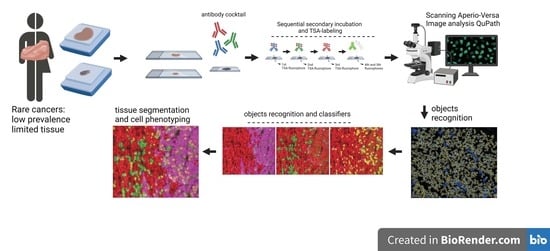
Simultaneous and Spatially-Resolved Analysis of T-Lymphocytes, Macrophages and PD-L1 Immune Checkpoint in Rare Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Case Selection
2.2. Sample Processing and Multiplexed Immunofluorescence
2.3. Cases and FFPE Cores Considerations
2.4. Slide Scanning and Image Generation
2.5. Analysis for Single Objects
2.6. Analysis for Segmentation and Masking
3. Results
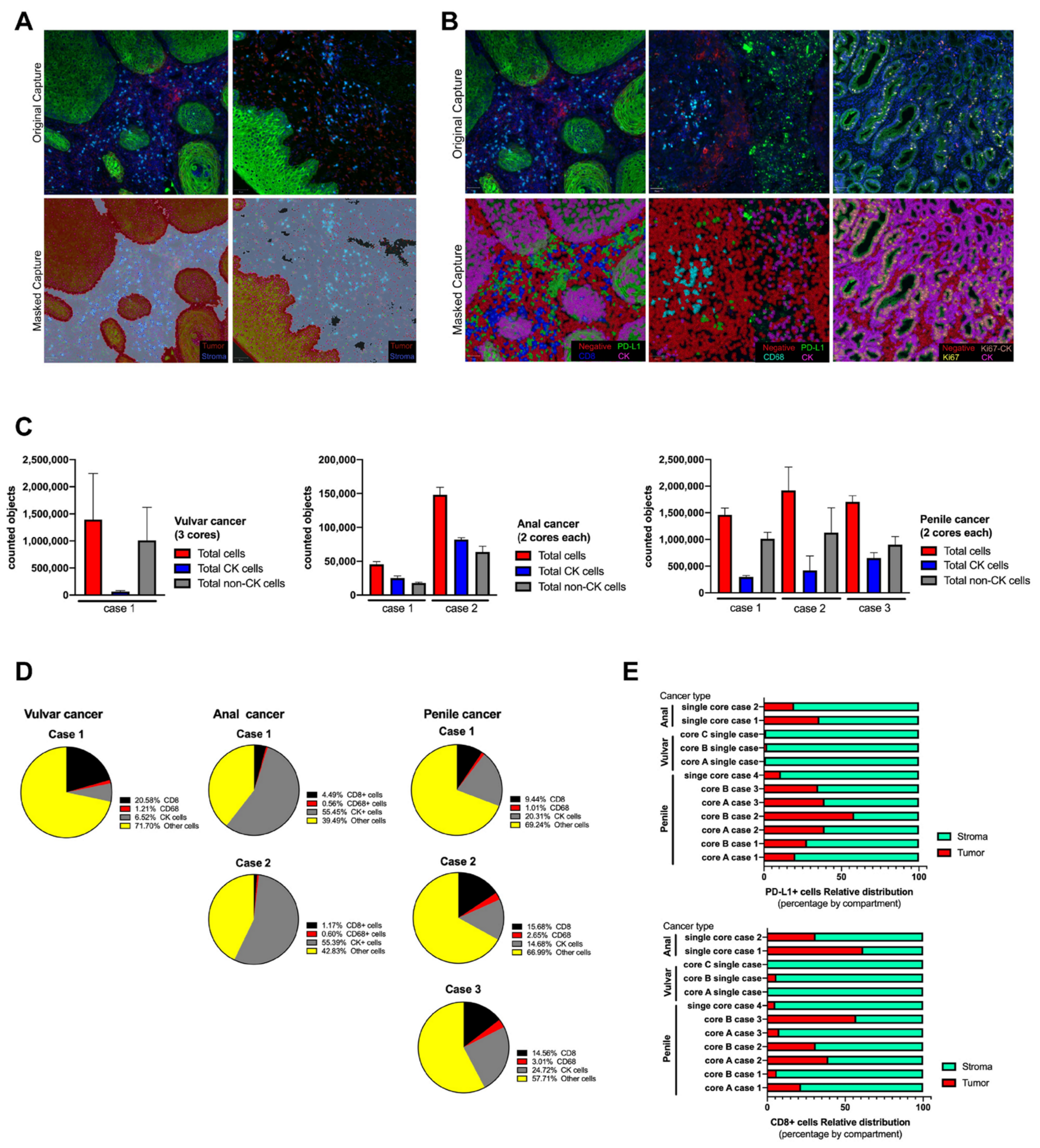
3.1. Immunostaining Verification
3.2. Multiplexed Immunofluorescence in Rare Cancer Cases Series
3.3. Image Analysis by Tissue Segmentation and Cell Phenotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globocan 2020 World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: https://gco.iarc.fr/ (accessed on 1 March 2020).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Douglawi, A.; Masterson, T.A. Updates on the Epidemiology and Risk Factors for Penile Cancer. Transl. Androl. Urol. 2017, 6, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.A.; Anderson, L.; Eva, L.; Scurry, J. Clinical and Molecular Classification of Vulvar Squamous Pre-Cancers. Int. J. Gynecol. Cancer 2019, 29, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-Wide Analysis of HPV Integration in Human Cancers Reveals Recurrent, Focal Genomic Instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodelon, C.; Untereiner, M.E.; Machiela, M.J.; Vinokurova, S.; Wentzensen, N. Genomic Characterization of Viral Integration Sites in HPV-Related Cancers. Int. J. Cancer 2016, 139, 2001–2011. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Dong, R.; Liu, J.; Lang, J.; Yang, J.; Wang, W.; Li, J.; Meng, B.; Tian, G. Accurate Detection of HPV Integration Sites in Cervical Cancer Samples Using the Nanopore MinION Sequencer Without Error Correction. Front. Genet. 2020, 11, 660. [Google Scholar] [CrossRef]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor Immunity and Immunotherapy for HPV-Related Cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef]
- Balermpas, P.; Martin, D.; Wieland, U.; Rave-Fränk, M.; Strebhardt, K.; Rödel, C.; Fokas, E.; Rödel, F. Human Papilloma Virus Load and PD-1/PD-L1, CD8+ and FOXP3 in Anal Cancer Patients Treated with Chemoradiotherapy: Rationale for Immunotherapy. Oncoimmunology 2017, 6, e1288331. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Allen, C.T. Immunotherapy for HPV Malignancies. Semin. Radiat. Oncol. 2021, 31, 361–370. [Google Scholar] [CrossRef]
- de Vries, H.M.; Ottenhof, S.R.; Horenblas, S.; van der Heijden, M.S.; Jordanova, E.S. Defining the Tumor Microenvironment of Penile Cancer by Means of the Cancer Immunogram. Eur. Urol. Focus 2019, 5, 718–721. [Google Scholar] [CrossRef] [Green Version]
