Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
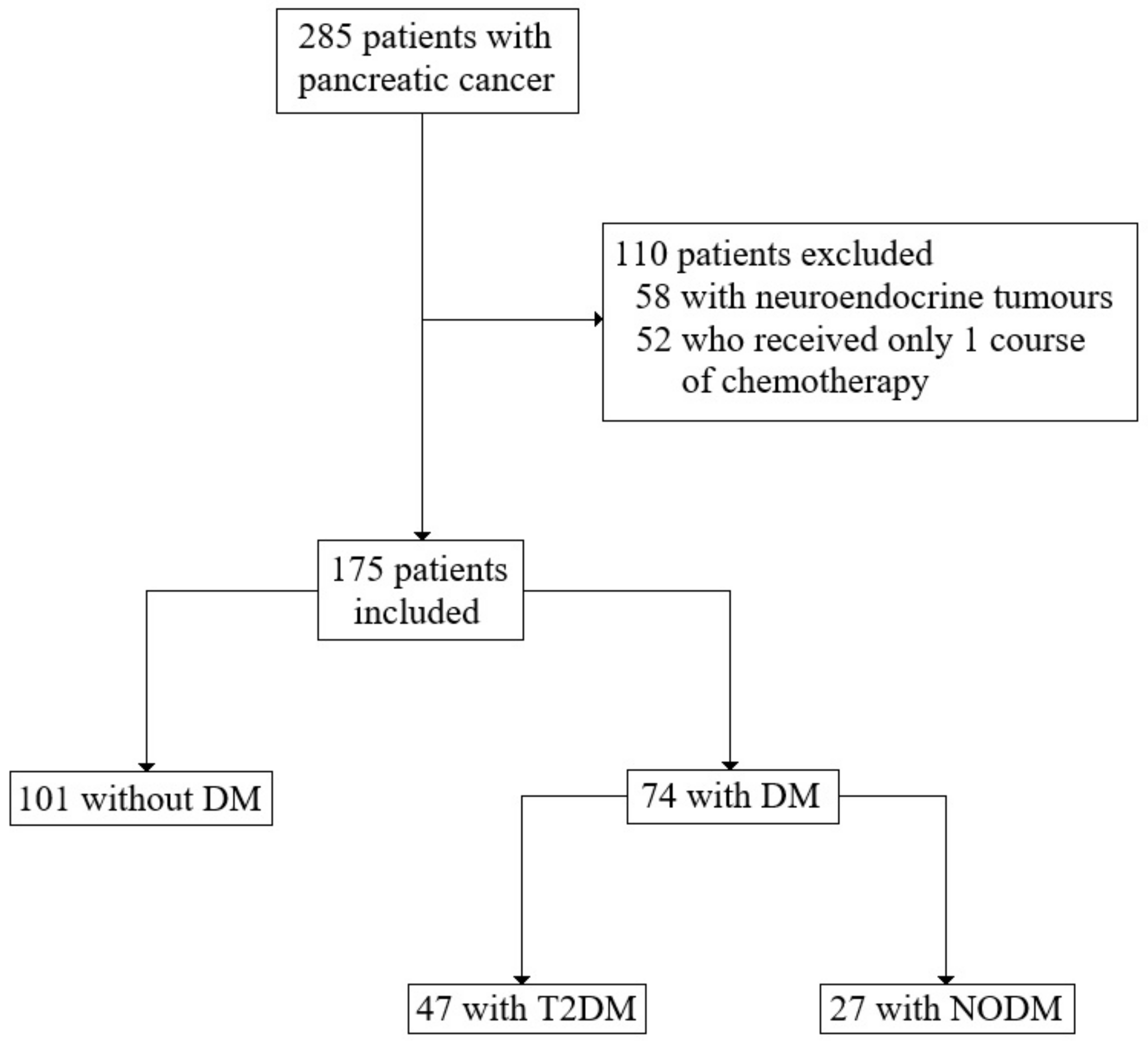
2.1. Patients and Data Collection
2.2. Study Design
- (i)
- Two consecutive fasting glucose levels ≥140 mg/dL (7.8 mmol/L);
- (ii)
- Random plasma glucose ≥200 mg/dL (11.1 mmol/L) in patients with classic symptoms of hyperglycaemia or hyperglycaemic crisis; or
- (iii)
- 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT).
