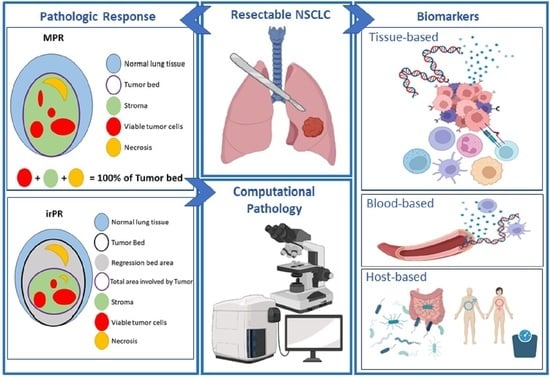
Pathological Response and Immune Biomarker Assessment in Non-Small-Cell Lung Carcinoma Receiving Neoadjuvant Immune Checkpoint Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathological Response
2.1. Pathological Response Evaluation in Tumors after Neoadjuvant Immunotherapy
2.2. Computational Pathology for the Assessment of MPR
3. Biomarkers for Potential Use as Predictors of Neoadjuvant Immunotherapy Efficacy
3.1. PD-L1 Expression in Tumor and Stromal Cells
3.2. Tumor Mutation Burden (TMB)
3.3. Oncogenic Driver Alterations
3.4. Tumor-Associated Immune Cells
3.5. The T Cell Receptor Repertoire
3.6. Tertiary Lymphoid Structures (TLS) and B-Lymphocytes
3.7. Circulating Tumor DNA
3.8. Circulating Peripheral Immune Cell Subsets and Cytokines
3.9. Complete Blood Count (CBC)
3.10. Gut Microbial-Derived Metabolites
3.11. Other Host-Related Biomarkers
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebuzzi, S.E.; Zullo, L.; Rossi, G.; Grassi, M.; Murianni, V.; Tagliamento, M.; Prelaj, A.; Coco, S.; Longo, L.; Dal Bello, M.G.; et al. Novel Emerging Molecular Targets in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 2625. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients with Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Wyld, L.; Audisio, R.A.; Poston, G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Ginsberg, R.J.; Venkatraman, E.S.; Bains, M.S.; Downey, R.J.; Korst, R.J.; Kris, M.G.; Rusch, V.W. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 1989–1995. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Shi, X.; Wang, G. Neoadjuvant immunotherapy for resectable non-small cell lung cancer. Am. J. Cancer Res. 2021, 11, 2521–2536. [Google Scholar]
- Nakagawa, K.; Watanabe, S.I.; Kunitoh, H.; Asamura, H. The Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group: Past activities, current status and future direction. Jpn. J. Clin. Oncol. 2017, 47, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Zhong, W.Z.; Chen, K.N.; Chen, C.; Gu, C.D.; Wang, J.; Yang, X.N.; Mao, W.M.; Wang, Q.; Qiao, G.B.; Cheng, Y.; et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J. Clin. Oncol. 2019, 37, 2235–2245. [Google Scholar] [CrossRef]
- Johnson, D.B.; Rioth, M.J.; Horn, L. Immune checkpoint inhibitors in NSCLC. Curr. Treat. Options. Oncol. 2014, 15, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Vansteenkiste, J.; Wauters, E.; Reymen, B.; Ackermann, C.J.; Peters, S.; De Ruysscher, D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann. Oncol. 2019, 30, 1244–1253. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Pradhan, M.; Chocry, M.; Gibbons, D.L.; Sepesi, B.; Cascone, T. Emerging biomarkers for neoadjuvant immune checkpoint inhibitors in operable non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 590–606. [Google Scholar] [CrossRef]
- Cascone, T.; Weissferdt, A.; Godoy, M.C.B.; William, W.N.; Leung, C.H.; Lin, H.Y.; Basu, S.; Yadav, S.S.; Pataer, A.; Mitchell, K.G.; et al. Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Vokes, N.I.; Liu, D.; Ricciuti, B.; Jimenez-Aguilar, E.; Rizvi, H.; Dietlein, F.; He, M.X.; Margolis, C.A.; Elmarakeby, H.; Girshman, J.; et al. Harmonization of Tumor Mutational Burden Quantification and Association With Response to Immune Checkpoint Blockade in Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Motoi, N. ES06.04 MPR and pCR as Primary Endpoints in Neaodjuvant Trials. J. Thorac. Oncol. 2021, 16, S71–S72. [Google Scholar] [CrossRef]
