) | |
| T2 | 9 (19.6) | 27 (13.3) | |
| T3 | 6 (13.0) | 124 (61.1) | |
| T4 | 0 (0.0) | 5 (2.5) | |
| Clinical N stage | <0.001 | ||
| N0 | 41 (89.1) | 45 (22.2) | |
| N1a | 5 (10.9) | 22 (10.8) | |
| N1b | 0 (0.0) | 136 (67.0) | |
| Clinical M stage | 0.201 | ||
| M0 | 46 (100.0) | 196 (96.6) | |
| M1 | 0 (0.0) | 7 (3.4) | |
| US-guided detection of aggressive tumors | 2 (4.3) | 76 (37.4) | <0.001 |
| HT Group (n = 46) | BTT Group (n = 203) | p-Value | |
|---|---|---|---|
| Pathologic cancer subtype | <0.001 | ||
| Diffuse sclerosing variant | 19 (41.3) | 163 (80.3) | |
| Solid variant | 19 (41.3) | 23 (11.3) | |
| Tall cell variant | 6 (13.0) | 13 (6.4) | |
| Hobnail variant | 1 (2.2) | 3 (1.5) | |
| Columnar cell variant | 1 (2.2) | 1 (0.5) | |
| Tumor size (cm) | 1.2 ± 0.8 (0.4–3.5) | 1.9 ± 1.2 (0.3–7.0) | 0.226 |
| Tumor number | <0.001 | ||
| Single | 35 (76.1) | 90 (44.3) | |
| Multiple | 11 (23.9) | 113 (55.7) | |
| Bilaterality | 3 (6.5) | 123 (60.0) | <0.001 |
| Variant only | 0 (0.0) | 73 (36.0) | |
| Conventional PTC mixed | 3 (6.5) | 50 (24.6) | |
| Metastatic LN positive | 19 (41.3) | 175 (86.2) | <0.001 |
| No. of harvested LNs | |||
| Positive nodes of CCND | 1.6 ± 2.4 | 6.7 ± 5.9 | <0.001 |
| Total node of CCND | 4.9 ± 2.9 | 11.1 ± 7.5 | 0.091 |
| Positive node of MRND | 10.2 ± 6.9 | ||
| Total node of MRND | 48.1 ± 26.5 | ||
| Perinodal soft tissue extension positive | 1 (2.2) | 108 (53.2) | <0.001 |
| Microscopic ETE positive | 18 (39.1) | 156 (76.8) | <0.001 |
| Gross ETE positive | 0 (0.0) | 47 (23.2) | <0.001 |
| LVI positive | 5 (10.9) | 46 (22.7) | 0.074 |
| BRAF mutation positive | 22 (47.8) | 45 (43.3) (n = 104) | 0.605 |
| TERT promoter mutation positive | 0 (0.0) | 4 (16.0) (n = 25) | 0.005 |
| HT Group (n = 46) | BTT Group (n = 203) | p-Value | |
|---|---|---|---|
| Postoperative adjuvant Tx | 0 (0.0) | 192 (94.6) | <0.001 |
| Low-dose RAI | 0 (0.0) | 49 (24.1) | |
| High-dose RAI | 0 (0.0) | 143 (70.4) | |
| Recurrence rate | 2 (4.3) | 22 (10.8) | 0.178 |