2.3. Statistical Analysis
2.4. Ethics Approval and Consent to Participate
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| PC | Pancreatic cancer |
| DM | Diabetes mellitus |
| NODM | New-onset diabetes mellitus |
| T2DM | Type 2 DM |
| OS | Overall survival |
| ICD-10 | The International Statistical Classification of Diseases and Related Health Problems |
| CSK | Central Clinical Hospital |
| MSWiA | Ministry of Interior and Administration |
| ECOG | Eastern Cooperative Oncology Group performance status |
| non-DM | Group without diabetes mellitus |
| OGTT | Oral glucose tolerance test |
| AJCC | American Joint Cancer Committee |
| CT | Computed tomography |
| SD | Standard deviation |
| HR | Hazard ratio |
| CRP | C reactive protein |
| CLR | CRP/lymphocytes ratio |
| M | Mean |
| n | Number |
| MD | Median |
| 95% CI | 95% Confidence Interval |
| DFS | Disease-free survival |
| PFS | Progression-free survival |
| CEA | Carcino-embryonic antigen |
| CA19-9 | Carbohydrate antigen 19-9 |
| TNM | TNM Classification of Malignant Tumors |
| T | Tumour size |
| N | Lymph nodal involvement |
| M | Distant metastasis |
| R | Resection margin |
| GH/IGF | Growth hormone/insulin-like growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| AGE | Advanced glycation end products |
| ASCO | American Society of Clinical Oncology |
| mTOR | The mammalian target of rapamycin |
| mFOLFIRINOX | A chemotherapy regimen: FOL—folinic acid |
| F | Fluorouracil (5-FU) |
| IRIN | Irinotecan |
| OX | Oxaliplatin |
| MMP9 | Matrix metalloproteinase-9 |
| AMP/ATP | Adenosine monophosphate/adenosine triphosphate |
| ADP/ATP | Adenosine diphosphate/adenosine triphosphate |
| AMPK | AMP-activated protein kinase |
| CAR | CRP/albumin ratio |
| HDL | High-density lipoproteins |
References
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Wangjam, T.; Zhang, Z.; Zhou, X.C.; Lyer, L.; Faisal, F.; Soares, K.C.; Fishman, E.; Hruban, R.H.; Herman, J.M.; Laheru, D.; et al. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget 2015, 6, 36903–36910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Khadka, R.; Tian, W.; Hao, X.; Koirala, R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018, 52, 342–346. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Adinolfi, V.; Morviducci, L.; Acquati, S.; Tuveri, E.; Ferrari, P.; Zatelli, M.C.; Faggiano, A.; Argentiero, A.; Natalicchio, A.; et al. Early prediction of pancreatic cancer from new-onset diabetes: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open 2021, 6, 100155. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Scholten, L.; Mungroop, T.H.; Haijtink, S.A.L.; Issa, Y.; van Rijssen, L.B.; Koerkamp, B.G.; van Eijck, C.H.; Busch, O.R.; DeVries, J.H.; Besselink, M.G. New-onset diabetes after pancreatoduodenectomy: A systematic review and meta-analysis. Surgery 2018, 164, 6–16. [Google Scholar] [CrossRef]
- Lee, W.; Yoon, Y.S.; Han, H.S.; Cho, J.Y.; Choi, Y.; Jang, J.Y.; Choi, H. Prognostic relevance of preoperative diabetes mellitus and the degree of hyperglycemia on the outcomes of resected pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2016, 113, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tao, M.; Jia, X.; Xu, H.; Chen, K.; Tang, H.; Li, D. Effect of Diabetes Mellitus on Survival in Patients with Pancreatic Cancer: A Systematic Review and Meta-analysis. Sci. Rep. 2015, 5, 17102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, G.; Ma, Q.; Liu, H.; Li, W.; Han, L. The bidirectional interation between pancreatic cancer and diabetes. World J. Surg. Oncol. 