- Pessia, B.; Romano, L.; Giuliani, A.; Lazzarin, G.; Carlei, F.; Schietroma, M. Squamous Cell Anal Cancer: Management and Therapeutic Options. Ann. Med. Surg. 2020, 55, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Vanthoor, J.; Vos, G.; Albersen, M. Penile Cancer: Potential Target for Immunotherapy? World J. Urol. 2021, 39, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, Z.; Kortekaas, K.E.; De Vos Van Steenwijk, P.J.; Van Der Burg, S.H.; Van Poelgeest, M.I.E. The Immune Microenvironment in Vulvar (Pre)Cancer: Review of Literature and Implications for Immunotherapy. Expert Opin. Biol. Ther. 2018, 18, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.M.; Chahoud, J.; Adashek, J.J.; Azizi, M.; Magliocco, A.; Ross, J.S.; Necchi, A.; Spiess, P.E. Understanding Genomics and the Immune Environment of Penile Cancer to Improve Therapy. Nat. Rev. Urol. 2020, 17, 555–570. [Google Scholar] [CrossRef]
- Chu, C.; Yao, K.; Lu, J.; Zhang, Y.; Chen, K.; Lu, J.; Zhang, C.Z.; Cao, Y. Immunophenotypes Based on the Tumor Immune Microenvironment Allow for Unsupervised Penile Cancer Patient Stratification. Cancers 2020, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Geltzeiler, C.B.; Xu, Y.; Carchman, E.; Ghouse, Y.; Beczkiewicz, J.; Son, J.; Voils, C.I.; Striker, R. CD4/CD8 Ratio as a Novel Marker for Increased Risk of High-Grade Anal Dysplasia and Anal Cancer in HIV+ Patients: A Retrospective Cohort Study. Dis. Colon Rectum 2020, 63, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Rotman, J.; Samuels, S.; Zijlmans, H.J.M.A.A.; Fons, G.; Van De Vijver, K.K.; Bleeker, M.C.G.; Kenter, G.G.; Jordanova, E.J.; De Gruijl, T.D. Immune Landscape in Vulvar Cancer-Draining Lymph Nodes Indicates Distinct Immune Escape Mechanisms in Support of Metastatic Spread and Growth. J. Immunother. Cancer 2021, 9, e003623. [Google Scholar] [CrossRef]
- Hu, W.H.; Miyai, K.; Cajas-Monson, L.C.; Luo, L.; Liu, L.; Ramamoorthy, S.L. Tumor-Infiltrating CD8+ T Lymphocytes Associated with Clinical Outcome in Anal Squamous Cell Carcinoma. J. Surg. Oncol. 2015, 112, 421–426. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Santegoets, S.J.; Abdulrahman, Z.; Van Ham, V.J.; Van Der Tol, M.; Ehsan, I.; Van Doorn, H.C.; Bosse, T.; Van Poelgeest, M.I.E.; Van Der Burg, S.H. High Numbers of Activated Helper T Cells Are Associated with Better Clinical Outcome in Early Stage Vulvar Cancer, Irrespective of HPV or P53 Status. J. Immunother. Cancer 2019, 7, 236. [Google Scholar] [CrossRef] [Green Version]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Józwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, J.; Wang, J.; Fu, J.; Li, T.; Zheng, X.; Wang, B.; Gu, S.; Jiang, P.; Fan, J.; et al. Publisher Correction: Landscape of B Cell Immunity and Related Immune Evasion in Human Cancers. Nat. Genet. 2019, 51, 1068. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, S.; Gong, Z.; Li, X.; Xiang, B.; Deng, H.; Zhou, M.; Li, G.; Li, Y.; Xiong, W.; et al. Emerging Role of Metabolic Reprogramming in Tumor Immune Evasion and Immunotherapy. Sci. China Life Sci. 2021, 64, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The Importance of Exosomal PDL1 in Tumour Immune Evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stanger, B.Z. How Tumor Cell Dedifferentiation Drives Immune Evasion and Resistance to Immunotherapy. Cancer Res. 2020, 80, 4037–4041. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Carlsson, J.; Giunchi, F.; Harlow, A.; Kirrander, P.; Rider, J.; Fiorentino, M.; Andrén, O. PD-L1 Expression in Men with Penile Cancer and Its Association with Clinical Outcomes. Eur. Urol. Oncol. 2019, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Udager, A.M.; Liu, T.Y.; Skala, S.L.; Magers, M.J.; McDaniel, A.S.; Spratt, D.E.; Feng, F.Y.; Siddiqui, J.; Cao, X.; Fields, K.L.; et al. Frequent PD-L1 Expression in Primary and Metastatic Penile Squamous Cell Carcinoma: Potential Opportunities for Immunotherapeutic Approaches. Ann. Oncol. 2016, 27, 1706–1712. [Google Scholar] [CrossRef] [Green Version]
- Choschzick, M.; Gut, A.; Fink, D. PD-L1 Receptor Expression in Vulvar Carcinomas Is HPV-Independent. Virchows Arch. 2018, 473, 513–516. [Google Scholar] [CrossRef]
- Thangarajah, F.; Morgenstern, B.; Pahmeyer, C.; Schiffmann, L.M.; Puppe, J.; Mallmann, P.; Hamacher, S.; Buettner, R.; Alidousty, C.; Holz, B.; et al. Clinical Impact of PD-L1 and PD-1 Expression in Squamous Cell Cancer of the Vulva. J. Cancer Res. Clin. Oncol. 2019, 145, 1651–1660. [Google Scholar] [CrossRef]
- Bucau, M.; Gault, N.; Sritharan, N.; Valette, E.; Charpentier, C.; Walker, F.; Couvelard, A.; Abramowitz, L. PD-1/PD-L1 Expression in Anal Squamous Intraepithelial Lesions. Oncotarget 2020, 11, 3582–3589. [Google Scholar] [CrossRef]
- Govindarajan, R.; Gujja, S.; Siegel, E.R.; Batra, A.; Saeed, A.; Lai, K.; James, J.D.; Fogel, B.J.; Williamson, S. Programmed Cell Death-Ligand 1 (PD-L1) Expression in Anal Cancer. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 638–642. [Google Scholar] [CrossRef]
- Varga, Z.; Diebold, J.; Dommann-Scherrer, C.; Frick, H.; Kaup, D.; Noske, A.; Obermann, E.; Ohlschlegel, C.; Padberg, B.; Rakozy, C.; et al. How Reliable Is Ki-67 Immunohistochemistry in Grade 2 Breast Carcinomas? A QA Study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS ONE 2012, 7, e37379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Herck, Y.; Antoranz, A.; Andhari, M.D.; Milli, G.; Bechter, O.; De Smet, F.; Bosisio, F.M. Multiplexed Immunohistochemistry and Digital Pathology as the Foundation for Next-Generation Pathology in Melanoma: Methodological Comparison and Future Clinical Applications. Front. Oncol. 2021, 11, 636681. [Google Scholar] [CrossRef] [PubMed]
- Cereceda, K.; Jorquera, R.; Villarroel-Espíndola, F. Advances in Mass Cytometry and Its Applicability to Digital Pathology in Clinical-Translational Cancer Research. Adv. Lab. Med. 2021, 3, 5–16. [Google Scholar] [CrossRef]
- Villarroel-Espindola, F.; Yu, X.; Datar, I.; Mani, N.; Sanmamed, M.; Velcheti, V.; Syrigos, K.; Toki, M.; Zhao, H.; Chen, L.; et al. Spatially Resolved and Quantitative Analysis of Vista/Pd-1h as a Novel Immunotherapy Target in Human Non–Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 1562–1573. [Google Scholar] [CrossRef] [Green Version]