- Weissferdt, A.; Pataer, A.; Vaporciyan, A.A.; Correa, A.M.; Sepesi, B.; Moran, C.A.; Wistuba, I.I.; Roth, J.A.; Shewale, J.B.; Heymach, J.V.; et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin. Lung Cancer 2020, 21, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.E.; Lipson, E.J.; Cottrell, T.R.; Forde, P.M.; Anders, R.A.; Cimino-Mathews, A.; Thompson, E.D.; Allaf, M.E.; Yarchoan, M.; Feliciano, J.; et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020, 26, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Nagano, T.; Tachihara, M.; Nishimura, Y. Molecular Mechanisms and Targeted Therapies Including Immunotherapy for Non-Small Cell Lung Cancer. Curr. Cancer Drug Targets. 2019, 19, 595–630. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef]
- Versluis, J.M.; Reijers, I.L.M.; Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Wouters, M.W.; Chng, S.; Saw, R.P.M.; Scolyer, R.A.; van de Wiel, B.A.; et al. Neoadjuvant ipilimumab plus nivolumab in synchronous clinical stage III melanoma. Eur. J. Cancer 2021, 148, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, B.; Mo, H.; Zhang, W.; Chen, X.; Wu, D.; Qu, D.; Wang, X.; Lan, B.; Yang, B.; et al. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin. Cancer Res. 2018, 24, 1296–1304. [Google Scholar] [CrossRef] [Green Version]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Montemurro, F.; Di Cosimo, S. Pathological complete response in breast cancer patients receiving neoadjuvant chemotherapy. Breast 2014, 23, 690–691. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Travis, W.D.; Dacic, S.; Wistuba, I.; Sholl, L.; Adusumilli, P.; Bubendorf, L.; Bunn, P.; Cascone, T.; Chaft, J.; Chen, G.; et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J. Thorac. Oncol. 2020, 15, 709–740. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xu, A.; Lin, Y.; Camidge, D.R.; Di Maio, M.; Califano, R.; Hida, T.; Rossi, A.; Guibert, N.; Zhu, C.; et al. A narrative review of primary research endpoints of neoadjuvant therapy for lung cancer: Past, present and future. Transl. Lung Cancer Res. 2021, 10, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Pataer, A.; Shao, R.; Correa, M.; Behrens, C.; Roth, J.; Vaporciyan, A.; Wistuba, I.; Swisher, S. Major pathologic response and RAD51 predict survival in lung cancer patients receiving neoadjuvant chemotherapy. Cancer Med. 2018, 7, 2405–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ma, K. Neoadjuvant Therapy in Lung Cancer: What Is Most Important: Objective Response Rate or Major Pathological Response? Curr. Oncol. 2021, 28, 4129–4138. [Google Scholar] [CrossRef]
- Parra, E.R.; Villalobos, P.; Behrens, C.; Jiang, M.; Pataer, A.; Swisher, S.G.; William, W.N.; Jr Zhang, J.; Lee, J.; Cascone, T.; et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J. Immunother. Cancer 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Pataer, A.; Weissferdt, A.; Vaporciyan, A.A.; Correa, A.M.; Sepesi, B.; Wistuba, I.I.; Heymach, J.V.; Cascone, T.; Swisher, S.G. Evaluation of Pathologic Response in Lymph Nodes of Patients with Lung Cancer Receiving Neoadjuvant Chemotherapy. J. Thorac. Oncol. 2021, 16, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, W.; Wu, J.; Feng, Y.; Mao, L.; Chen, M.; Yang, X.; Wang, H.; Chi, K.; Yang, Y.; et al. Major pathologic response assessment and clinical significance of metastatic lymph nodes after neoadjuvant therapy for non-small cell lung cancer. Mod. Pathol. 2021, 34, 1990–1998. [Google Scholar] [CrossRef]