2012, 10, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, C.J.; Falasca, M.; Chari, S.T.; Greenfield, J.R.; Xu, Z.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers 2021, 13, 5224. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, M.; Turati, F.; Rosato, V.; La Vecchia, C. Exploring the link between diabetes and pancreatic cancer. Expert. Rev. Anticancer Ther. 2019, 19, 681–687. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, E.; Vetter, S.W. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim. Biophys. Acta 2015, 1852, 2706–2711. [Google Scholar] [CrossRef] [Green Version]
- Hank, T.; Sandini, M.; Qadan, M.; Weniger, M.; Ciprani, D.; Li, A.; Ferrone, C.R.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-Del Castillo, C. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology 2020, 20, 125–131. [Google Scholar] [CrossRef]
- Chu, C.K.; Mazo, A.E.; Goodman, M.; Egnatashvili, V.; Sarmiento, J.M.; Staley, C.A.; Galloway, J.R.; Adsay, N.V.; Jacobs, S.; Kooby, D.A. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann. Surg. Oncol. 2010, 17, 502–513. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Jiang, Y.; Yuan, Y.; Wang, K.; Fang, J.; Li, Z. Clinical profiles and long-term outcomes of patients with pancreatic ductal adenocarcinoma and diabetes mellitus. Diabetes Metab. Res. Rev. 2012, 28, 169–176. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Postoperative mortality in cancer patients with preexisting diabetes: Systematic review and meta-analysis. Diabetes Care 2010, 33, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.K.; Mazo, A.E.; Sarmiento, J.M.; Staley, C.A.; Adsay, N.V.; Umpierrez, G.E.; Kooby, D.A. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J. Am. Coll. Surg. 2010, 210, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mao, Y.; Chang, P.; Liu, C.; Hassan, M.M.; Yeung, S.J.; Abbruzzese, J.L. Impacts of new-onset and long-term diabetes on clinical outcome of pancreatic cancer. Am. J. Cancer Res. 2015, 5, 3260–3269. [Google Scholar] [PubMed]
- Yuan, C.; Rubinson, D.A.; Qian, Z.R.; Wu, C.; Kraft, P.; Bao, Y.; Ogino, S.; Ng, K.; Clancy, T.E.; Swanson, R.S.; et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J. Clin. Oncol. 2015, 33, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhan, M.; Wang, W.; Yang, D.; Wang, J. Impact of diabetes mellitus on the survival of pancreatic cancer: A meta-analysis. Onco. Targets Ther. 2016, 9, 1679–1688. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, M.R.; Mascitelli, L.; Pezzetta, F. Obesity and survival among patients with pancreatic cancer. JAMA 2009, 302, 1752, author reply 1752–1753. [Google Scholar] [CrossRef]
- Karlin, N.J.; Amin, S.B.; Kosiorek, H.E.; Buras, M.R.; Verona, P.M.; Cook, C.B. Survival and glycemic control outcomes among patients with coexisting pancreatic cancer and diabetes mellitus. Future Sci. OA 2018, 4, Fso291. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.M.; Wang, H.H.; Wang, W.L.; Lee, C.T.; Tai, C.M.; Tseng, C.H.; Chen, C.C.; Tsai, Y.N.; Sun, M.S.; Hsu, Y.C. Prognostic Impact of Diabetes Mellitus on Overall Survival in A Nationwide Population-Based Cohort of Patients with Pancreatic Cancer. Endocr. Pract. 2020, 26, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.H.; Chou, J.F.; Ludwig, E.; O’Reilly, E.; Allen, P.J.; Jarnagin, W.R.; Bayuga, S.; Simon, J.; Gonen, M.; Reisacher, W.R.; et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int. J. Cancer 2010, 127, 2412–2419. [Google Scholar] [CrossRef]