- Pelekanou, V.; Villarroel-espindola, F.; Schalper, K.A.; Pusztai, L.; Rimm, D.L. CD68, CD163, and matrix metalloproteinase 9 (MMP-9) Co-Localization in Breast Tumor Microenvironment Predicts Survival Differently in ER-Positive and -Negative Cancers. Breast Cancer Res. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Taube, J.M.; Akturk, G.; Angelo, M.; Engle, E.L.; Gnjatic, S.; Greenbaum, S.; Greenwald, N.F.; Hedvat, C.V.; Hollmann, T.J.; Juco, J.; et al. The Society for Immunotherapy in Cancer Statement on Best Practices for Multiplex Immunohistochemistry (IHC) and Immunofluorescence (IF) Staining and Validation. J. Immunother. Cancer 2020, 8, e000155. [Google Scholar] [CrossRef]
- Eslick, G.D. What Is a Rare Cancer? Hematol. Oncol. Clin. N. Am. 2012, 26, 1137–1141. [Google Scholar] [CrossRef]
- Bondei, I.T.; Suteu, O.; Todor, N.; Nagy, V.M. Rare Tumors: A Comprehensive Analysis of Cancer. J. BUON 2019, 24, 2173–2179. [Google Scholar]
- Wong, P.F.; Wei, W.; Smithy, J.W.; Acs, B.; Toki, M.I.; Blenman, K.R.M.; Zelterman, D.; Kluger, H.M.; Rimm, D.L. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Clin. Cancer Res. 2019, 25, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Viratham Pulsawatdi, A.; Craig, S.G.; Bingham, V.; McCombe, K.; Humphries, M.P.; Senevirathne, S.; Richman, S.D.; Quirke, P.; Campo, L.; Domingo, E.; et al. A Robust Multiplex Immunofluorescence and Digital Pathology Workflow for the Characterisation of the Tumour Immune Microenvironment. Mol. Oncol. 2020, 14, 2384–2402. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.P.; Maxwell, P.; Salto-Tellez, M. QuPath: The Global Impact of an Open Source Digital Pathology System. Comput. Struct. Biotechnol. J. 2021, 19, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Wildiers, H.; Marcelis, L.; Antoranz, A.; Bosisio, F.; Hatse, S.; Floris, G. Computerised Scoring Protocol for Identification and Quantification of Different Immune Cell Populations in Breast Tumour Regions by the Use of QuPath Software. Histopathology 2020, 77, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, M.B.; Bankhead, P.; Coleman, H.G.; Hagan, R.S.; Craig, S.; McCorry, A.M.B.; Gray, R.T.; McQuaid, S.; Dunne, P.D.; Hamilton, P.W.; et al. Validation of the Systematic Scoring of Immunohistochemically Stained Tumour Tissue Microarrays Using QuPath Digital Image Analysis. Histopathology 2018, 73, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhun, I.; Shepherd, D.; Hung, Y.P.; Madrigal, E.; Le, L.P.; Mino-Kenudson, M. Digital Image Analysis for Estimating Stromal CD8+ Tumor-Infiltrating Lymphocytes in Lung Adenocarcinoma. J. Pathol. Inform. 2021, 12, 28. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Petropoulou, P.I.; Rodriguez-Calvo, T. Whole-Slide Image Analysis of Human Pancreas Samples to Elucidate the Immunopathogenesis of Type 1 Diabetes Using the QuPath Software. Front. Mol. Biosci. 2021, 8, 503. [Google Scholar] [CrossRef]
- Courtney, J.M.; Morris, G.P.; Cleary, E.M.; Howells, D.W.; Sutherland, B.A. An Automated Approach to Improve the Quantification of Pericytes and Microglia in Whole Mouse Brain Sections. eNeuro 2021, 8, ENEURO.0177–21.2021. [Google Scholar] [CrossRef]