- Cottrell, T.R.; Thompson, E.D.; Forde, P.M.; Stein, J.E.; Duffield, A.S.; Anagnostou, V.; Rekhtman, N.; Anders, R.A.; Cuda, J.D.; Illei, P.B.; et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 2018, 29, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, N.; Li, L.; Guo, C.; Wei, J.; Yuan, P.; Tan, F.; Tao, X.; Wang, S.; Wang, Z.; et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis. Oncol. 2020, 4, 32. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, A.W.; Marjanski, T.; Falcoz, P.-E.; Tsuboi, M.; Wu, Y.-L.; Sun, S.W.; Gitlitz, B.J. Important Surgical and Clinical End Points in Neoadjuvant Immunotherapy Trials in Resectable NSCLC. JTO Clin. Res. Rep. 2021, 2, 100221. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N.; Jr Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ock, C.-Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial Intelligence–Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022. JCO.21.02010. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ferrara, R.; Caramella, C.; Besse, B.; Champiat, S. Pseudoprogression in Non-Small Cell Lung Cancer upon Immunotherapy: Few Drops in the Ocean? J. Thorac. Oncol. 2019, 14, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar] [PubMed]
- Chen, M.-Y.; Zeng, Y.-C. Pseudoprogression in lung cancer patients treated with immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 169, 103531. [Google Scholar] [CrossRef]
- Abels, E.; Pantanowitz, L.; Aeffner, F.; Zarella, M.D.; van der Laak, J.; Bui, M.M.; Vemuri, V.N.; Parwani, A.V.; Gibbs, J.; Agosto-Arroyo, E.; et al. Computational pathology definitions, best practices, and recommendations for regulatory guidance: A white paper from the Digital Pathology Association. J. Pathol. 2019, 249, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Roa, A.; Gilmore, H.; Basavanhally, A.; Feldman, M.; Ganesan, S.; Shih, N.N.C.; Tomaszewski, J.; González, F.A.; Madabhushi, A. Accurate and reproducible invasive breast cancer detection in whole-slide images: A Deep Learning approach for quantifying tumor extent. Sci. Rep. 2017, 7, 46450. [Google Scholar] [CrossRef] [Green Version]
- Tellez, D.; Balkenhol, M.; Otte-Holler, I.; van de Loo, R.; Vogels, R.; Bult, P.; Wauters, C.; Vreuls, W.; Mol, S.; Karssemeijer, N.; et al. Whole-Slide Mitosis Detection in H&E Breast Histology Using PHH3 as a Reference to Train Distilled Stain-Invariant Convolutional Networks. IEEE Trans. Med. Imaging 2018, 37, 2126–2136. [Google Scholar] [CrossRef] [Green Version]
- Qaiser, T.; Mukherjee, A.; Reddy Pb, C.; Munugoti, S.D.; Tallam, V.; Pitkäaho, T.; Lehtimäki, T.; Naughton, T.; Berseth, M.; Pedraza, A.; et al. HER2 challenge contest: A detailed assessment of automated HER2 scoring algorithms in whole slide images of breast cancer tissues. Histopathology 2018, 72, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehteshami Bejnordi, B.; Veta, M.; Johannes van Diest, P.; van Ginneken, B.; Karssemeijer, N.; Litjens, G.; van der Laak, J.; Hermsen, M.; Manson, Q.F.; Balkenhol, M.; et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women with Breast Cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef]