| Recurrence site | 0.397 | ||
| Local | 2 (4.3) | 11 (5.4) | |
| Distant | 0 (0.0) | 7 (3.4) | |
| Local and distant | 0 (0.0) | 4 (2.0) | |
| Additional Tx after recurrence | 0.605 | ||
| Reoperation | 0 (0.0) | 2 (1.0) | |
| High-dose RAI | 0 (0.0) | 6 (3.0) | |
| Reoperation with postoperative high-dose RAI | 2 (4.3) | 11 (5.4) | |
| Other Tx | 0 (0.0) | 3 (1.5) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | 1.0 | 0.9–1.0 | 0.570 | |||
| Female sex | 0.5 | 0.2–1.3 | 0.155 | |||
| Incidentaloma as diagnostic Sx | 0.3 | 0.1–0.7 | 0.006 | 0.4 | 0.2–0.9 | 0.033 |
| Palpable neck mass as diagnostic Sx | 2.9 | 1.3–6.9 | 0.013 | 2.7 | 1.1–6.4 | 0.025 |
| FHx positive | 1.3 | 0.4–3.8 | 0.651 | |||
| Clinical T stage | ||||||
| T1 | 1.0 | Reference | - | 1.0 | Reference | - |
| T2 | 4.7 | 0.4–51.6 | 0.207 | 1.7 | 0.1–21.3 | 0.687 |
| T3 | 9.0 | 1.2–67.4 | 0.032 | 6.6 | 0.8–53.0 | 0.075 |
| T4 | 16.2 | 1.0–260.2 | 0.049 | 5.2 | 0.3–92.3 | 0.265 |
| Clinical N stage | ||||||
| N0 | 1.0 | Reference | - | 1.0 | Reference | - |
| N1a | 9.3 | 1.0–89.6 | 0.053 | 8.5 | 0.9–82.4 | 0.065 |
| N1b | 12.7 | 1.7–94.9 | 0.013 | 8.3 | 1.1–63.4 | 0.041 |
| US-guided aggressiveness detection | 0.8 | 0.3–2.0 | 0.679 | |||
| Operation type | ||||||
| HT | 1.0 | Reference | - | |||
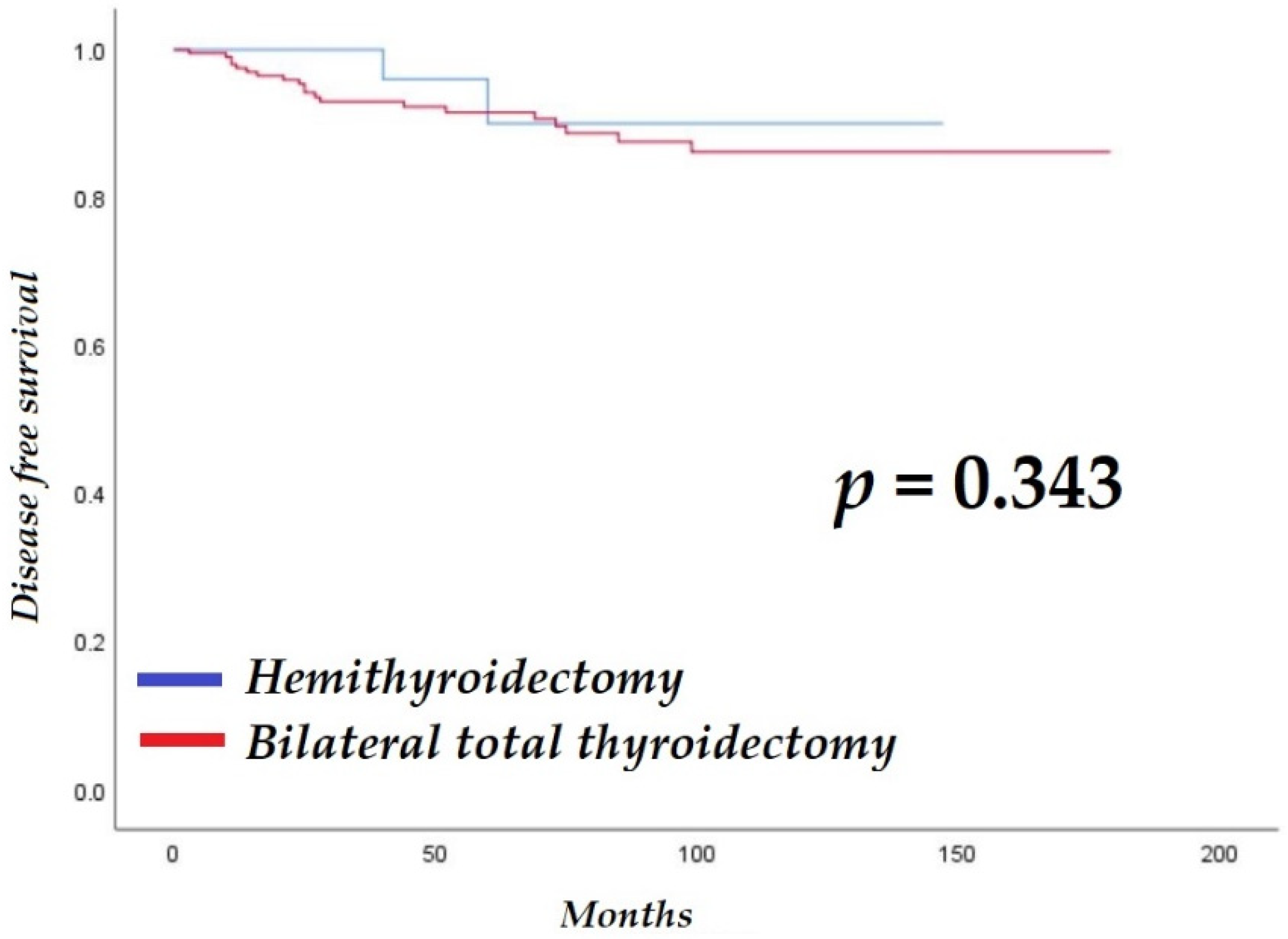
| BTT | 2.0 | 0.5–8.5 | 0.353 | |||
| Subtype of AVPTC | ||||||
| DSV | 1.0 | Reference | - | |||
| SV | 0.8 | 0.2–2.8 | 0.759 | |||
| TCV | 1.8 | 0.4–7.7 | 0.443 | |||
| HV | <0.01 | N/A | 0.988 | |||
| CCV | <0.01 | N/A | 0.989 | |||
| Tumor size (cm) | 1.4 | 1.1–1.8 | 0.006 | 1.3 | 1.0–1.7 | 0.036 |
| Perinodal soft tissue extension positive | 1.9 | 0.9–4.4 | 0.116 | |||
| Microscopic ETE positive | 10.2 | 1.4–75.3 | 0.023 | 4.4 | 0.6–34.2 | 0.161 |
| Gross ETE positive | 4.4 | 2.0–9.9 | <0.001 | 3.1 | 1.4–7.0 | 0.007 |
| Multiplicity positive | 1.2 | 0.5–2.6 | 0.733 | |||
| Bilaterality positive | 1.5 | 0.6–3.3 | 0.381 | |||
| LVI positive | 3.0 | 1.2–7.5 | 0.019 | 2.4 | 0.9–6.6 | 0.080 |