- Linette, G.P.; Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malig. Rep. 2019, 14, 286–291. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.K.; Rana, B.; Rana, A. Tumor-Infiltrating CD8(+) T Cell Antitumor Efficacy and Exhaustion: Molecular Insights. Drug Discov. Today 2021, 26, 951–967. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Nie, X.; Desai, S.S.; Villaroel-Espindola, F.; Badri, T.; Zhao, D.; Kim, A.W.; Ji, L.; Zhang, T.; Quinlan, E.; et al. A Burned-out Cd8+ t-Cell Subset Expands in the Tumor Microenvironment and Curbs Cancer Immunotherapy. Cancer Discov. 2021, 11, 1700–1715. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Garasa, S.; Barbes, B.; Solorzano, J.L.; Perez-Gracia, J.L.; Labiano, S.; Sanmamed, M.F.; Azpilikueta, A.; Bolaños, E.; et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory MAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016, 76, 5994–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Li, Z.; Guo, S.; Chen, P.; Chen, X.; Zhou, Q.; Chen, J.; Yu, X.; Wu, X.; Ma, W.; et al. Tumor PD-L1 Expression Is Correlated with Increased TILs and Poor Prognosis in Penile Squamous Cell Carcinoma. Oncoimmunology 2017, 6, e1269047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohneis, P.; Boral, S.; Kaufmann, A.M.; Lehmann, A.; Schewe, C.; Dietel, M.; Anagnostopoulos, I.; Jöhrens, K. Human Papilloma Virus Status of Penile Squamous Cell Carcinoma Is Associated with Differences in Tumour-Infiltrating T Lymphocytes. Virchows Arch. 2015, 466, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Lérias, S.; Esteves, S.; Silva, F.; Cunha, M.; Cochicho, D.; Martins, L.; Félix, A. CD274 (PD-L1), CDKN2A (P16), TP53, and EGFR Immunohistochemical Profile in Primary, Recurrent and Metastatic Vulvar Cancer. Mod. Pathol. 2020, 33, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Trafalis, D.T.; Alifieris, C.E.; Kalantzis, A.; Verigos, K.E.; Vergadis, C.; Sauvage, S. Evidence for Efficacy of Treatment with the Anti-PD-1 Mab Nivolumab in Radiation and Multichemorefractory Advanced Penile Squamous Cell Carcinoma. J. Immunother. 2018, 41, 300–305. [Google Scholar] [CrossRef]
- Mantovani, G.; Fragomeni, S.M.; Inzani, F.; Fagotti, A.; Della Corte, L.; Gentileschi, S.; Tagliaferri, L.; Zannoni, G.F.; Scambia, G.; Garganese, G. Molecular Pathways in Vulvar Squamous Cell Carcinoma: Implications for Target Therapeutic Strategies. J. Cancer Res. Clin. Oncol. 2018, 146, 1647–1658. [Google Scholar] [CrossRef]
- Iseas, S.; Golubicki, M.; Robbio, J.; Ruiz, G.; Guerra, F.; Mariani, J.; Salanova, R.; Cabanne, A.; Eleta, M.; Gonzalez, J.V.; et al. A Clinical and Molecular Portrait of Non-Metastatic Anal Squamous Cell Carcinoma. Transl. Oncol. 2021, 14, 101084. [Google Scholar] [CrossRef]
- Wessely, A.; Heppt, M.V.; Kammerbauer, C.; Steeb, T.; Kirchner, T.; Flaig, M.J.; French, L.E.; Berking, C.; Schmoeckel, E.; Reinholz, M. Evaluation of PD-L1 Expression and HPV Genotyping in Anal Squamous Cell Carcinoma. Cancers 2020, 12, 2516. [Google Scholar] [CrossRef]