- Dacic, S.; Travis, W.D.; Giltnane, J.M.; Abel, J.; Kos, F.; Hilz, S.; Hennek, S.; Fujimoto, J.; Sholl, L.M.; Khalil, F.; et al. Artificial intelligence (AI)–powered pathologic response (PathR) assessment of resection specimens after neoadjuvant atezolizumab in patients with non-small cell lung cancer: Results from the LCMC3 study. J. Clin. Oncol. 2021, 39, 106. [Google Scholar] [CrossRef]
- Tizhoosh, H.R.; Pantanowitz, L. Artificial Intelligence and Digital Pathology: Challenges and Opportunities. J. Pathol. Inform. 2018, 9, 38. [Google Scholar] [CrossRef]
- Zarella, M.D.; Quaschnick, M.R.; Breen, D.E.; Garcia, F.U. Estimation of Fine-Scale Histologic Features at Low Magnification. Arch. Pathol. Lab. Med. 2018, 142, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.J.; Mornin, J.D.; Biswas, S.K.; Beck, L.E.; Bauer, D.P.; Raj, A.; Bianco, S.; Gartner, Z.J. Quanti.us: A tool for rapid, flexible, crowd-based annotation of images. Nat. Methods 2018, 15, 587–590. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendry, S.; Byrne, D.J.; Wright, G.M.; Young, R.J.; Sturrock, S.; Cooper, W.A.; Fox, S.B. Comparison of Four PD-L1 Immunohistochemical Assays in Lung Cancer. J. Thorac. Oncol. 2018, 13, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef]
- Chaft, J.E. Neoadjuvant and adjuvant approaches in surgically resectable NSCLC. Clin. Adv. Hematol. Oncol. 2021, 19, 631–633. [Google Scholar]
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Ying, J.; Wang, S.; Tao, X.; Zhao, J.; Mao, Y.; Wang, B.; et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2020, 15, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Campesato, L.F.; Barroso-Sousa, R.; Jimenez, L.; Correa, B.R.; Sabbaga, J.; Hoff, P.M.; Reis, L.F.; Galante, P.A.; Camargo, A.A. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget 2015, 6, 34221–34227. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients with Non-Small-Cell Lung Cancer Profiled with Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Rusch, V.W.; Chaft, J.E.; Johnson, B.E.; Nicholas, A.; Wistuba, I.I.; Merritt, R.; Lee, J.M.; Bunn, P.A.; Tang, Y.; et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J. Clin. Oncol. 2019, 37, 8503. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Brown, S.D.; Warren, R.L.; Gibb, E.A.; Martin, S.D.; Spinelli, J.J.; Nelson, B.H.; Holt, R.A. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014, 24, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- DRUG FUSF. FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors. 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 29 April 2022).
- Friedlaender, A.; Nouspikel, T.; Christinat, Y.; Ho, L.; McKee, T.; Addeo, A. Tissue-Plasma TMB Comparison and Plasma TMB Monitoring in Patients with Metastatic Non-small Cell Lung Cancer Receiving Immune Checkpoint Inhibitors. Front. Oncol. 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Velcheti, V.; Mekhail, T.; Yun, C.; Shagan, S.M.; Hu, S.; Chae, Y.K.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: The phase 2 B-F1RST trial. Nat. Med. 2022, 28, 939–945. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.-J.; Qu, Z.; Song, C.-Y.; Sun, Y.; Lai, A.-L.; Luo, M.-Y.; Ying, Y.-Z.; Meng, H.; Liang, Z.; He, Y.-J.; et al. NeoPeptide: An immunoinformatic database of T-cell-defined neoantigens. Database J. Biol. Databases Curation 2019, 2019, baz128. [Google Scholar] [CrossRef]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the Immunotarget registry. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef]
