Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Anatomical, Physiological, and Pathological Features of PMCs
2.1. Anatomy
2.2. Physiology
2.3. Embryology
2.4. Pathology
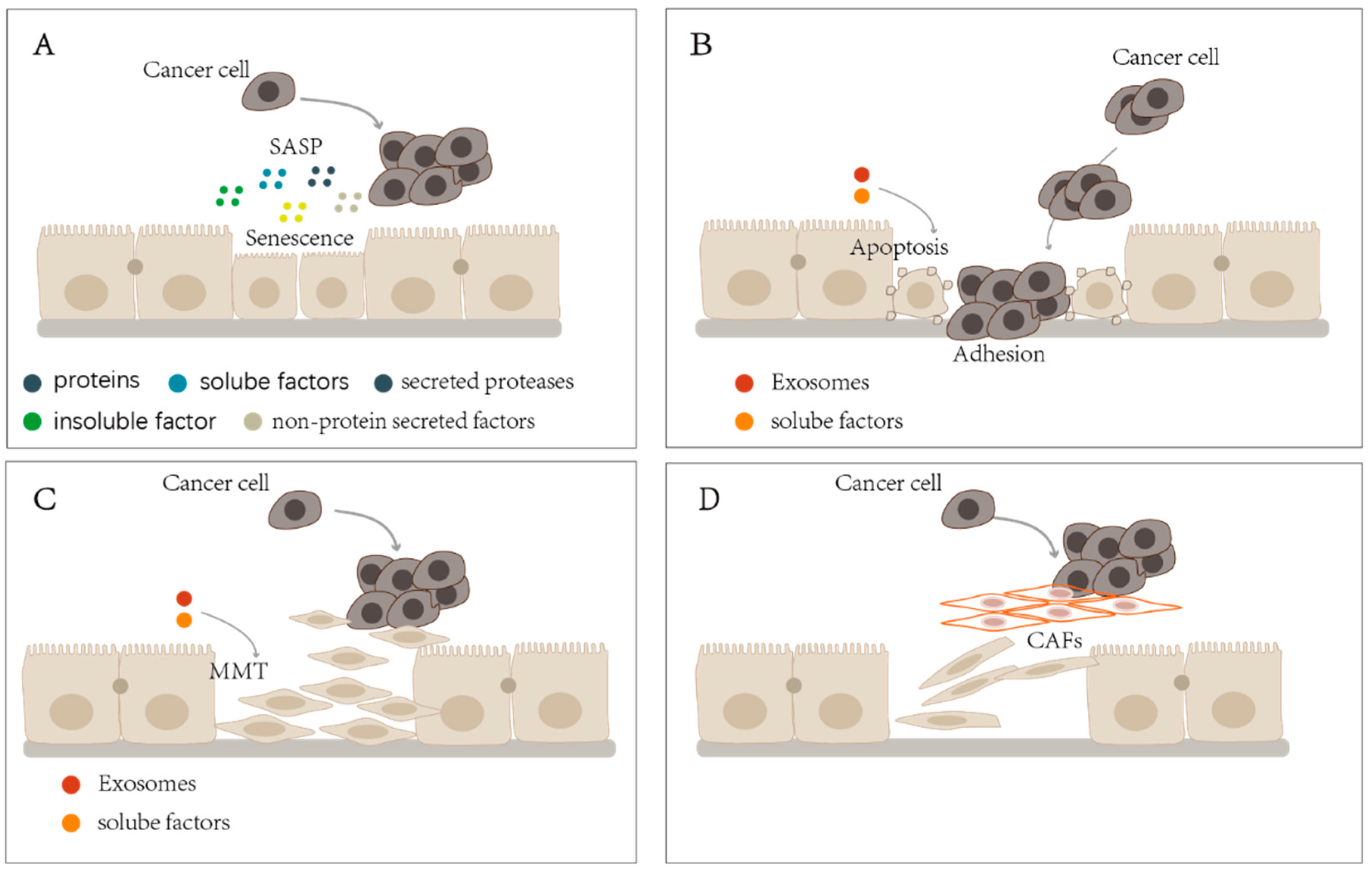
3. PMCs Inhibit Tumor Progression in the Peritoneal Cavity
4. Aging PMCs Promote Tumor Progression in the Peritoneal Cavity
5. Apoptotic PMCs Promote Tumor Progression in the Peritoneal Cavity
6. MMT of PMCs Promotes Tumor Progression in the Peritoneal Cavity
7. PMCs’ Transition Promotes Tumor Progression in the Peritoneal Cavity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isaza-Restrepo, A.; Martin-Saavedra, J.S.; Velez-Leal, J.L.; Vargas-Barato, F.; Riveros-Duenas, R. The Peritoneum: Beyond the Tissue—A Review. Front. Physiol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Baal, J.O.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Ujszaszi, A.; Wallwiener, M.; Nyarangi-Dix, J.; Sallay, P.; Burkhardt, D.; Querfeld, U.; Pfeifle, V.; et al. Quantitative Histomorphometry of the Healthy Peritoneum. Sci. Rep. 2016, 6, 21344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordillo, C.H.; Sandoval, P.; Munoz-Hernandez, P.; Pascual-Anton, L.; Lopez-Cabrera, M.; Jimenez-Heffernan, J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers 2020, 12, 499. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Anton, L.; Cardenes, B.; Sainz de la Cuesta, R.; Gonzalez-Cortijo, L.; Lopez-Cabrera, M.; Cabanas, C.; Sandoval, P. Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 11496. [Google Scholar] [CrossRef]
- Kastelein, A.W.; Vos, L.M.C.; de Jong, K.H.; van Baal, J.; Nieuwland, R.; van Noorden, C.J.F.; Roovers, J.W.R.; Lok, C.A.R. Embryology, anatomy, physiology and pathophysiology of the peritoneum and the peritoneal vasculature. Semin. Cell Dev. Biol. 2019, 92, 27–36. [Google Scholar] [CrossRef]
- Zarogiannis, S.; Liakopoulos, V.; Hatzoglou, C.; Kourti, P.; Vogiatzidis, K.; Potamianos, S.; Eleftheriadis, T.; Gourgoulianis, K.; Molyvdas, P.A.; Stefanidis, I. Effect of sodium-potassium pump inhibition by ouabain on the permeability of isolated visceral sheep peritoneum. Adv. Perit. Dial. 2007, 23, 43–47. [Google Scholar]
- Twardowski, Z.J. Pathophysiology of peritoneal transport. Contrib. Nephrol. 2006, 150, 13–19. [Google Scholar] [CrossRef]
- Negoi, D.; Khanna, R.J.N. History of peritoneal dialysis. In Nolph and Gokal’s Textbook of Peritoneal Dialysis; Springer: Berlin, Germany, 2020; pp. 1–26. [Google Scholar]
- Bartosova, M.; Herzog, R.; Ridinger, D.; Levai, E.; Jenei, H.; Zhang, C.; Gonzalez Mateo, G.T.; Marinovic, I.; Hackert, T.; Bestvater, F.; et al. Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules 2020, 10, 1178. [Google Scholar] [CrossRef]
- Baturina, G.S.; Katkova, L.E.; Schmitt, C.P.; Solenov, E.I.; Zarogiannis, S.G. Comparison of Isotonic Activation of Cell Volume Regulation in Rat Peritoneal Mesothelial Cells and in Kidney Outer Medullary Collecting Duct Principal Cells. Biomolecules 2021, 11, 1452. [Google Scholar] [CrossRef]
- Stefanidis, I.; Liakopoulos, V.; Kourti, P.; Zarogiannis, S.; Poultsidi, A.; Mertems, P.R.; Salmas, M.; Hatzoglou, C.; Gourgoulianis, K.; Molyvdas, P.A. Amiloride-sensitive sodium channels on the parietal human peritoneum: Evidence by ussing-type chamber experiments. ASAIO J. 2007, 53, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, I.; Zarogiannis, S.; Hatzoglou, C.; Liakopoulos, V.; Kourti, P.; Poultsidi, A.; Mertens, P.R.; Gourgoulianis, K.; Molyvdas, P.A. Enhancement of the transmesothelial resistance of the parietal sheep peritoneum by epinephrine in vitro: Ussing-type chamber experiments. Artif. Organs 2005, 29, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Zarogiannis, S.; Kourti, P.; Hatzoglou, C.; Liakopoulos, V.; Poultsidi, A.; Gourgoulianis, K.; Molyvdas, P.A.; Stefanidis, I. Influence of the sodium transport inhibition by amiloride on the transmesothelial resistance of isolated visceral sheep peritoneum. Adv. Perit. Dial. 2005, 21, 5–8. [Google Scholar]