- Mizuno, S.; Nakai, Y.; Isayama, H.; Takahara, N.; Miyabayashi, K.; Yamamoto, K.; Kawakubo, K.; Mohri, D.; Kogure, H.; Sasaki, T.; et al. Diabetes is a useful diagnostic clue to improve the prognosis of pancreatic cancer. Pancreatology 2013, 13, 285–289. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Kim, T.Y.; Oh, D.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Bang, Y.J. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res. Treat 2016, 48, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Choi, Y.; Kim, T.-Y.; Lee, K.-H.; Han, S.-W.; Im, S.-A.; Kim, T.-Y.; Bang, Y.-J. The impact of diabetes mellitus and metformin on survival of patients with advanced pancreatic cancer receiving chemotherapy. J. Clin. Oncol. 2013, 31, 4044. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Gu, G.; Yang, X.; Qian, Z. Metformin Increases the Chemosensitivity of Pancreatic Cancer Cells to Gemcitabine by Reversing EMT Through Regulation DNA Methylation of miR-663. Onco. Targets Ther. 2020, 13, 10417–10429. [Google Scholar] [CrossRef]
- Lu, R.; Yang, J.; Wei, R.; Ke, J.; Tian, Q.; Yu, F.; Liu, J.; Zhang, J.; Hong, T. Synergistic anti-tumor effects of liraglutide with metformin on pancreatic cancer cells. PLoS ONE 2018, 13, e0198938. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, Y.C.; Yang, S.F.; Yen, H.H.; Tsai, H.D.; Hsieh, M.C.; Hsiao, Y.H. Pitavastatin and metformin synergistically activate apoptosis and autophagy in pancreatic cancer cells. Environ. Toxicol. 2021, 36, 1491–1503. [Google Scholar] [CrossRef]
- Chen, K.; Qian, W.; Jiang, Z.; Cheng, L.; Li, J.; Sun, L.; Zhou, C.; Gao, L.; Lei, M.; Yan, B.; et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol. Cancer 2017, 16, 131. [Google Scholar] [CrossRef]
- Pretta, A.; Ziranu, P.; Puzzoni, M.; Lai, E.; Orsi, G.; Liscia, N.; Molinaro, E.; Mariani, S.; Riggi, L.; Rovesti, G.; et al. Retrospective survival analysis in patients with metastatic pancreatic ductal adenocarcinoma with insulin-treated type 2 diabetes mellitus. Tumori 2021, 107, 550–555. [Google Scholar] [CrossRef]
- Pircher, A.; Zieher, M.; Eigentler, A.; Pichler, R.; Schäfer, G.; Fritz, J.; Puhr, M.; Steiner, E.; Horninger, W.; Klocker, H.; et al. Antidiabetic drugs influence molecular mechanisms in prostate cancer. Cancer Biol. Ther. 2018, 19, 1153–1161. [Google Scholar] [CrossRef]
- Winer, L.K.; Dhar, V.K.; Wima, K.; Morris, M.C.; Lee, T.C.; Shah, S.A.; Ahmad, S.A.; Patel, S.H. The Impact of Tumor Location on Resection and Survival for Pancreatic Ductal Adenocarcinoma. J. Surg. Res. 2019, 239, 60–66. [Google Scholar] [CrossRef]
- Meng, Z.; Cao, M.; Zhang, Y.; Liu, Z.; Wu, S.; Wu, H. Tumor location as an indicator of survival in T1 resectable pancreatic ductal adenocarcinoma: A propensity score-matched analysis. BMC Gastroenterol. 2019, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.M.; Singh, H.; Turner, A.P.; Ceppa, E.P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M. Extended distal pancreatectomy for pancreatic adenocarcinoma with splenic vein thrombosis and/or adjacent organ invasion. Am. J. Surg. 2015, 209, 564–569. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.; et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, F.; Hong, J.; Ng, D.M.; Yang, T.; Zhou, X.; Jin, J.; Zhou, F.; Chen, P.; Xu, Y. The role of FOLFIRINOX in metastatic pancreatic cancer: A meta-analysis. World J. Surg. Oncol. 2021, 19, 182. [Google Scholar] [CrossRef]
- Freeman, R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014, 126, 63–79. [Google Scholar] [CrossRef]
- Sahin, I.H.; Shama, M.A.; Tanaka, M.; Abbruzzese, J.L.; Curley, S.A.; Hassan, M.; Li, D. Association of diabetes and perineural invasion in pancreatic cancer. Cancer Med. 2012, 1, 357–362. [Google Scholar] [CrossRef]