- Denis, C.; Sakalihasan, S.; Frères, P.; Withofs, N.; Sautois, B. Cemiplimab for Cisplatin Resistant Metastatic Penile Cancer. Case Rep. Oncol. 2021, 14, 972–976. [Google Scholar] [CrossRef]
- Hahn, A.W.; Chahoud, J.; Campbell, M.T.; Karp, D.D.; Wang, J.; Stephen, B.; Tu, S.M.; Pettaway, C.A.; Naing, A. Pembrolizumab for Advanced Penile Cancer: A Case Series from a Phase II Basket Trial. Investig. New Drugs 2021, 39, 1405–1410. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in Vaginal and Vulvar Squamous Cell Carcinoma: A Case Series from a Phase II Basket Trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients with Recurrent Carcinoma of the Anal Canal. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.G.; Steigen, S.E.; Deutsch, E.; Martinelli, E.; Arnold, D. Anal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Ngoi, N.Y.L.; Lim, D.; Poon, M.L.M.; Thian, Y.L.; Lim, Y.W.; Lim, S.E.; Tong, P.; Lum, J.H.Y.; Ng, J.; et al. PD-L1 Expressing Recurrent Clear Cell Carcinoma of the Vulva with Durable Partial Response to Pembrolizumab: A Case Report. Onco. Targets. Ther. 2021, 14, 3921–3928. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Fu, C.; Xiao, M.; Wang, C. Recurrent Metastatic Penile Cancer Patient with Positive Pd-L1 Expression Obtained Significant Benefit from Immunotherapy: A Case Report and Literature Review. Onco. Targets. Ther. 2020, 13, 3319–3324. [Google Scholar] [CrossRef] [Green Version]
- Tostes, F.T.; Fernandes, I.; Segatelli, V.; Callegaro, D.; Carmagnani Pestana, R. Response to Pembrolizumab in Advanced Anal Squamous Cell Carcinoma With High TMB and PD-L1 and PD-L2 Amplification. Clin. Colorectal Cancer 2021, 20, 350–353. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, R.; Zhang, B.; Zhou, H.; Lin, Z.; Yao, T. Pembrolizumab in FIGO IVB Verrucous Carcinoma of the Vulva: A Case Report. Front. Oncol. 2021, 11, 1689. [Google Scholar] [CrossRef]
- Yeku, O.; Russo, A.L.; Lee, H.; Spriggs, D. A Phase 2 Study of Combined Chemo-Immunotherapy with Cisplatin-Pembrolizumab and Radiation for Unresectable Vulvar Squamous Cell Carcinoma. J. Transl. Med. 2020, 18, 350. [Google Scholar] [CrossRef]
- Reiser, J.; Banerjee, A. Effector, Memory, and Dysfunctional CD8+ T Cell Fates in the Antitumor Immune Response. J. Immunol. Res. 2016, 2016, 8941260. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Fu, T.; Dai, L.J.; Wu, S.Y.; Xiao, Y.; Ma, D.; Jiang, Y.Z.; Shao, Z.M. Spatial Architecture of the Immune Microenvironment Orchestrates Tumor Immunity and Therapeutic Response. J. Hematol. Oncol. 2021, 14, 98. [Google Scholar] [CrossRef] [PubMed]


| Tumor Type | Patient ID * | FFPE Block | Age at Diagnosis | Gender | Vital Status | Histology | Grade | Stage | TNM | Other Conditions |
|---|---|---|---|---|---|---|---|---|---|---|
| Penile | Case 1 | Core A | 72 | Male | Deceased | Squamous cell carcinoma | Well differentiated | III | T3N2M0 | None |
| Core B | ||||||||||
| Case 2 | Core A | 57 | Male | Deceased | Squamous cell carcinoma | Poorly differentiated | III | T3N1M0 | None | |