- Kourti, P.; Zarogiannis, S.G.; Liakopoulos, V.; Karioti, A.; Eleftheriadis, T.; Hatzoglou, C.; Gourgoulianis, K.; Molyvdas, P.A.; Stefanidis, I. Endothelin-1 acutely reduces the permeability of visceral sheep peritoneum in vitro through both endothelin-A and endothelin-B receptors. Artif. Organs 2013, 37, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Chapuli, R.; Perez-Pomares, J.M.; Macias, D.; Garcia-Garrido, L.; Carmona, R.; Gonzalez, M. Differentiation of hemangioblasts from embryonic mesothelial cells? A model on the origin of the vertebrate cardiovascular system. Differentiation 1999, 64, 133–141. [Google Scholar] [CrossRef]
- Perez-Pomares, J.M.; Carmona, R.; Gonzalez-Iriarte, M.; Atencia, G.; Wessels, A.; Munoz-Chapuli, R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002, 46, 1005–1013. [Google Scholar]
- Li, Y.; Wang, J.; Asahina, K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc. Natl. Acad. Sci. USA 2013, 110, 2324–2329. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.; Balogh, P.; Kiss, A.L. Mesothelial cells can detach from the mesentery and differentiate into macrophage-like cells. APMIS 2011, 119, 782–793. [Google Scholar] [CrossRef]
- Herzog, R.; Sacnun, J.M.; Gonzalez-Mateo, G.; Bartosova, M.; Bialas, K.; Wagner, A.; Unterwurzacher, M.; Sobieszek, I.J.; Daniel-Fischer, L.; Rusai, K.; et al. Lithium preserves peritoneal membrane integrity by suppressing mesothelial cell αB-crystallin. Sci. Transl. Med. 2021, 13, aaz9705. [Google Scholar] [CrossRef]
- Zindel, J.; Mittner, J.; Bayer, J.; April-Monn, S.L.; Kohler, A.; Nusse, Y.; Dosch, M.; Buchi, I.; Sanchez-Taltavull, D.; Dawson, H.; et al. Intraperitoneal microbial contamination drives post-surgical peritoneal adhesions by mesothelial EGFR-signaling. Nat. Commun. 2021, 12, 7316. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Zsiros, V.; Kiss, A.L. Under inflammatory stimuli mesenteric mesothelial cells transdifferentiate into macrophages and produce pro-inflammatory cytokine IL-6. Inflamm. Res. 2019, 68, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Horne, A.W. The role of the peritoneum in the pathogenesis of endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, V.; Kiss, A.L. Cellular and molecular events of inflammation induced transdifferentiation (EMT) and regeneration (MET) in mesenteric mesothelial cells. Inflamm. Res. 2020, 69, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Archid, R.; Reymond, M.A. Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition. Int. J. Mol. Sci. 2020, 21, 4158. [Google Scholar] [CrossRef]
- Bellingan, G.J.; Xu, P.; Cooksley, H.; Cauldwell, H.; Shock, A.; Bottoms, S.; Haslett, C.; Mutsaers, S.E.; Laurent, G.J. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 2002, 196, 1515–1521. [Google Scholar] [CrossRef]
- Pronk, A.; de Groot, P.G.; Hoynck van Papendrecht, A.A.; Verbrugh, H.A.; Leguit, P.; van Vroonhoven, T.J.; Sixma, J.J. Thrombogenicity and procoagulant activity of human mesothelial cells. Arterioscler. Thromb. 1992, 12, 1428–1436. [Google Scholar] [CrossRef] [Green Version]