- Jian, L.; Yang, G. Identification of Key Genes Involved in Diabetic Peripheral Neuropathy Progression and Associated with Pancreatic Cancer. Diabetes Metab. Syndr. Obes. 2020, 13, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Eibl, G.; Rozengurt, E. Metformin: Review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadhurst, P.J.; Hart, A.R. Metformin as an Adjunctive Therapy for Pancreatic Cancer: A Review of the Literature on Its Potential Therapeutic Use. Dig. Dis. Sci. 2018, 63, 2840–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, A.; Khawaja, K.I.; Masud, F.; Saif, M.W. Metformin and pancreatic cancer: Is there a role? Cancer Chemother. Pharmacol. 2016, 77, 235–242. [Google Scholar] [CrossRef]
- Duan, W.; Chen, K.; Jiang, Z.; Chen, X.; Sun, L.; Li, J.; Lei, J.; Xu, Q.; Ma, J.; Li, X.; et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017, 385, 225–233. [Google Scholar] [CrossRef]
- Ren, D.; Qin, G.; Zhao, J.; Sun, Y.; Zhang, B.; Li, D.; Wang, B.; Jin, X.; Wu, H. Metformin activates the STING/IRF3/IFN-β pathway by inhibiting AKT phosphorylation in pancreatic cancer. Am. J. Cancer Res. 2020, 10, 2851–2864. [Google Scholar]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer 2018, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, Y.; Miyazaki, K.; Yoshikawa, M.; Yamada, S.; Saito, Y.; Ikemoto, T.; Imura, S.; Morine, Y.; Shimada, M. Value of the CRP-albumin ratio in patients with resectable pancreatic cancer. J. Med. Investig. 2021, 68, 244–255. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, K.; Gong, Y.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Luo, G.; et al. The CRP/Albumin Ratio Predicts Survival And Monitors Chemotherapeutic Effectiveness In Patients With Advanced Pancreatic Cancer. Cancer Manag. Res. 2019, 11, 8781–8788. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jin, K.; Guo, M.; Long, J.; Liu, L.; Liu, C.; Xu, J.; Ni, Q.; Luo, G.; Yu, X. Prognostic Value of the CRP/Alb Ratio, a Novel Inflammation-Based Score in Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Luo, G.; Gong, Y.; Xu, H.; Qian, Y.; Deng, S.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; et al. Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 4017–4025. [Google Scholar] [CrossRef] [PubMed]
- Strijker, M.; van Veldhuisen, E.; van der Geest, L.G.; Busch, O.R.; Bijlsma, M.F.; Haj Mohammad, N.; Homs, M.Y.; van Hooft, J.E.; Verheij, J.; de Vos-Geelen, J.; et al. Readily available biomarkers predict poor survival in metastatic pancreatic cancer. Biomarkers 2021, 26, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.G.; Maingay, J.; Sangster, K.; Fearon, K.C.; Ross, J.A. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: Relationship to acute phase response and survival. Oncol. Rep. 2009, 21, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Heinemann, V.; Kullmann, F.; Laubender, R.P.; Klose, C.; Bruns, C.J.; Holdenrieder, S.; Modest, D.P.; Schulz, C.; Boeck, S. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: Results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J. Cancer Res. Clin. Oncol. 2013, 139, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- You, L.; Ning, L.; Xie, W.; Lang, J.; Huang, Y. Metabolic syndrome and pancreatic cancer risk: A systematic review and meta-analysis. Oncotarget 2018, 5. [Google Scholar] [CrossRef]
- Xia, B.; He, Q.; Pan, Y.; Gao, F.; Liu, A.; Tang, Y.; Chong, C.; Teoh, A.Y.B.; Li, F.; He, Y.; et al. Metabolic syndrome and risk of pancreatic cancer: A population-based prospective cohort study. Int. J. Cancer 2020, 147, 3384–3393. [Google Scholar] [CrossRef]