| Core B | ||||||||||
| Case 3 | Core A | 61 | Male | Deceased | Squamous cell carcinoma | Moderately differentiated | IV | T4N2M1 | None | |
| Core B | ||||||||||
| Case 4 | Core A | 31 | Male | Deceased | Squamous cell carcinoma | Moderately differentiated | IV | T4N1M1 | HIV infection | |
| Vulva | Case 1 | Core A | 67 | Female | Deceased | Squamous cell carcinoma | Poorly differentiated | IV | T4N2M1 | Hypertension |
| Core B | ||||||||||
| Core C | ||||||||||
| Anal | Case 1 | Core A | 69 | Female | Deceased | Squamous cell carcinoma | Poorly differentiated | II | T2N1M0 | Hypertension |
| Case 2 | Core A | 74 | Female | Deceased | Squamous cell carcinoma | Moderately differentiated | III | T3N1M0 | Meningioma |
| Tumor Cell Phenotyping * | Counted Cells | Counted CK (+) | % Ki67 (−)/PD-L1 (−) | % Ki67 (+) Cells | % PD-L1 (+) Cells | % Ki67 (+)/PDL1 (+) | ||
|---|---|---|---|---|---|---|---|---|
| penile cancer | Case 1 | Core A | 1,301,254 | 306,091 | 95.15 | 4.13 | 1.39 | 0.60 |
| Core B | 1,365,949 | 294,761 | 95.80 | 4.13 | 0.12 | 0.04 | ||
| Case 2 | Core A | 1,894,818 | 400,175 | 69.79 | 28.19 | 3.63 | 0.51 | |
| Core B | 1,622,523 | 897,117 | 93.09 | 6.67 | 0.37 | 0.10 | ||
| Case 3 | Core A | 1,597,643 | 679,128 | 96.28 | 3.49 | 0.28 | 0.03 | |
| Core B | 1,713,451 | 839,073 | 96.90 | 2.92 | 0.24 | 0.05 | ||
| Case 4 | Single core | 181,154 | 32,011 | 93.89 | 4.62 | 0.84 | 2.48 | |
| vulvar cancer | Case 1 | Core A | 2,258,207 | 60,982 | 96.22 | 3.35 | 0.02 | 0.48 |
| Core B | 402,220 | 46,374 | 99.80 | 0.06 | 0.01 | 0.15 | ||
| Core C | 1,052,697 | 27,993 | 99.61 | 0.06 | 0.01 | 0.34 | ||
| anal cancer | Case 1 | Single core | 49,977 | 37,524 | 72.04 | 23.81 | 1.62 | 8.00 |
| Case 2 | Single core | 158,127 | 89,776 | 93.44 | 5.34 | 0.60 | 1.94 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cereceda, K.; Bravo, N.; Jorquera, R.; González-Stegmaier, R.; Villarroel-Espíndola, F. Simultaneous and Spatially-Resolved Analysis of T-Lymphocytes, Macrophages and PD-L1 Immune Checkpoint in Rare Cancers. Cancers 2022, 14, 2815. https://doi.org/10.3390/cancers14112815
Cereceda K, Bravo N, Jorquera R, González-Stegmaier R, Villarroel-Espíndola F. Simultaneous and Spatially-Resolved Analysis of T-Lymphocytes, Macrophages and PD-L1 Immune Checkpoint in Rare Cancers. Cancers. 2022; 14(11):2815. https://doi.org/10.3390/cancers14112815
Chicago/Turabian StyleCereceda, Karina, Nicolas Bravo, Roddy Jorquera, Roxana González-Stegmaier, and Franz Villarroel-Espíndola. 2022. "Simultaneous and Spatially-Resolved Analysis of T-Lymphocytes, Macrophages and PD-L1 Immune Checkpoint in Rare Cancers" Cancers 14, no. 11: 2815. https://doi.org/10.3390/cancers14112815
APA StyleCereceda, K., Bravo, N., Jorquera, R., González-Stegmaier, R., & Villarroel-Espíndola, F. (2022). Simultaneous and Spatially-Resolved Analysis of T-Lymphocytes, Macrophages and PD-L1 Immune Checkpoint in Rare Cancers. Cancers, 14(11), 2815. https://doi.org/10.3390/cancers14112815