- Iakhiaev, A.; Idell, S. Activation and degradation of protein C by primary rabbit pleural mesothelial cells. Lung 2006, 184, 81–88. [Google Scholar] [CrossRef]
- Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int. J. Mol. Sci. 2019, 20, 5765. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Guiral, D.; Hubner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Kucinska, M.; Tykarski, A.; Ksiazek, K. The protective activity of mesothelial cells against peritoneal growth of gastrointestinal tumors: The role of soluble ICAM-1. Int. J. Biochem. Cell Biol. 2017, 86, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Feichtinger, H.; Mikuz, G.; Marth, C.; Zeimet, A.G.; Herold, M.; Knabbe, C.; Offner, F.A. Interactions of human peritoneal mesothelial cells with serous ovarian cancer cell spheroids—Evidence for a mechanical and paracrine barrier function of the peritoneal mesothelium. Int. J. Gynecol. Cancer 2014, 24, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.A.; Brugge, J.S. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011, 1, 144–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, H.A.; Nieman, K.M.; Mitra, A.K.; Lengyel, E. The first line of intra-abdominal metastatic attack: Breaching the mesothelial cell layer. Cancer Discov. 2011, 1, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Braig, M.; Lee, S.; Loddenkemper, C.; Rudolph, C.; Peters, A.H.; Schlegelberger, B.; Stein, H.; Dorken, B.; Jenuwein, T.; Schmitt, C.A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005, 436, 660–665. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Narita, M.; Lowe, S.W. Senescence comes of age. Nat. Med. 2005, 11, 920–922. [Google Scholar] [CrossRef]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Hunter, K.D.; Fonseca, F.P.; Radhakrishnan, R. Emerging role of cellular senescence in the pathogenesis of oral submucous fibrosis and its malignant transformation. Head Neck 2021, 43, 3153–3164. [Google Scholar] [CrossRef]
- Kitawaki, Y.; Nakamura, Y.; Kubota-Nakayama, F.; Yamazaki, Y.; Miki, Y.; Hata, S.; Ise, K.; Kikuchi, K.; Morimoto, R.; Satoh, F.; et al. Tumor microenvironment in functional adrenocortical adenomas: Immune cell infiltration in cortisol-producing adrenocortical adenoma. Hum. Pathol. 2018, 77, 88–97. [Google Scholar] [CrossRef]
- Singh, A.J.; Gray, J.W. Chemokine signaling in cancer-stroma communications. J. Cell Commun. Signal. 2021, 15, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Sosinska, P.; Maksin, K.; Kucinska, M.G.; Piotrowska, H.; Murias, M.; Wozniak, A.; Szpurek, D.; Ksiazek, K. Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget 2015, 6, 29178–29195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.G.; Jackson, J.G. SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer 2016, 2, 676–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jia, Y.; Shen, S.; Kim, J.; Wang, X.; Lee, E.; Brownell, I.; Cho-Vega, J.H.; Lewis, C.; Homsi, J.; et al. Merkel Cell Polyomavirus Small T Antigen Activates Noncanonical NF-κB Signaling to Promote Tumorigenesis. Mol. Cancer Res. 2020, 18, 1623–1637. [Google Scholar] [CrossRef]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. IGF Binding Protein-5 Induces Cell Senescence. Front. Endocrinol. 2018, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, H.M.; Birnbaum, R.S.; Poot, M.; Quinn, L.S.; Swisshelm, K. Insulin-like growth factor binding protein-related protein 1 inhibits proliferation of MCF-7 breast cancer cells via a senescence-like mechanism. Cell Growth Differ. 2002, 13, 205–213. [Google Scholar] [PubMed]