- Rosato, V.; Tavani, A.; Bosetti, C.; Pelucchi, C.; Talamini, R.; Polesel, J.; Serraino, D.; Negri, E.; La Vecchia, C. Metabolic syndrome and pancreatic cancer risk: A case-control study in Italy and meta-analysis. Metabolism 2011, 60, 1372–1378. [Google Scholar] [CrossRef]


| Variable | Non-DM (n = 101) | DM (n = 74) | T2DM (n = 47) | NODM (n = 27) | |||
|---|---|---|---|---|---|---|---|
| M ± SD/n (%)/ MD (95% CI) | M ± SD/n (%)/ MD (95% CI) | p * | M ± SD/n (%)/ MD (95% CI) | p ** | M ± SD/n (%)/ MD (95% CI) | p *** | |
| Gender (male) | 47 (46.5%) | 40 (54.1%) | 0.326 | 27 (57.4%) | 0.216 | 13 (48.1%) | 0.881 |
| Age (years) | 63.41 ± 10.68 | 64.30 ± 8.23 | 0.550 | 65.66 ± 7.99 | 0.200 | 61.93 ± 8.26 | 0.506 |
| ECOG (0/1/2) | 6/75/17 | 6/54/14 | 0.927 | 5/35/7 | 0.893 | 1/20/6 | 0.441 |
| History of other cancers | 9 (8.9%) | 8 (11.1%) | 0.632 | 7 (15.2%) | 0.255 | 1 (3.8%) | 0.686 |
| Autoimmune disease | 11 (10.9%) | 9 | 0.936 | 7 | 0.717 | 2 | 1.000 |
| Hypertension | 44 (43.6%) | 45 (60.8%) | 0.024 | 31 (66.0%) | 0.011 | 14 (51.9%) | 0.442 |
| Adverse effects—adjuvant chemotherapy | 44 (83.0%) | 35 (74.5%) | 0.295 | 24 (82.8%) | 1.000 | 11 (61.1%) | 0.099 |
| Neurological | 4 (7.5%) | 2 (4.3%) | 0.681 | 2 (6.9%) | 1.000 | 0 | 0.566 |
| Neutropenia | 38 (71.7%) | 24 (51.1%) | 0.034 | 17 (58.6%) | 0.228 | 7 (38.9%) | 0.013 |
| Hepatological | 2 (3.8%) | 4 (8.5%) | 0.416 | 2 (6.9%) | 0.612 | 2 (11.1%) | 0.265 |
| Adverse effects—palliative chemotherapy | 67 (83.8%) | 41 (71.9%) | 0.095 | 24 (68.6%) | 0.065 | 17 (77.3%) | 0.531 |
| Neurological | 14(17.5%) | 7 (12.3%) | 0.403 | 4 (11.4%) | 0.410 | 3 (13.6%) | 1.000 |
| Neutropenia | 44 (55.0%) | 22 (38.6%) | 0.058 | 11 (31.4%) | 0.020 | 11 (50%) | 0.677 |
| Hepatological | 5 (6.3%) | 6 (10.5%) | 0.525 | 5 (14.3%) | 0.170 | 1 (4.5%) | 1.000 |
| Operative complications | 5 (7.0%) | 3 (5.6%) | 1.000 | 2 (6.3%) | 1.000 | 1 (4.5%) | 1.000 |
| Neuroinvasion | 43 (79.6%) | 35 (87.5%) | 0.315 | 24 (88.9%) | 0.365 | 11 (84.6%) | 1.000 |
| Angioinvasion | 46 (85.2%) | 29 (69.0%) | 0.058 | 21 (72.4%) | 0.160 | 8 (61.5%) | 0.110 |
| Grading (1/2/3) | 11/51/14 | 9/39/12 | 0.964 | 5/27/7 | 0.965 | 4/12/5 | 0.632 |
| T (1/2/3/4) | 2/15/51/5 | 1/15/34/2 | 0.689 | 1/11/19/2 | 0.519 | 0/4/15/0 | 0.825 |
| N (0/1/2) | 13/43/16 | 17/25/11 | 0.181 | 9/17/6 | 0.508 | 8/8/5 | 0.121 |
| M (0/1) | 56/45 | 52/22 | 0.046 | 32/15 | 0.145 | 20/7 | 0.080 |
| TNM Stage (IA/IB/IIA/IIB/III/IV) | 1/3/5/31/12/45 | 1/6/6/22/10/21 | 0.268 | 1/5/2/15/5/15 | 0.354 | 0/1/4/7/5/6 | 0.156 |
| Localisation | 77/9/7/3/2/3 | 57/4/5/0/4/4 | 0.475 | 33/3/5/0/3/3 | 0.414 | 24/1/0/0/1/1 | 0.579 |
| Metastases | 29/4/30/3/9 | 25/6/11/3/3 | 0.131 | 16/4/7/2/2 | 0.219 | 9/2/4/1/1 | 0.440 |
| CEA > 5 ng/mL | 22 (32.8%) | 19 (36.5%) | 0.673 | 15 (40.5%) | 0.432 | 4 (26.7%) | 0.765 |
| CA19-9 > 37 IU/mL | 51 (56.7%) | 38 (58.6%) | 0.824 | 26 (57.8%) | 0.902 | 12 (60.0%) | 0.785 |
| CRP/lymphocytes ratio > 1.8 | 37 (66.1%) | 21 (46.7%) | 0.050 | 17 (50.0%) | 0.131 | 4 (36.4%) | 0.092 |
| Lymphocytes > 1 × 103/µL | 93 (92.1%) | 66 (91.7%) | 0.922 | 42 (89.4%) | 0.552 | 24 (96.0%) | 0.687 |
| Haemoglobin > 12 g/dL | 63 (63.6%) | 51 (70.8%) | 0.324 | 33 (70.2%) | 0.434 | 18 (72.0%) | 0.432 |
| Platelets > 400 × 103/µL | 18 (17.8%) | 11 (15.3%) | 0.659 | 6 (12.8%) | 0.437 | 5 (20.0%) | 0.777 |
| CRP > 5 mg/L | 28 (59.0%) | 18 (40.0%) | 0.316 | 15 (44.1%) | 0.588 | 3 (27.3%) | 0.167 |
| OS | 18 (15.7–20.3) | 22 (18.4–26.6) | 0.050 | 20 (15.9–24.1) | 0.462 | 23 (16.0–30.0) | 0.017 |