- Carbone, D.; Lee, J.; Kris, M.; Wistuba, I.; Kwiatkowski, D.; Owen, D.; Bunn, P.; Johnson, B.; Oezkan, F.; Tang, Y.; et al. OA06.06 Clinical/Biomarker Data for Neoadjuvant Atezolizumab in Resectable Stage IB-IIIB NSCLC: Primary Analysis in the LCMC3 Study. J. Thorac. Oncol. 2021, 16, S115–S116. [Google Scholar] [CrossRef]
- Reuss, J.E.; Anagnostou, V.; Cottrell, T.R.; Smith, K.N.; Verde, F.; Zahurak, M.; Lanis, M.; Murray, J.C.; Chan, H.Y.; McCarthy, C.; et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e001282. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front. Immunol. 2018, 9, 3101. [Google Scholar] [CrossRef] [Green Version]
- Federico, L.; McGrail, D.J.; Bentebibel, S.E.; Haymaker, C.; Ravelli, A.; Forget, M.A.; Karpinets, T.; Jiang, P.; Reuben, A.; Negrao, M.V.; et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2, TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1, Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Parra, E.R.; Jiang, M.; Solis, L.; Mino, B.; Laberiano, C.; Hernandez, S.; Gite, S.; Verma, A.; Tetzlaff, M.; Haymaker, C.; et al. Procedural Requirements and Recommendations for Multiplex Immunofluorescence Tyramide Signal Amplification Assays to Support Translational Oncology Studies. Cancers 2020, 12, 255. [Google Scholar] [CrossRef] [Green Version]
- Decalf, J.; Albert, M.L.; Ziai, J. New tools for pathology: A users review of a highly multiplexed method for in situ analysis of protein and RNA expression in tissue. J. Pathol. 2019, 247, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Francisco-Cruz, A.; Parra, E.R.; Tetzlaff, M.T.; Wistuba, I.I. Multiplex Immunofluorescence Assays. Methods Mol. Biol. 2020, 2055, 467–495. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Kidman, J.; Principe, N.; Watson, M.; Lassmann, T.; Holt, R.A.; Nowak, A.K.; Lesterhuis, W.J.; Lake, R.A.; Chee, J. Characteristics of TCR Repertoire Associated with Successful Immune Checkpoint Therapy Responses. Front. Immunol. 2020, 11, 2668. [Google Scholar] [CrossRef]
- Werner, F.; Wagner, C.; Simon, M.; Glatz, K.; Mertz, K.D.; Laubli, H.; Griss, J.; Wagner, S.N. A Standardized Analysis of Tertiary Lymphoid Structures in Human Melanoma: Disease Progression- and Tumor Site-Associated Changes with Germinal Center Alteration. Front. Immunol. 2021, 12, 2522. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Erazo, L.; Rhodes, J.L.; Marion, V.C.; Kemp, R.A. Tertiary lymphoid structures in cancer-considerations for patient prognosis. Cell. Mol Immunol. 2020, 17, 570–575. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Z.; Caushi, J.X.; El Asmar, M.; Anagnostou, V.; Cottrell, T.R.; Chan, H.Y.; Suri, P.; Guo, H.; Merghoub, T.; et al. Compartmental Analysis of T-cell Clonal Dynamics as a Function of Pathologic Response to Neoadjuvant PD-1 Blockade in Resectable Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Caushi, J.X.; Zhang, J.; Ji, Z.; Vaghasia, A.; Zhang, B.; Hsiue, E.H.-C.; Mog, B.J.; Hou, W.; Justesen, S.; Blosser, R.; et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 2021, 596, 126–132. [Google Scholar] [CrossRef]
- Casarrubios, M.; Cruz-Bermúdez, A.; Nadal, E.; Insa, A.; García Campelo, M.D.R.; Lázaro, M.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Pretreatment Tissue TCR Repertoire Evenness Is Associated with Complete Pathologic Response in Patients with NSCLC Receiving Neoadjuvant Chemoimmunotherapy. Clin. Cancer Res. 2021, 27, 5878–5890. [Google Scholar] [CrossRef]