- Mabrouk, N.; Ghione, S.; Laurens, V.; Plenchette, S.; Bettaieb, A.; Paul, C. Senescence and Cancer: Role of Nitric Oxide (NO) in SASP. Cancers 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Pulido, T.; Valdovinos, A.; Alptekin, S.; Chang, E.; Jones, E.; Diaz, D.A.; Flores, J.; Velarde, M.C.; Demaria, M.; et al. Cellular Senescence Promotes Skin Carcinogenesis through p38MAPK and p44/42MAPK Signaling. Cancer Res. 2020, 80, 3606–3619. [Google Scholar] [CrossRef]
- Takasugi, M.; Yoshida, Y.; Hara, E.; Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2022. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, D.; Yu, B.P. Senescent cells in cancer therapy: Why and how to remove them. Cancer Lett. 2021, 520, 68–79. [Google Scholar] [CrossRef]
- Marquard, S.; Thomann, S.; Weiler, S.M.E.; Bissinger, M.; Lutz, T.; Sticht, C.; Toth, M.; de la Torre, C.; Gretz, N.; Straub, B.K.; et al. Yes-associated protein (YAP) induces a secretome phenotype and transcriptionally regulates plasminogen activator Inhibitor-1 (PAI-1) expression in hepatocarcinogenesis. Cell Commun. Signal. 2020, 18, 166. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Quintanilla, R.; Olate-Briones, A.; Venturini, W.; Mancilla, D.; Cayo, A.; Moore-Carrasco, R.; Brown, N.E. SASP-Dependent Interactions between Senescent Cells and Platelets Modulate Migration and Invasion of Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5292. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.C.; Yang, C.H.; Cheng, L.H.; Chang, W.T.; Lin, Y.R.; Cheng, H.C. Fibronectin in Cancer: Friend or Foe. Cells 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Bhatiya, M.; Pathak, S.; Banerjee, A. Oxidative Stress and Cellular Senescence: The Key Tumor-Promoting Factors in Colon Cancer and Beneficial Effects of Polyphenols in Colon Cancer Prevention. Curr. Cancer Ther. Rev. 2021, 17, 292–303. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.F.; Wu, J.H.; Wang, Z.N.; Zhao, T.T.; Xu, H.M.; Song, Y.X.; Xing, Y.N.; Huang, J.Y.; Zhang, J.Y.; Liu, X.Y.; et al. Endoglin overexpression mediates gastric cancer peritoneal dissemination by inducing mesothelial cell senescence. Hum. Pathol. 2016, 51, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikula-Pietrasik, J.; Sosinska, P.; Wierzchowski, M.; Piwocka, K.; Ksiazek, K. Synthetic resveratrol analogue, 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene, accelerates senescence in peritoneal mesothelium and promotes senescence-dependent growth of gastrointestinal cancers. Int. J. Mol. Sci. 2013, 14, 22483–22498. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Matuszkiewicz, K.; Szubert, S.; Moszynski, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell Oncol. 2016, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Sosinska, P.; Maksin, K.; Piotrowska-Kempisty, H.; Kucinska, M.; Murias, M.; Szubert, S.; Wozniak, A.; Szpurek, D.; et al. Senescent peritoneal mesothelium creates a niche for ovarian cancer metastases. Cell Death Dis. 2016, 7, e2565. [Google Scholar] [CrossRef] [Green Version]
- Ksiazek, K.; Mikula-Pietrasik, J.; Korybalska, K.; Dworacki, G.; Jorres, A.; Witowski, J. Senescent peritoneal mesothelial cells promote ovarian cancer cell adhesion: The role of oxidative stress-induced fibronectin. Am. J. Pathol. 2009, 174, 1230–1240. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Ohuchida, K.; Koikawa, K.; Endo, S.; Okumura, T.; Sada, M.; Horioka, K.; Zheng, B.; Moriyama, T.; Nakata, K.; et al. Cancer-associated peritoneal mesothelial cells lead the formation of pancreatic cancer peritoneal dissemination. Int. J. Oncol. 