| DFS | 12 (9.0–15.0) | 9 (7.7–10.3) | 0.533 | 9 (4.9–13.1) | 0.706 | 7 (4.9–9.1) | 0.580 |
| PFS | 5 (3.9–6.1) | 6 (4.34–7.6) | 0.206 | 6 (4.6–7.4) | 0.389 | 7 (4.7–9.3) | 0.230 |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 0.986 (0.952–1.021) | 0.424 |
| Gender | - | - |
| Female | Ref | - |
| Male | 1.194 (0.690–2.063) | 0.526 |
| Autoimmune disease | 0.660 (0.260–1.676) | 0.382 |
| Hypertension | 0.643 (0.372–1.112) | 0.114 |
| T | - | - |
| T1 | Ref | - |
| T2 | 0.336 (0.041–2.729) | 0.308 |
| T3 | 0.254 (0.032–1.996) | 0.192 |
| T4 | 0.456 (0.040–5.222) | 0.528 |
| N | - | - |
| N0 | Ref | - |
| N1 | 1.692 (0.768–3.730) | 0.192 |
| N2 | 3.163 (1.200–8.338) | 0.020 |
| M | - | - |
| M0 | Ref | - |
| M1 | 1.524 (0.852–2.725) | 0.156 |
| TNM stage | - | - |
| IA | Ref | - |
| IB | 0.310 (0.033–2.862) | 0.301 |
| IIA | 0.149 (0.015–1.465) | 0.103 |
| IIB | 0.302 (0.039–2.359) | 0.254 |
| III | 0.551 (0.067–4.513) | 0.579 |
| IV | 0.450 (0.058–3.450) | 0.449 |
| Angioinvasion | 1.193 (0.525–2.712) | 0.674 |
| Neuroinvasion | 0.563 (0.192–1.653) | 0.296 |
| R | - | 0.081 |
| R0 | Ref | |
| R1 | 1.789 (0.930–3.442) | |
| Grading | - | - |
| G1 | Ref | - |
| G2 | 1.691 (0.659–4.339) | 0.275 |
| G3 | 2.155 (0.711–6.531) | 0.175 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.809 (0.999–3.277) | 0.050 |
| Operation type | - | - |
| Whipple | Ref | - |
| Other | 1.367 (0.688–2.717) | 0.372 |
| Vascular reconstruction | 1.242 (0.543–2.842) | 0.608 |
| Adverse effects—adjuvant chemotherapy | 1.521 (0.650–3.557) | 0.333 |
| Neutropenia | 1.673 (0.792–3.535) | 0.178 |
| Hepatological | 1.113 (0.263–4.722) | 0.883 |
| Adverse effects—palliative chemotherapy | 1.025 (0.544–1.931) | 0.939 |
| Neutropenia | 0.651 (0.356–1.191) | 0.163 |
| Hepatological | 1.182 (0.418–3.345) | 0.751 |
| Neurological | 0.490 (0.173–1.386) | 0.179 |
| History of other cancers | 1.907 (0.838–4.337) | 0.124 |
| CEA | 1.004 (1.001–1.007) | 0.019 |
| CA 19-9 | 1.000 (0.999–1.002) | 0.059 |
| Lymphocytes | 0.956 (0.844–1.082) | 0.475 |
| CRP/lymphocytes ratio | 1.032 (1.013–1.053) | 0.001 |
| Haemoglobin | 0.954 (0.720–1.263) | 0.741 |
| Platelets | 1.001 (0.999–1.003) | 0.249 |
| Calcium | 0.446 (0.051–3.899) | 0.465 |
| CRP | 1.017 (1.008–1.025) | <0.001 |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Step 1 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 1.180 (0.182–7.667) | 0.862 |
| N2 | 1.239 (0.126–12.214) | 0.854 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.149 (0.226–5.833) | 0.867 |
| CEA | 1.006 (0.983–1.030) | 0.620 |
| CRP/lymphocytes ratio | 0.984 (0.900–1.075) | 0.718 |
| CRP | 1.027 (0.965–1.092) | 0.401 |
| Step 2 | ||
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.030 (0.352–3.011) | 0.957 |
| CEA | 1.006 (0.984–1.028) | 0.621 |
| CRP/lymphocytes ratio | 0.988 (0.917–1.065) | 0.756 |
| CRP | 1.024 (0.971–1.079) | 0.381 |
| Step 3 | ||
| CEA | 1.005 (0.984–1.028) | 0.623 |
| CRP/lymphocytes ratio | 0.988 (0.917–1.065) | 0.988 |
| CRP | 1.024 (0.972–1.079) | 0.369 |
| Step 4 | ||
| CEA | 1.005 (0.984–1.027) | 0.633 |
| CRP | 1.016 (0.997–1.035) | 0.095 |
| Step 5 | ||
| CRP | 1.017 (1.008–1.025) | <0.001 |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age | 0.563 (0.229–1.383) | 0.210 |
| Gender | - | 0.187 |
| Female | Ref | |
| Male | 0.517 (0.194–1.377) | |
| Hypertension | 0.418 (0.164–1.069) | 0.069 |
| Nodal involvement | 3.142 (0.808–12.216) | 0.098 |
| TNM stage (IA/IB/IIA/IIB/III/IV) | 1.774 (1.116–2.822) | 0.015 |
| R | - | 0.081 |
| R0 | Ref | |
| R1 | 1.287 (0.443–3.737) |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 0.978 (0.926–1.033) | 0.426 |