- Qin, M.; Jin, Y.; Pan, L.Y. Tertiary lymphoid structure and B-cell-related pathways: A potential target in tumor immunotherapy. Oncol. Lett. 2021, 22, 836. [Google Scholar] [CrossRef]
- Sautes-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Goc, J.; Giraldo, N.A.; Sautes-Fridman, C.; Fridman, W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014, 35, 571–580. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Lund, F.E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: Their role and application in anti-tumor immunity in lung cancer. Cell. Mol. Immunol. 2019, 16, 6–18. [Google Scholar] [CrossRef]
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Giroux Leprieur, E.; Herbretau, G.; Dumenil, C.; Julie, C.; Giraud, V.; Labrune, S.; Dumoulin, J.; Tisserand, J.; Emile, J.F.; Blons, H.; et al. Circulating tumor DNA evaluated by Next-Generation Sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. Oncoimmunology 2018, 7, e1424675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M.; et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Nadal, E.; Serna, R.; Insa, A.; Campelo, M.R.G.; Benito, C.; Domine, M.; Majem, M.; Abreu, D.R.; Martinez-Marti, A.; et al. OA20.02 Pre-Treatment Levels of ctDNA for Long-Term Survival Prediction in Stage IIIA NSCLC Treated with Neoadjuvant Chemo-Immunotherapy. J. Thorac. Oncol. 2021, 16, S883–S884. [Google Scholar] [CrossRef]
- Oezkan, F.; He, K.; Owen, D.; Pietrzak, M.; Rusch, V.W.; Chaft, J.E.; Kitzler, R.; Nicholas, A.; Schulze, K.; Johnson, A.; et al. Neoadjuvant Atezolizumab in Resectable NSCLC Patients: Immunophenotyping Results from the Interim Analysis of the Multicenter Trail LCMC3. J. Thorac. Oncol. 2019, 14, S242–S243. [Google Scholar] [CrossRef]
- Laza-Briviesca, R.; Casarrubios, M.; Nadal, E.; Insa, A.; Garcia-Campelo, R.; Alvarez, N.d.; Domine, M.; Massuti, B.; Majem, M.; Rodriguez-Abreu, D.; et al. Biomarkers of Pathological Response on Neo-Adjuvant Chemo-Immunotherapy Treatment for Resectable Stage IIIA NSCLC Patients. J. Thorac. Oncol. 2019, 14, e20026. [Google Scholar] [CrossRef]
- Ren, F.; Zhao, T.; Liu, B.; Pan, L. Neutrophil-lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). OncoTargets Ther. 2019, 12, 4235–4244. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Cowley, L.A.; Liu, X.-S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Behrens, C.; Rocha, P.; Parra, E.R.; Feng, L.; Rodriguez-Canales, J.; Solis, L.M.; Mino, B.; Zhang, J.; Gibbons, D.L.; Sepesi, B.; et al. Female Gender Predicts Augmented Immune Infiltration in Lung Adenocarcinoma. Clin. Lung Cancer 2021, 22, e415–e424. [Google Scholar] [CrossRef]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336–99346. [Google Scholar] [CrossRef]
- Xiao, D.; Pan, H.; Li, F.; Wu, K.; Zhang, X.; He, J. Analysis of ultra-deep targeted sequencing reveals mutation burden is associated with gender and clinical outcome in lung adenocarcinoma. Oncotarget 2016, 7, 22857–22864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, J.M.; Cooper, J.D.; Penninx, B.W.; Bahn, S. Variation in serum biomarkers with sex and female hormonal status: Implications for clinical tests. Sci. Rep. 2016, 6, 26947. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex Differences in Tolerability to Anti-Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-Small Cell Lung Cancer: Are We All Equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauklin, S.; Sernández, I.V.; Bachmann, G.; Ramiro, A.R.; Petersen-Mahrt, S.K. Estrogen directly activates AID transcription and function. J. Exp. Med. 2009, 206, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beagley, K.W.; Gockel, C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 2003, 38, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Kugel, C.H., 3rd; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Moschos, S.J. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2016, 45, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018, 359, 582–587. [Google Scholar] [CrossRef] [Green Version]




| Trial Name (Registry Number) Phase | Tumor Stage | Patient N | Neoadjuvant Treatment | MPR | pCR | Outcome | PD-L1 (IHC) | Correlative Studies |
|---|---|---|---|---|---|---|---|---|
| >Checkmate 159 (NCT02259621) Phase 2 | IB–IIIA | 45 | Arm A: Nivolumab Arm B: Nivolumab + Carboplatin + Paclitaxel | 45% | 22% | 30-months disease-free: 15/20 patients Median RFS: not reached-24-months RFS: 69% | Yes % PD-L1+(≥1%): 46.6% (7/15) | Tumor mutation burden Molecular mutations Circulating tumor DNA Tumor infiltrating lymphocytes TCR repertoire |
| NEOSTAR (NCT03158129) Phase 2 | IA–IIIA | 88 | Arm A: Nivolumab Arm B: Nivolumab + Ipilimumab Arm C: Nivolumab + Platinum doublet CT Arm D: Nivolumab + Ipilimumab + Platinum doublet CT | Arm A: 22% Arm B: 38% Arm C and D: not reported | Arm A: 9% Arm B: 29% Arm C and D: not reported | Median OS and Lung cancer-related RFS: not reached after a median follow-up of 22.2 months | Pretherapy tumor PD-L1: MPR: median, 3% No MPR: median, 0% Posttherapy tumor PD-L1: MPR: median, 5% No MPR: median, 0% | Flow cytometry Multiplex immunofluorescence T-cell receptor sequencing Gut microbiome |
| LCMC3 (NCT02927301) Phase 2 | IB–IIIB (resectable) | 179 | Atezolizumab | 20% | 7% | Not reported | Yes PD-L1+ (≥1%): 19.5% (35/179) | Multiplex immunofluorescence Tumor mutation burden Molecular mutations RNA sequencing Flow cytometry |
| NADIM (NCT03081689) Phase 2 | IIIA | 46 | Nivolumab + Carboplatin + Paclitaxel | 83% | 71% | PFS (24 months): 7% OS (12 months):97.8% OS (18 months):93.5% OS (24 months): 89.9% | Yes PD-L1 + (≥1%)): 39% (18/46) | Multiplex Immunofluorescence T-cell receptor sequencing Tumor mutation burden Molecular mutations Circulating tumor DNA |
| MK3475-223 (NCT02938624) Phase 1 | I-II | 28 | Pembrolizumab | 40% | Not reported | Not reported | Yes PD-L1 + (≥1%): 18% (5/28) | Not reported |
| NEOCOAST (NCT03794544) Phase 2 | I–IIIA (resectable) | 160 | Arm A: Durvalumab Arm B: Durvalumab + Oleclumab Arm C: Durvalumab + Monalizumab Arm D: Durvalumab + Danvatirsen | Not reported | Not reported | Not reported | Yes, not reported | Tumor genomics Tumor microenvironment and T cell population |
| PRINCEPS (NCT02994576) Phase 2 | resectable | 60 | Atezolizumab | 14% | Not observed | Not reported | Yes, not reported | Multiplex Immunofluorescence Molecular mutations SUVmax |
| SAKK (NCT02572843) Phase 2 | IIIA (resectable) | 67 | Durvalumab + CT | 62% | 18% | 1-year EFS: 73% Median EFS and OS: not reached after 28.6 months follow-up. | Yes, not reported | Not reported |