2017, 50, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Demuytere, J.; Ceelen, W.; Van Dorpe, J.; Hoorens, A. The role of the peritoneal microenvironment in the pathogenesis of colorectal peritoneal carcinomatosis. Exp. Mol. Pathol. 2020, 115, 104442. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Hu, C.; Chen, S.; Du, Y.; Huang, L.; Shi, C.; Mo, S.; Cheng, X. Peritoneal metastatic gastric carcinoma cells exhibit more malignant behavior when co-cultured with HMrSV5 cells. Aging 2020, 12, 3238–3248. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Heath, R.M.; Jayne, D.G.; O’Leary, R.; Morrison, E.E.; Guillou, P.J. Tumour-induced apoptosis in human mesothelial cells: A mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br. J. Cancer 2004, 90, 1437–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besso, M.J.; Rosso, M.; Lapyckyj, L.; Moiola, C.P.; Matos, M.L.; Mercogliano, M.F.; Schillaci, R.; Reventos, J.; Colas, E.; Gil-Moreno, A.; et al. FXYD5/Dysadherin, a Biomarker of Endometrial Cancer Myometrial Invasion and Aggressiveness: Its Relationship With TGF-β1 and NF-κB Pathways. Front. Oncol. 2019, 9, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.; Xu, S.; Nguyen, T.T.; Quan, X.; Choi, S.K.; Kim, S.J.; Lee, E.Y.; Cha, S.K.; Park, K.S. Transforming Growth Factor β1-induced Apoptosis in Podocytes via the Extracellular Signal-regulated Kinase-Mammalian Target of Rapamycin Complex 1-NADPH Oxidase 4 Axis. J. Biol. Chem. 2015, 290, 30830–30842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ashmawy, N.E.; Khedr, N.F.; Mansour, M.G.; Al-Ashmawy, G.M. TNM staging for GIT cancers is correlated with the level of MMPs and TGF-β1. Clin. Exp. Med. 2020, 20, 545–555. [Google Scholar] [CrossRef]
- Na, D.; Lv, Z.D.; Liu, F.N.; Xu, Y.; Jiang, C.G.; Sun, Z.; Miao, Z.F.; Li, F.; Xu, H.M. Transforming growth factor beta1 produced in autocrine/paracrine manner affects the morphology and function of mesothelial cells and promotes peritoneal carcinomatosis. Int. J. Mol. Med. 2010, 26, 325–332. [Google Scholar]
- Wu, P.; Wang, J.; Mao, X.; Xu, H.; Zhu, Z. PDCD4 regulates apoptosis in human peritoneal mesothelial cells and promotes gastric cancer peritoneal metastasis. Histol. Histopathol. 2021, 36, 447–457. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.I.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Qu, J.; Zhang, Y.; Che, X.; Cheng, Y.; Fan, Y.; Zhang, S.; Na, D.; Liu, Y.; Qu, X. Gastric cancer-derived exosomes promote peritoneal metastasis by destroying the mesothelial barrier. FEBS Lett. 2017, 591, 2167–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Zhang, N.; He, S.; Lu, X. Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7. Cell Cycle 2020, 19, 1200–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Miao, Z.F.; Zhao, T.T.; Wang, Z.N.; Xu, Y.Y.; Gao, J.; Wu, J.H.; You, Y.; Xu, H.; Xu, H.M. Milky spot macrophages remodeled by gastric cancer cells promote peritoneal mesothelial cell injury. Biochem. Biophys. Res. Commun. 2013, 439, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Serrati, S.; Porcelli, L.; Fragassi, F.; Garofoli, M.; Di Fonte, R.; Fucci, L.; Iacobazzi, R.M.; Palazzo, A.; Margheri, F.; Cristiani, G.; et al. The Interaction between Reactive Peritoneal Mesothelial Cells and Tumor Cells via Extracellular Vesicles Facilitates Colorectal Cancer Dissemination. Cancers 2021, 13, 2505. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Lara-Pezzi, E.; Selgas, R.; Ramirez-Huesca, M.; Dominguez-Jimenez, C.; Jimenez-Heffernan, J.A.; Aguilera, A.; Sanchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Gao, L.; Nie, X.; Gou, R.; Hu, Y.; Dong, H.; Li, X.; Lin, B. Exosomal ANXA2 derived from ovarian cancer cells regulates epithelial-mesenchymal plasticity of human peritoneal mesothelial cells. J. Cell. Mol. Med. 2021, 25, 10916–10929. [Google Scholar] [CrossRef]