| Gender | - | - |
| Female | Ref | - |
| Male | 0.898 (0.639–1.263) | 0.536 |
| Autoimmune disease | 0.962 (0.334–2.771) | 0.942 |
| Hypertension | 0.732 (0.358–1.500) | 0.393 |
| T | - | - |
| T1 | Ref | - |
| T2 | 0.268 (0.030–2.383) | 0.238 |
| T3 | 0.196 (0.025–1.702) | 0.139 |
| T4 | 0.358 (0.030–4.325) | 0.419 |
| N | - | - |
| N0 | Ref | - |
| N1 | 1.383 (0.465–4.111) | 0.560 |
| N2 | 5.342 (1.264–22.564) | 0.023 |
| M | - | - |
| M0 | Ref | - |
| M1 | 1.524 (0.852–2.725) | 0.156 |
| TNM stage | - | - |
| IA | Ref | - |
| IB | 0.179 (0.017–1.900) | 0.154 |
| IIB | 0.215 (0.025–1.827) | 0.159 |
| III | 0.734 (0.079–6.829) | 0.786 |
| IV | 0.255 (0.029–2.172) | 0.211 |
| Angioinvasion | 2.023 (0.670–6.109) | 0.212 |
| Neuroinvasion | 0.865 (0.194–3.863) | 0.849 |
| R | - | 0.064 |
| R0 | Ref | |
| R1 | 2.185 (0.956–4.996) | |
| Grading | - | - |
| G1 | Ref | - |
| G2 | 2.111 (0.494–9.014) | 0.313 |
| G3 | 1.433 (0.259–7.920) | 0.680 |
| Tumour site | - | - |
| Head | Ref | - |
| Other | 1.705 (0.841–3.454) | 0.139 |
| Operation type | - | - |
| Whipple | Ref | - |
| Other | 1.307 (0.567–3.184) | 0.555 |
| Vascular reconstruction | 0.877 (0.295–2.606) | 0.814 |
| DM treatment | - | - |
| Insulin | Ref | - |
| Metformin | 0.726 (0.363–1.451) | 0.364 |
| Adverse effects—adjuvant chemotherapy | 0.833 (0.237–2.934) | 0.776 |
| Neutropenia | 1.055 (0.376–2.957) | 0.919 |
| Adverse effects—palliative chemotherapy | 0.745 (0.336–1.650) | 0.468 |
| Neutropenia | 0.541 (0.215–1.259) | 0.191 |
| Hepatological | 0.763 (0.228–2.556) | 0.662 |
| Neurological | 0.224 (0.030–1.669) | 0.144 |
| History of other cancers | 1.748 (0.695–4.396) | 0.235 |
| CEA | 1.012 (0.978–1.048) | 0.493 |
| Lymphocytes | 0.964 (0.840–1.107) | 0.607 |
| CRP/lymphocytes ratio | 1.013 (1.004–1.022) | 0.004 |
| Haemoglobin | 0.900 (0.639–1.263) | 0.536 |
| Calcium | 0.738 (0.050–10.854) | 0.825 |
| CRP | 1.018 (1.007–1.028) | <0.001 |
| Variable | HR (95% CI) | p-Value |
|---|---|---|
| Step 1 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 0.620 (0.169–2.280 | 0.472 |
| N2 | 2.116 (0.390–11.471) | 0.385 |
| CRP/lymphocytes ratio | 0.998 (0.904–1.102) | 0.971 |
| CRP | 1.017 (0.950–1.089) | 0.629 |
| Step 2 | ||
| N | - | - |
| N0 | Ref | - |
| N1 | 0.616 (0.177–2.139) | 0.445 |
| N2 | 2.097 (0.416–10.562) | 0.370 |
| CRP | 1.016 (0.995–1.037) | 0.134 |
| Step 3 | ||
| CRP | 1.018 (1.007–1.028) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badowska-Kozakiewicz, A.; Fudalej, M.; Kwaśniewska, D.; Durlik, M.; Nasierowska-Guttmejer, A.; Mormul, A.; Włoszek, E.; Czerw, A.; Banaś, T.; Deptała, A. Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers 2022, 14, 2840. https://doi.org/10.3390/cancers14122840
Badowska-Kozakiewicz A, Fudalej M, Kwaśniewska D, Durlik M, Nasierowska-Guttmejer A, Mormul A, Włoszek E, Czerw A, Banaś T, Deptała A. Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers. 2022; 14(12):2840. https://doi.org/10.3390/cancers14122840
Chicago/Turabian StyleBadowska-Kozakiewicz, Anna, Marta Fudalej, Daria Kwaśniewska, Marek Durlik, Anna Nasierowska-Guttmejer, Agata Mormul, Emilia Włoszek, Aleksandra Czerw, Tomasz Banaś, and Andrzej Deptała. 2022. "Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes" Cancers 14, no. 12: 2840. https://doi.org/10.3390/cancers14122840
APA StyleBadowska-Kozakiewicz, A., Fudalej, M., Kwaśniewska, D., Durlik, M., Nasierowska-Guttmejer, A., Mormul, A., Włoszek, E., Czerw, A., Banaś, T., & Deptała, A. (2022). Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers, 14(12), 2840. https://doi.org/10.3390/cancers14122840