| Pathologic T stage | ||||||
| T1 | 1.0 | Reference | - | 1.0 | Reference | - |
| T2 | 2.0 | 0.5–7.8 | 0.343 | 1.2 | 0.3–5.0 | 0.800 |
| T3a | 5.0 | 1.3–20.1 | 0.023 | 2.7 | 0.6–12.0 | 0.185 |
| T3b | 5.0 | 1.5–16.4 | 0.008 | 3.4 | 1.0–11.4 | 0.045 |
| T4a | 8.3 | 2.8–24.8 | <0.001 | 6.0 | 1.9–18.8 | 0.002 |
| Pathologic N stage | ||||||
| N0 | 1.0 | Reference | - | 1.0 | Reference | - |
| N1a | 4.6 | 0.5–38.9 | 0.167 | 8.9 | 0.9–87.3 | 0.060 |
| N1b | 7.4 | 1.0–55.2 | 0.052 | 7.2 | 0.9–55.9 | 0.059 |
| Postoperative adjuvant Tx | ||||||
| No treatment | 1.0 | Reference | - | |||
| Low-dose RAI | 0.5 | 0.1–3.2 | 0.491 | |||
| High-dose RAI | 2.2 | 0.6–7.4 | 0.208 | |||
| HR | 95% CI | p-Value | Harrell’s c-Index | |
|---|---|---|---|---|
| Tumor size (continuous) | 1.4 | 1.1–1.8 | 0.006 | 0.692 |
| Tumor size (binary) | ||||
| <1.4 cm | 1.0 | Reference | - | 0.666 |
| ≥1.4 cm | 3.3 | 1.2–9.0 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.A.; Moon, G.; Kang, S.; Lee, K.H.; Lee, S.M.; Kim, J.K.; Lee, C.R.; Kang, S.-W.; Jeong, J.J.; Nam, K.-H.; et al. Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer. Cancers 2022, 14, 2757. https://doi.org/10.3390/cancers14112757
Lee IA, Moon G, Kang S, Lee KH, Lee SM, Kim JK, Lee CR, Kang S-W, Jeong JJ, Nam K-H, et al. Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer. Cancers. 2022; 14(11):2757. https://doi.org/10.3390/cancers14112757
Chicago/Turabian StyleLee, In A, Gilseong Moon, Seokmin Kang, Kang Hee Lee, Sun Min Lee, Jin Kyong Kim, Cho Rok Lee, Sang-Wook Kang, Jong Ju Jeong, Kee-Hyun Nam, and et al. 2022. "Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer" Cancers 14, no. 11: 2757. https://doi.org/10.3390/cancers14112757
APA StyleLee, I. A., Moon, G., Kang, S., Lee, K. H., Lee, S. M., Kim, J. K., Lee, C. R., Kang, S.-W., Jeong, J. J., Nam, K.-H., & Chung, W. Y. (2022). Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer. Cancers, 14(11), 2757. https://doi.org/10.3390/cancers14112757