- Jiang, C.G.; Lv, L.; Liu, F.R.; Wang, Z.N.; Na, D.; Li, F.; Li, J.B.; Sun, Z.; Xu, H.M. Connective tissue growth factor is a positive regulator of epithelial-mesenchymal transition and promotes the adhesion with gastric cancer cells in human peritoneal mesothelial cells. Cytokine 2013, 61, 173–180. [Google Scholar] [CrossRef]
- Zhu, A.K.; Shan, Y.Q.; Zhang, J.; Liu, X.C.; Ying, R.C.; Kong, W.C. Exosomal NNMT from peritoneum lavage fluid promotes peritoneal metastasis in gastric cancer. Kaohsiung J. Med. Sci. 2021, 37, 305–313. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Li, Q.; Wei, S.; He, Z.; Huang, X.; Wang, L.; Xia, Y.; Xu, Z.; Li, Z.; et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018, 9, 854. [Google Scholar] [CrossRef] [Green Version]
- Mogi, K.; Yoshihara, M.; Iyoshi, S.; Kitami, K.; Uno, K.; Tano, S.; Koya, Y.; Sugiyama, M.; Yamakita, Y.; Nawa, A.; et al. Ovarian Cancer-Associated Mesothelial Cells: Transdifferentiation to Minions of Cancer and Orchestrate Developing Peritoneal Dissemination. Cancers 2021, 13, 1352. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zong, Z.H.; Liu, Y.; Chen, S.; Wang, L.L.; Zhao, Y. circPUM1 Promotes Tumorigenesis and Progression of Ovarian Cancer by Sponging miR-615-5p and miR-6753-5p. Mol. Ther. Nucleic Acids 2019, 18, 882–892. [Google Scholar] [CrossRef] [Green Version]
- Zong, Z.H.; Du, Y.P.; Guan, X.; Chen, S.; Zhao, Y. CircWHSC1 promotes ovarian cancer progression by regulating MUC1 and hTERT through sponging miR-145 and miR-1182. J. Exp. Clin. Cancer Res. 2019, 38, 437. [Google Scholar] [CrossRef] [PubMed]
- Umakoshi, M.; Takahashi, S.; Itoh, G.; Kuriyama, S.; Sasaki, Y.; Yanagihara, K.; Yashiro, M.; Maeda, D.; Goto, A.; Tanaka, M. Macrophage-mediated transfer of cancer-derived components to stromal cells contributes to establishment of a pro-tumor microenvironment. Oncogene 2019, 38, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Gunjigake, K.; Kinoshita, J.; Yamaguchi, T.; Saito, H.; Fujimori, D.; Horiike, T.; Harada, S.; Tajima, H.; Ninomiya, I.; Ohta, T.; et al. Interleukin-17A derived from mast cells contributes to fibrosis in gastric cancer with peritoneal dissemination. Gastric Cancer 2021, 24, 31–44. [Google Scholar] [CrossRef]
- Tanaka, M.; Kuriyama, S.; Itoh, G.; Maeda, D.; Goto, A.; Tamiya, Y.; Yanagihara, K.; Yashiro, M.; Aiba, N. Mesothelial Cells Create a Novel Tissue Niche That Facilitates Gastric Cancer Invasion. Cancer Res. 2017, 77, 684–695. [Google Scholar] [CrossRef] [Green Version]
- Kawka, E.; Witowski, J.; Sandoval, P.; Rudolf, A.; Vidal, A.R.; Cabrera, M.L.; Jorres, A. Epithelial-to-Mesenchymal Transition and Migration of Human Peritoneal Mesothelial Cells Undergoing Senescence. Perit. Dial. Int. 2019, 39, 35–41. [Google Scholar] [CrossRef]