| Checkmate 816 (NCT02998528) Phase 3 | IB–IIIA | 358 | Platinum doublet CT Nivolumab + platinum doublet CT | Platinum doublet CT: 8.9% Nivolumab + platinum doublet CT: 36.9% | Platinum doublet CT: 2.2% Nivolumab + platinum doublet CT: 24.0% | Platinum doublet CT: Median EFS: 20.8 months 1-year OS: 63.4% 2-year OS:45.3% Nivolumab + platinum doublet CT: Median EFS: 31.6 months 1-year OS: 76.1% 2-year OS: 63.8% | Yes PD-L1 + (≥1%): 49% (178/358) | Tumor mutation burden Circulating Tumor DNA |
| Impower 030 (NCT03456063) Phase 3 | II–IIIA-selected IIIB | 451 | Arm A: Atezolizumab + platinum doublet CT Arm B: Placebo + platinum doublet CT | ongoing, end date April 2024 | ongoing, end date April 2024 | Not reported | Yes, not reported | Not reported |
| AEGEAN (NCT03800134) Phase 3 | II–III | 824 | Arm 1: Durvalumab + platinum doublet CT Arm 2: placebo + platinum doublet CT | ongoing, end date April 2024 | ongoing, end date April 2024 | Not reported | Yes, not reported | Not reported |
| Biomarker | Source | Gold Standard | In Development |
|---|---|---|---|
| PD-L1 expression | Tissue | Immunohistochemistry | Multiplex immunoflourescence |
| Tumor-infiltrating lymphocytes (TILs) | Tissue | H&E stain: Pathology analysis | Immunohistochemistry Multiplex immunofluorescence/High-plex technologies Next Generation Sequencing Flow Cytometry/CyTOF TCR and BCR sequencing |
| Tertiary lymphoid structures (TLSs) | Tissue | H&E stain: Pathology analysis | Immunohistochemistry Multiplex immunofluorescence/High-plex technologies Next Generation Sequencing |
| Immune cell subsets | Tissue | Immunohistochemistry | Multiplex immunofluorescence/High-plex technologies Next Generation Sequencing Flow Cytometry TCR and BCR sequencing |
| Circulating immune cell subsets | Blood | Flow Cytometry | Functional T cells assays ELISPOT Next Generation Sequencing Cytokines/chemokines CyTOF |
| T cell receptor repertoire (TCR) | Tissue, Blood | None | TCR and BCR sequencing |
| Tumor mutation burden (TMB) | Tissue, Blood | Whole exome sequencing (WES) | Next Generation Sequencing |
| Complete Blood Count (CBC) | Blood | Hemogram | |
| Circulating tumor DNA (ctDNA) | Blood | None | Next Generation Sequencing |
| Gut microbiota | Stool | None | Next Generation Sequencing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, F.; Parra, E.R.; Wistuba, I.I.; Haymaker, C.; Solis Soto, L.M. Pathological Response and Immune Biomarker Assessment in Non-Small-Cell Lung Carcinoma Receiving Neoadjuvant Immune Checkpoint Inhibitors. Cancers 2022, 14, 2775. https://doi.org/10.3390/cancers14112775
Rojas F, Parra ER, Wistuba II, Haymaker C, Solis Soto LM. Pathological Response and Immune Biomarker Assessment in Non-Small-Cell Lung Carcinoma Receiving Neoadjuvant Immune Checkpoint Inhibitors. Cancers. 2022; 14(11):2775. https://doi.org/10.3390/cancers14112775
Chicago/Turabian StyleRojas, Frank, Edwin Roger Parra, Ignacio Ivan Wistuba, Cara Haymaker, and Luisa Maren Solis Soto. 2022. "Pathological Response and Immune Biomarker Assessment in Non-Small-Cell Lung Carcinoma Receiving Neoadjuvant Immune Checkpoint Inhibitors" Cancers 14, no. 11: 2775. https://doi.org/10.3390/cancers14112775
APA StyleRojas, F., Parra, E. R., Wistuba, I. I., Haymaker, C., & Solis Soto, L. M. (2022). Pathological Response and Immune Biomarker Assessment in Non-Small-Cell Lung Carcinoma Receiving Neoadjuvant Immune Checkpoint Inhibitors. Cancers, 14(11), 2775. https://doi.org/10.3390/cancers14112775