- Dong, D.; Tang, L.; Li, Z.Y.; Fang, M.J.; Gao, J.B.; Shan, X.H.; Ying, X.J.; Sun, Y.S.; Fu, J.; Wang, X.X.; et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 2019, 30, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Tykarski, A.; Ksiazek, K. The peritoneal “soil” for a cancerous “seed”: A comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell. Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Endo, S.; Ikenaga, M.; Ohta, K.; Ueda, M.; Tsuda, Y.; Kato, R.; Itakura, H.; Matsuyama, J.; Nishikawa, K.; Yamada, T. Prognostic factors for cytology-positive gastric cancer. Surg. Today 2019, 49, 56–64. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Jia, S.; Bu, Z.; Zhang, L.; Wu, X.; Li, S.; Shan, F.; Ji, X.; Ji, J. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin. J. Cancer Res. 2017, 29, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.J.; Blumenthaler, A.N.; Das, P.; Minsky, B.D.; Blum, M.; Roy-Chowdhuri, S.; Ajani, J.A.; Ikoma, N.; Mansfield, P.F.; Badgwell, B.D. Staging laparoscopy and peritoneal cytology in patients with early stage gastric adenocarcinoma. World J. Surg. Oncol. 2020, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Nishikawa, K.; Ikenaga, M.; Fujitani, K.; Kawada, J.; Yamatsuji, T.; Kubota, H.; Higashida, M.; Fujiwara, Y.; Ueno, T. Prognostic factors for cytology-positive gastric cancer: A multicenter retrospective analysis. Int. J. Clin. Oncol. 2021, 26, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Nussbaum, D.P.; Speicher, P.J.; Czito, B.G.; Tyler, D.S.; Blazer, D.G., 3rd. Neoadjuvant radiation therapy does not increase perioperative morbidity among patients undergoing gastrectomy for gastric cancer. J. Surg. Oncol. 2015, 112, 46–50. [Google Scholar] [CrossRef]
- Valletti, M.; Eshmuminov, D.; Gnecco, N.; Gutschow, C.A.; Schneider, P.M.; Lehmann, K. Gastric cancer with positive peritoneal cytology: Survival benefit after induction chemotherapy and conversion to negative peritoneal cytology. World J. Surg. Oncol. 2021, 19, 245. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takashima, A.; Nagashima, K.; Terashima, M.; Aizawa, M.; Ohashi, M.; Tanaka, R.; Yamada, T.; Kinoshita, T.; Matsushita, H.; et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): A multi-institutional retrospective study. Gastric Cancer 2021, 24, 701–709. [Google Scholar] [CrossRef]
- Matsui, S.; Fukunaga, Y.; Sugiyama, Y.; Iwagami, M.; Nagasaki, T.; Akiyoshi, T.; Konishi, T.; Kawachi, H. Incidence and Prognostic Value of Lavage Cytology in Colorectal Cancer. Dis. Colon Rectum 2022, 65, 894–900. [Google Scholar] [CrossRef]
- Yu, J.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; He, X.; et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019, 321, 1983–1992. [Google Scholar] [CrossRef] [Green Version]
- Min, B.H.; Kim, E.R.; Kim, K.M.; Park, C.K.; Lee, J.H.; Rhee, P.L.; Kim, J.J. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015, 47, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment—Accomplices in tumor malignancy. Cell. Immunol. 2019, 343, 103729. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kobayashi, H.; Iida, T.; Asai, N.; Masamune, A.; Hara, A.; Esaki, N.; Ushida, K.; Mii, S.; Shiraki, Y.; et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res. 2019, 79, 5367–5381. [Google Scholar] [CrossRef] [Green Version]
- Tsang, M.; Quesnel, K.; Vincent, K.; Hutchenreuther, J.; Postovit, L.M.; Leask, A. Insights into Fibroblast Plasticity: Cellular Communication Network 2 Is Required for Activation of Cancer-Associated Fibroblasts in a Murine Model of Melanoma. Am. J. Pathol. 2020, 190, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Spaw, M.; Anant, S.; Thomas, S.M. Stromal contributions to the carcinogenic process. Mol. Carcinog. 2017, 56, 1199–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Moon, A. Inflammatory fibroblasts in cancer. Arch. Pharm. Res. 2016, 39, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef] [Green Version]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Herrick, S.E.; Mutsaers, S.E. Mesothelial progenitor cells and their potential in tissue engineering. Int. J. Biochem. Cell Biol. 2004, 36, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Lansley, S.M.; Searles, R.G.; Hoi, A.; Thomas, C.; Moneta, H.; Herrick, S.E.; Thompson, P.J.; Newman, M.; Sterrett, G.F.; Prele, C.M.; et al. Mesothelial cell differentiation into osteoblast- and adipocyte-like cells. J. Cell. Mol. Med. 2011, 15, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Pomares, J.M.; Carmona, R.; Gonzalez-Iriarte, M.; Macias, D.; Guadix, J.A.; Munoz-Chapuli, R. Contribution of mesothelium-derived cells to liver sinusoids in avian embryos. Dev. Dyn. 2004, 229, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Chen, J.Y.; Lin, J.K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003, 63, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Chang, Y.T.; Pan, S.Y.; Chou, Y.H.; Chang, F.C.; Yeh, P.Y.; Liu, Y.H.; Chiang, W.C.; Chen, Y.M.; Wu, K.D.; et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J. Am. Soc. Nephrol. 2014, 25, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Wilm, T.P.; Tanton, H.; Mutter, F.; Foisor, V.; Middlehurst, B.; Ward, K.; Benameur, T.; Hastie, N.; Wilm, B. Restricted differentiative capacity of Wt1-expressing peritoneal mesothelium in postnatal and adult mice. Sci. Rep. 2021, 11, 15940. [Google Scholar] [CrossRef]
- Lv, Z.D.; Wang, H.B.; Dong, Q.; Kong, B.; Li, J.G.; Yang, Z.C.; Qu, H.L.; Cao, W.H.; Xu, H.M. Mesothelial cells differentiate into fibroblast-like cells under the scirrhous gastric cancer microenvironment and promote peritoneal carcinomatosis in vitro and in vivo. Mol. Cell. Biochem. 2013, 377, 177–185. [Google Scholar] [CrossRef]
- Wei, M.; Yang, T.; Chen, X.; Wu, Y.; Deng, X.; He, W.; Yang, J.; Wang, Z. Malignant ascites-derived exosomes promote proliferation and induce carcinoma-associated fibroblasts transition in peritoneal mesothelial cells. Oncotarget 2017, 8, 42262–42271. [Google Scholar] [CrossRef]
- Lv, Z.D.; Na, D.; Liu, F.N.; Du, Z.M.; Sun, Z.; Li, Z.; Ma, X.Y.; Wang, Z.N.; Xu, H.M. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J. Exp. Clin. Cancer Res. 2010, 29, 139. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, T.; Fushida, S.; Harada, S.; Yagi, Y.; Kinoshita, J.; Oyama, K.; Tajima, H.; Fujita, H.; Ninomiya, I.; Fujimura, T.; et al. The role of human peritoneal mesothelial cells in the fibrosis and progression of gastric cancer. Int. J. Oncol. 2012, 41, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Rynne-Vidal, A.; Au-Yeung, C.L.; Jimenez-Heffernan, J.A.; Perez-Lozano, M.L.; Cremades-Jimeno, L.; Barcena, C.; Cristobal-Garcia, I.; Fernandez-Chacon, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, P.; Jimenez-Heffernan, J.A.; Rynne-Vidal, A.; Perez-Lozano, M.L.; Gilsanz, A.; Ruiz-Carpio, V.; Reyes, R.; Garcia-Bordas, J.; Stamatakis, K.; Dotor, J.; et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J. Pathol. 2013, 231, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Guo, T. Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases. Cancers 2022, 14, 2856. https://doi.org/10.3390/cancers14122856
Li J, Guo T. Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases. Cancers. 2022; 14(12):2856. https://doi.org/10.3390/cancers14122856
Chicago/Turabian StyleLi, Junliang, and Tiankang Guo. 2022. "Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases" Cancers 14, no. 12: 2856. https://doi.org/10.3390/cancers14122856
APA StyleLi, J., & Guo, T. (2022). Role of Peritoneal Mesothelial Cells in the Progression of Peritoneal Metastases. Cancers, 14(12), 2856. https://doi.org/10.3390/cancers14122856





